Abstract
Recently, we reported that dyspnea on exertion is strongly associated with an increased oxygen cost of breathing in otherwise healthy obese women; the mechanism of dyspnea on exertion in obese men is unknown. Obese men underwent measurements of body composition, fat distribution, pulmonary function, steady state and maximal graded cycle ergometry, and oxygen cost of breathing. Nine men (34±8yr, 35±4 BMI) with ratings of perceived breathlessness of ≤ 2 during cycling, and ten men (36±9yr, 38±5 BMI) with ratings of perceived breathlessness ≥ 4 were studied (ratings of perceived breathlessness: 1.8±0.4 vs. 4.7±0.8, respectively; p<0.0001). Groups had only minor differences in fat distribution, pulmonary function, and steady state exercise. There was no association between ratings of perceived breathlessness and oxygen cost of breathing; but ratings of perceived breathlessness was strongly correlated with ratings of perceived exertion (RPE, rho=0.87, p<0.0001). The differences in exercise intensity, ventilatory demand, cardiovascular conditioning and/or the quality of respiratory sensation did not appear to play a role in the development of dyspnea on exertion. The mechanism of dyspnea on exertion in obese men seems unrelated to the oxygen cost of breathing.
Keywords: Obesity, Exercise, Oxygen cost of breathing, Breathlessness
1. INTRODUCTION1
Recently, we reported that dyspnea on exertion is strongly associated with an increased oxygen cost of breathing in otherwise healthy obese women (Babb et al., 2008a). The prevalence of dyspnea on exertion and its association with the oxygen cost of breathing are unknown in obese men but could be an extremely important factor in prescribing exercise as a treatment of obese men. Although the physiological mechanism of this relationship remains unclear in obese women, the associations among dyspnea on exertion, the oxygen cost of breathing, and pulmonary function could be quite different in obese men since fat distribution is dissimilar between obese men and women.
In obese adults, even a slight increase in ventilation (V̇E) from resting levels can yield a considerable increase in the oxygen cost of breathing (i.e., the rate of increase in oxygen uptake [V̇O2] from respiratory work) (Cherniack, 1959a; Gilbert et al., 1961; Kress et al., 1999). This rate increases precipitously at higher levels of V̇E, such as those encountered during exercise. In normal weight subjects, the oxygen cost of breathing at low ventilatory levels is an average of 1.2mL of oxygen/L of V̇E (Cherniack, 1959a; Cherniack, 1959b; Lorenzo and Babb, 2011) and 2.6mL/L at a V̇E of 100 L/min (Coast et al., 1993). In obese subjects the oxygen cost of breathing at low ventilatory levels can be as much as 3 times higher, at a rate of 3.0mL/L in otherwise healthy women (Babb et al. 2008a) to 3.5mL/L independent of gender and it increases disproportionately with further increases in ventilation (Kaufman et al., 1959; Cherniack, 1959a). When the oxygen cost of breathing is increased, the level of central respiratory motor output required to obtain a given level of ventilatory output rises, and if the oxygen cost of breathing and/or respiratory drive are out of proportion to the exercise intensity, this could elicit dyspnea (Meek et al., 1999). Thus, an increased oxygen cost of breathing could be an important factor in the manifestation of dyspnea on exertion in obese men just as in obese women.
Alternatively, in many cases obese patients with dyspnea on exertion are considered to be deconditioned. However, in contrast to conventional thinking, in our earlier studies in obese adults and obese women with dyspnea on exertion, we have not found them to be deconditioned. Thus, it remains unclear if dyspnea on exertion in otherwise healthy obese men is due to cardiovascular deconditioning or to obesity-related changes in respiratory function. Dyspnea on exertion is not only an important and prolific clinical concern in obese men, it is an obstacle to the prevention and treatment of obesity (U.S.Department of Health and Human Services -National Institutes of Health, 2004).
Lastly, breathlessness can be described in terms of intensity, i.e. how weak or strong the sensation is, as well as in terms of quality, i.e. what the sensation feels like. It has been shown that patients with shortness of breath can be distinguished based on their qualitative descriptors of breathlessness (Simon et al., 1989; Mahler et al., 1996; O’Donnell et al., 1997). The perception of the quality of respiratory sensations may be associated with the origin of the intensity of breathlessness. While the intensity of breathlessness may be increased in some obese individuals it is unknown if the quality of breathing sensation differs between obese men with or without dyspnea on exertion.
We hypothesized that in obese men with dyspnea on exertion (+DOE) as compared with obese men without dyspnea on exertion (−DOE): 1) peak oxygen uptake would not be decreased, 2) the oxygen cost of breathing would be increased; 3) the ratings of perceived breathlessness during exercise would be associated with the increased oxygen cost of breathing, and 4) the quality of respiratory sensations would be different.
2. METHODS
Written informed consent was obtained before participation (University of Texas Southwestern Medical Center IRB Committee, approval #0703438). Volunteers were recruited by advertisements, flyers, and word-of-mouth. Volunteers were screened by body mass index (BMI) (≥30≤45) and pulmonary function (no significant obstruction, FEV1/FVC > 0.7). No subject had a history of asthma, cardiovascular disease, or musculoskeletal abnormalities that would preclude maximal exercise. None of the subjects participated in regular vigorous exercise for the last 6 months, or had a significant smoking history (<5 pack year, n=2). After screening, the participants returned on three separate occasions for fat distribution measurements, exercise testing, and determination of the oxygen cost of breathing.
2.1. Subjects
As subjects were enrolled, they were assigned to one of two groups according to their ratings of perceived breathlessness (0–10 Borg Scale) during steady state cycling at 90W. Those with a ratings of perceived breathlessness of ≥ 4 were designated as obese men with dyspnea on exertion (+DOE, n=10) and those with a ratings of perceived breathlessness of ≤ 2 were designated as obese men without dyspnea on exertion (−DOE, n=9). To better delineate differences between groups, men with a rating of perceived breathlessness of 3 were excluded from further study (n=8, 30%). The work rate and grouping was based on our previous finding that obese men have an average ratings of perceived breathlessness of 2 ± 2 at ventilatory threshold during incremental exercise, which occurred at 105 ± 16W (DeLorey et al., 2005).
2.2. Body Composition and Fat Distribution
Measurements of height, weight, body circumference, and underwater weighing (%body fat, lean body mass, and total body fat mass (Babb et al. 2008a; Babb et al. 2008b)) were collected.
Multiple MRI scans (i.e., whole body magnet) through the chest and abdomen were used to estimate subcutaneous chest fat, anterior subcutaneous abdominal fat, visceral fat, posterior subcutaneous fat, and peripheral fat as previously described (Abate et al., 1994; Abate et al., 1995; Abate et al., 1996; Abate et al., 1997). The images were analyzed with interactive software (Wafter, version 1.3, Dallas, TX) (Thomas et al., 1998); (Perry et al., 2000). The data were similar to those produced by comparable MRI techniques (Ross et al., 1992; Thomas et al. 1998; Kamel et al., 2000).
2.3. Pulmonary Function
All subjects underwent standard spirometry, lung volume, and diffusing capacity determinations (model V62W body plethysmograph, SensorMedics, Yorba Linda, CA) according to American Thoracic Society guidelines (American Thoracic Society, 1995). Predicted values were based on published norms (Goldman and Becklake, 1959; Burrows et al., 1961; Knudson et al., 1976; Knudson et al., 1983). Resting maximal flow-volume loops were measured in a pressure-corrected volume-displacement body plethysmograph to eliminate the gas compression artifact (SensorMedics 6200).
2.4. Cardio-respiratory Responses during Exercise at 90W
Testing began with the subjects seated on the cycle ergometer (model CPE 2000; MedGraphics); after 3 min of baseline measurements, subjects performed a 6-min steady state exercise cycling test at 90W. Ventilation (V̇E), gas exchange (V̇O2 and V̇CO2) and breathing mechanics were measured at rest and during exercise as previously described (Babb, 1997b). Inspiratory capacity (IC) was measured at rest and during the last 20s of each exercise increment to determine placement of tidal flow-volume loops within the maximal flow-volume loop as previously described (Babb, 1997a; Babb, 1997b). Measurement of IC was performed by having the subject, on cue from the investigator, inhale maximally to total lung capacity (TLC) (Babb and Rodarte, 1993). End-expiratory lung volume (EELV) was estimated from IC during exercise and TLC during body plethysmography (EELV=TLC-IC) and reported as %TLC (Babb et al., 2002; DeLorey et al. 2005). Expiratory flow limitation was defined as the percentage of Vt (%Vt) where tidal expiratory flow impinged on maximal expiratory flow
2.5. Intensity and Quality of Respiratory Sensations during Exercise at 90W
All participants were given detailed written instructions for rating the intensity of breathlessness and exertion (RPE) during exercise as described previously (Babb et al. 2008a). Scales (0–10 Borg scale for rating of perceived breathlessness, 6–20 Borg scales for RPE) with verbal expressions of severity anchored to specific numbers were used (Borg, 1982). Ratings of perceived breathlessness and RPE were collected every two minutes of the test and the last value recorded was used for analysis. Following the exercise test subjects were seated in a chair and asked to complete a breathlessness questionnaire to examine the quality of the respiratory sensations. The questionnaire consisted of 15 descriptors relating to breathlessness adapted from Simon et al (Simon et al., 1990) and Mahler et al (Mahler et al. 1996) (Table 1). Subjects were instructed to select the “best three descriptors” that most closely described their respiratory sensations experienced during the exercise.
Table 1.
Breathlessness descriptors to examine the quality of respiratory sensations
| Breathlessness Descriptors | |
|---|---|
| 1 | My breathing requires effort |
| 2 | I feel out of breath |
| 3 | My breathing requires work |
| 4 | I cannot get enough air |
| 5 | I feel that I am smothering |
| 6 | I feel that I am suffocating |
| 7 | My breath does not go out all the way |
| 8 | My chest feels tight |
| 9 | My chest is constricted |
| 10 | My breath does not go in all the way |
| 11 | My breathing is shallow |
| 12 | I feel that my breathing is rapid |
| 13 | I feel that I am breathing more |
| 14 | My breathing is heavy |
| 15 | I feel hunger for air |
2.6. Peak Oxygen Uptake
Following the steady state exercise test and a short rest period, peak oxygen uptake was determined by graded cycle ergometry to volitional exhaustion or pedal rate ≤ 50 rpm. Testing began with the subjects seated on the cycle ergometer for 3 min of baseline measurements. Subjects started pedaling at 60–65 rpm with an initial work rate of 30 W. Work rate was increased by 30 W each minute until termination of test and evidenced by approaching predicted peak heart rate (HR), peak work rate, peakV̇O2, and/or RER>1.1.
2.7. Oxygen Cost of Breathing
Oxygen cost of breathing was determined from 6-min measurements of V̇O2 and V̇E at rest and 4-min measurements during eucapnic voluntary hyperventilation (EVH) at 60L/min and 90L/min as previously described (Babb, 1997b; Rundell et al., 2004; Babb et al. 2008a). The oxygen cost of breathing was assessed by calculating the slope of the linear regression between V̇O2 (mL/min) versus V̇E (L/min) at rest and during the two levels of EVH.
2.8. Data Analysis
Differences between groups were determined by independent t-test. Relationships among variables were determined with Pearson or Spearman correlation coefficients. Fisher’s exact tests were used to compare selection frequencies of breathlessness descriptors between groups. The primary variable of this study was the oxygen cost of breathing between obese men with and without dyspnea on exertion. Based on our previously published findings in obese women (Babb et al. 2008a), we expected a difference in means of O2 cost slopes between +DOE and −DOE groups of 0.74 and a pooled SD of 0.60. Under these assumptions, only 12 subjects (six per group) were required to achieve 80% power at a nominal alpha of 0.05 using independent t-test sample size calculation. Values are reported as mean±SD. A p value of <0.05 was considered significant.
3. RESULTS
3.1. Subjects Characteristics, Body Composition, and Fat Distribution
Subject characteristics were not significantly different between the obese men −DOE and +DOE with the exception of waist circumference, which was larger in men +DOE (Table 2). Nine of the obese men were classified as mild (Class I), 5 as moderate (Class II), and 5 as extreme (Class III) based on the NHLBI clinical guidelines for BMI. While visceral fat and total abdominal fat were significantly higher (p < 0.05) in the obese men +DOE, abdominal fat as a percent of total fat weight was not different, indicating abdominal fat distribution was not significantly greater in these men (Table 3). There was no significant correlation between the total abdominal fat (as percent of total fat weight) and the ratings of perceived breathlessness (p > 0.05).
Table 2.
Subject Characteristics
| Group | Obese −DOE (n = 9) | Obese +DOE (n = 10) | P value |
|---|---|---|---|
| Age (yr) | 34 ± 8 | 36 ± 9 | 0.58 |
| Height (cm) | 179 ± 7 | 180 ± 4 | 0.66 |
| Weight (kg) | 112 ± 19 | 125 ± 14 | 0.12 |
| Body Mass Index (kg/m2) | 35 ± 4 | 38 ± 5 | 0.10 |
| Body Fat (%) | 36 ± 4 | 40 ± 5 | 0.11 |
| Fat Weight (kg) | 41 ± 12 | 50 ± 11 | 0.11 |
| Lean Body Mass (kg) | 72 ± 8 | 75 ± 6 | 0.29 |
| Chest circumference (cm) | 114 ± 9 | 122 ± 8 | 0.07 |
| Waist circumference (cm) | 115 ± 10 | 127 ± 13 | 0.04 |
| Hip circumference (cm) | 118 ± 11 | 126 ± 09 | 0.09 |
| Waist/Hip (ratio) | 0.98 ± 0.06 | 1.01 ± 0.06 | 0.37 |
Values are means ± SD. DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; Significant, p≤0.05.
Table 3.
Fat Distribution
| Group | Obese −DOE (n = 9) | Obese +DOE (n = 10) | P value |
|---|---|---|---|
| Chest Fat (kg) | 5.2 ± 2.0 | 6.5 ± 1.4 | 0.12 |
| Chest Fat (%FW) | 13 ± 2 | 13 ± 2 | 0.56 |
| Ant Sub Q Abd Fat (kg) | 5.1 ± 1.8 | 6.7 ± 3.2 | 0.20 |
| Ant Sub Q Abd Fat | 12 ± 2 | 13 ± 4 | 0.64 |
| Visceral Fat (kg) | 5.3 ± 2.0 | 8.1 ± 2.5 | 0.02 |
| Visceral Fat (%FW) | 13 ± 4 | 17 ± 6 | 0.12 |
| Post Sub Q Fat (kg) | 6.6 ± 2.4 | 8.1 ± 2.8 | 0.24 |
| Post Sub Q Fat (%FW) | 16 ± 3 | 16 ± 3 | 0.88 |
| Total Chest Wall Fat | 22.2 ± 7.3 | 29.3 ± 8.0 | 0.06 |
| Total Chest Wall Fat | 54 ± 7 | 58 ± 6 | 0.14 |
| Peripheral Fat (kg) | 18.8 ± 6.0 | 20.5 ± 4.8 | 0.52 |
| Peripheral Fat (%FW) | 46 ± 7 | 41 ± 6 | 0.14 |
| Total Abdominal Fat | 10.4 ± 3.5 | 14.8 ± 4.1 | 0.02 |
| Total Abdominal Fat | 30 ± 4 | 25 ± 5 | 0.06 |
Values are means ± SD. DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; %FW, percent fat weight; Chest, rib cage fat; Ant SubQ Abd fat, anterior subcutaneous abdominal fat; Post SubQ, posterior subcutaneous fat; Peripheral = total fat – chest fat - anterior subcutaneous abdominal fat - visceral fat - posterior subcutaneous fat. Total abdominal fat = Ant SubQ Abd fat + visceral fat. Significant, p≤0.05.
3.2. Pulmonary Function
All subjects had normal pulmonary function, but forced vital capacity (FVC) and TLC (%predicted) were lower (p < 0.05) in the obese men +DOE (Table 4). Maximal inspiratory and expiratory pressures were normal with no significant differences between groups. BMI and %body fat were not significantly correlated with FVC, but BMI and TLC (%predicted) were moderately correlated (r = −0.50, p = 0.03), as was %body fat and FRC (%TLC) (r = −0.51, p = 0.03).
Table 4.
Pulmonary Function
| Group | Obese −DOE (n = 9) | Obese +DOE (n = 10) | P value |
|---|---|---|---|
| FVC (L) | 5.6 ± 0.8 | 5.1 ± 0.4 | 0.14 |
| FVC (% pred) | 105 ± 7 | 95 ± 8 | 0.01 |
| FEV1 (L) | 4.3 ± 0.6 | 4.1 ± 0.3 | 0.38 |
| FEV1 (% pred) | 98 ± 9 | 92 ± 8 | 0.15 |
| FEV1/FVC (%) | 77 ± 6 | 80 ± 6 | 0.29 |
| PEF (L/min) | 10.0 ± 1.4 | 9.9 ± 0.9 | 0.79 |
| PEF (% pred) | 106 ± 15 | 102 ± 9 | 0.52 |
| MVV (L/min) | 164 ± 18 | 158 ± 21 | 0.52 |
| MVV (% pred) | 94 ± 9 | 90 ± 12 | 0.54 |
| DLco (% pred) | 88 ± 5 | 82 ± 9 | 0.14 |
| DLco/VA (% pred) | 108 ± 12 | 113 ± 10 | 0.32 |
| TLC (% pred) | 102 ± 9 | 93 ± 9 | 0.05 |
| FRC (%TLC) | 36 ± 4 | 35 ± 5 | 0.47 |
| RV (% pred) | 83 ± 20 | 72 ± 16 | 0.21 |
| RV/TLC (%) | 22 ± 4 | 22 ± 5 | 0.80 |
| FRC (% pred TLC) | 37 ± 6 | 32 ± 6 | 0.13 |
| MIP (% pred) | 115 ± 19 | 99 ± 21 | 0.09 |
| MEP (% pred) | 105 ± 20 | 103 ± 31 | 0.82 |
| ERV (%TLC) | 15 ± 4 | 12 ± 6 | 0.22 |
Values are mean ± SD. DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; FVC, forced vital capacity; L, liter; %pred, percent of predicted; FEV1, forced expiratory volume in one second; PEF, peak expiratory flow; MVV, measured maximal voluntary ventilation; DLco, diffusing capacity of the lung; VA, alveolar volume; TLC, total lung capacity; FRC, functional residual capacity (note: reported as %TLC); RV, residual volume; and ns, non-significant. MIP, maximal inspiratory pressure; MEP, Maximal expiratory pressure. Predicted values for spirometry, lung volumes, and diffusing capacity were based on the norms of Knudson et al., 28,29, Goldman and Becklake 31, and Burrows et al., 30, respectively.
3.3. Cardio-respiratory Responses during Exercise at 90W
Cardio-respiratory measurements made during steady state exercise at 90W were not significantly different between the men +DOE and −DOE, with the exception of V̇O2 (L/min) (Table 5). Based on V̇O2 (%peakV̇O2), HR (%peakHR), V̇E/V̇CO2, and V̇E (V̇E/maximal voluntary ventilation (MVV) ratio), the relative intensity of exercise and ventilatory demand were not different between the two groups during exercise at 90W. RPE during steady state exercise was 10±2 for the obese men −DOE and 14±1 for the obese men +DOE (p<0.001). Ratings of perceived breathlessness during steady state exercise was not strongly correlated with any cardio-respiratory measurement, with the exception of RPE (rho = 0.87, p < 0.0001).
Table 5.
Steady State Exercise at 90W
| Group | Obese −DOE (n = 9) | Obese +DOE (n = 10) | P value |
|---|---|---|---|
| V̇O2 (L/min) | 1.60 ± 0.10 | 1.74 ± 0.14 | 0.03 |
| V̇O2 (%Peak) | 55 ± 9a | 59 ± 7 | 0.32 |
| V̇CO2 (L/min) | 1.56 ± 0.16 | 1.69 ± 0.16 | 0.09 |
| V̇E (L/min) | 44 ± 8 | 49 ± 7 | 0.23 |
| VT (L) | 2.20 ± 0.38 | 2.44 ± 0.54 | 0.28 |
| Fb (bpm) | 21 ± 7 | 21 ± 7 | 0.95 |
| V̇E/V̇CO2 | 28 ± 4 | 29 ± 2 | 0.71 |
| PETCO2 (torr) | 45 ± 4 | 45 ± 3 | 0.88 |
| V̇E/MVV (%) | 28 ± 7 | 31 ± 6 | 0.22 |
| EELV (%TLC) | 40 ± 4a | 35 ± 5 | 0.07 |
| Resting EELV | 42 ± 6 | 37 ± 6 | 0.08 |
| HR (% Peak) | 68 ± 9 | 70 ± 6 | 0.62 |
| VT (%TLC) | 30 ± 5 | 36 ± 7 | 0.08 |
| RER | 0.97 ± 0.07 | 0.98 ± 0.06 | 0.92 |
| EILV (%TLC) | 71 ± 6a | 71 ± 9 | 0.86 |
| RPB | 1.78 ± 0.44 | 4.70 ± 0.82 | < 0.0001 |
| RPE | 10 ± 2 | 14 ± 1 | < 0.001 |
Values are mean ± SD. DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; V̇O2, oxygen uptake; V̇CO2, carbon dioxide production; V̇E, minute ventilation; VT, tidal volume; Fb, breathing frequency; V̇E/V̇CO2, ventilatory equivalent for CO2; PETCO2, end-tidal CO2, MVV, maximal voluntary ventilation; RER, respiratory exchange rate; EILV, end-inspiratory lung volume; EELV, end-expiratory lung volume; HR, heart rate percent of peak heart rate; and ns, non-significant; RPB, ratings of perceived breathlessness; RPE, ratings of perceived exertion;
n=8.
EELV (%TLC) during steady state cycling was not significantly different between the obese men +DOE and −DOE (Table 5). However, further examination of the flow-volume loops revealed that the men +DOE were breathing closer to their residual volume (RV) (i.e., bottom of their FVC) due to a lower (p = 0.05) expiratory reserve volume (ERV, %TLC) during exercise at 90W (11±6% compared with 16±4% in men −DOE). The smaller ERV places the tidal flow-volume loop in the lower portion of the FVC where there is greater potential for tidal expiratory flow limitation. However, only two men +DOE (15 and 22%tidal volume (VT)) and two men −DOE (25 and 26 %VT) had expiratory flow limitation during exercise at 90W.
3.4. Intensity and Quality of Respiratory Sensation during Exercise at 90W
Ten (37%) of the twenty-seven subjects recruited had a rating of perceived breathlessness ≥ 4 (+DOE) during steady state cycling at 90W as compared with nine men −DOE (33%). Overall, ratings of perceived breathlessness during exercise was more than twice as high (p < 0.01) in +DOE (4.7 ± 0.8) than in −DOE (1.8 ± 0.4). Ratings of perceived breathlessness were not strongly correlated with any measurements of waist circumference, body composition, fat distribution, or pulmonary function.
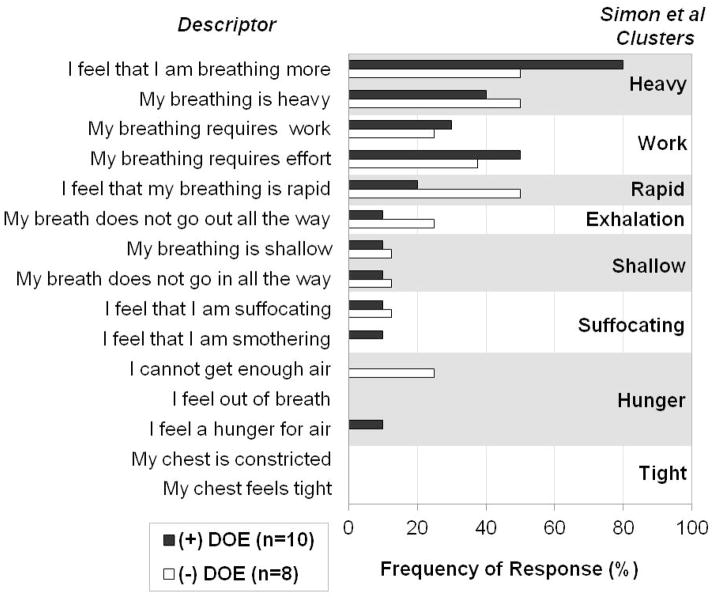
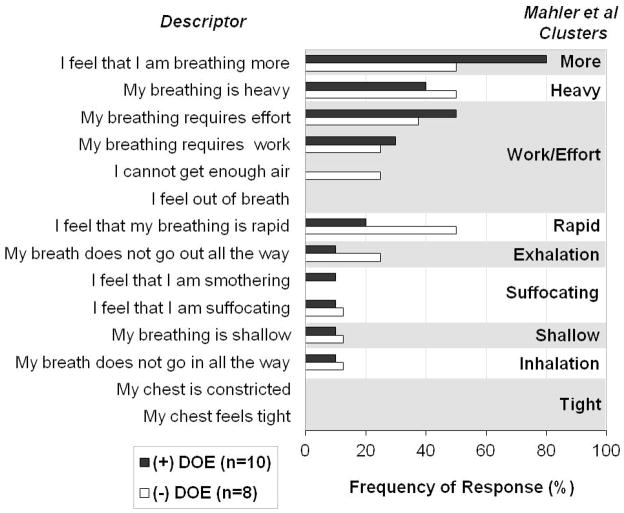
65% of all subjects selected the breathlessness descriptors “I feel that I am breathing more”, 44% chose “my breathing is heavy”, and 44% picked “my breathing requires effort”. Both +DOE and −DOE groups chose descriptors with similar frequency, only “I feel that I am breathing more” (80% of +DOE subjects selected this statement, 50% of −DOE) and “I feel that my breathing is rapid” (+DOE 20%, −DOE 50%) differed slightly but not significantly between the groups (p=0.321). The 15 descriptors were compared with the cluster analyses published by Simon et al (Simon et al. 1990) and Mahler et al (Mahler et al. 1996) (Figure 1 and 2). The +DOE group was characterized by the clusters “Breathing more” (80%), “Work/Effort” (50%), and “Breathing heavy” (40%), while the −DOE group was most closely associated with the clusters “Breathing heavy” (50%), “Breathing more” (50%), and “Breathing rapid” (50%).
Figure 1.
Most common descriptors and clusters selected by obese men with (+DOE) and without dyspnea on exertion (−DOE) based on cluster analysis by Simon et al (Simon et al. 1990). Percentage of subjects per group who chose the descriptors as one of the “best three” that applied to their respiratory sensations they felt during steady state cycling exercise at 90W.
Figure 2.
Most common descriptors and clusters selected by obese men with (+DOE) and without dyspnea on exertion (−DOE) based on cluster analysis by Mahler et al (Mahler et al. 1996). Percentage of subjects per group who chose the descriptors as one of the “best three” that applied to their respiratory sensations they felt during steady state cycling exercise at 90W.
3.5. Peak Oxygen Uptake
Exercise capacity was not different between the men +DOE and −DOE based on peak work rate, exercise time, HR, and V̇O2 (Table 6). None of the cardiorespiratory measures at peak exercise was different. In mL/min/kg (Table 6), exercise capacity was lower in the obese men +DOE (p < 0.05); but, we consider this measurement misleading due to slight increases in age, and body weight in the men +DOE, and not truly representative of cardiovascular conditioning (Lorenzo and Babb, 2011). In percent predicted (Wasserman et al., 2005), peakV̇O2 was not different between the obese men +DOE and −DOE (Table 6). There was no significant correlation between ratings of perceived breathlessness measured during exercise at 90W and any measure of peak exercise.
Table 6.
Peak Exercise
| Group | Obese −DOE (n = 9) | Obese +DOE (n = 10) | P value |
|---|---|---|---|
| Work Rate (W) | 250 ± 45 | 240 ± 28 | 0.56 |
| Exercise Time (min) | 8.2 ±1.6 | 7.9 ± 1.0 | 0.57 |
| Heart Rate (% pred) | 96 ± 6 | 95 ± 7 | 0.69 |
| V̇O2 (L/min) | 3.06 ± 0.52 | 3.00 ± 0.38 | 0.77 |
| RER | 1.22 ± 0.06 | 1.25 ± 0.08 | 0.32 |
| V̇E (L/min) | 121 ± 20 | 130 ± 19 | 0.35 |
| VT (L) | 2.96 ± 0.56 | 3.00 ± 0.84 | 0.89 |
| Fb (bpm) | 42 ± 8 | 46 ± 11 | 0.37 |
| V̇E/V̇CO2 | 33 ± 1 | 35 ± 4 | 0.11 |
| PETCO2 (torr) | 36 ± 3 | 34 ± 4 | 0.30 |
| V̇E/MVV (%) | 74 ± 7 | 83 ± 16 | 0.10 |
| RPE (6–20) | 18 ± 1 | 19 ± 1 | 0.13 |
| RPB (0–10) | 8 ± 2 | 8 ± 2 | 0.49 |
| VT (%TLC) | 40.61 ± 3.80 | 43.84 ± 9.58 | 0.35 |
| EELV | 43 ± 4 | 41 ± 3 | 0.20 |
| V̇O2 (mL/min/kg) | 27.0 ± 2.4 | 24.1 ± 3.4 | 0.05 |
| V̇O2 (mL/min/kgLBM) | 42.6 ± 2.8 | 39.8 ± 3.5 | 0.08 |
| V̇O2 (% pred) | 104 ± 12 | 104 ± 14 | 0.96 |
| V̇O2 (% pred + corr) | 96 ± 11 | 94 ± 13 | 0.72 |
Values are mean ± SD. DOE, dyspnea on exertion; DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; Exercise Time, exercise time to peak; V̇O2, oxygen uptake; RER, respiratory exchange ratio; V̇E, minute ventilation; VT, tidal volume; Fb, breathing frequency; bpm, breaths per minute; MVV, measured maximal voluntary ventilation; RPE, ratings of perceived exertion; RPB, ratings of perceived breathlessness; PETCO2, end-tidal CO2; LBM, lean body mass; %Pred, percent predicted; Corr, corrected; and ns, non-significant. While interpretation of peakV̇O2 in determining cardiovascular conditioning in obesity is a complex issue, the recommendation is to use a method whereby peakV̇O2 is compared with an age, gender, and weight-corrected predicted peakV̇O2 51,52,53. Thus, we used the following equation adapted from Wasserman et al., [predicted peak V̇O2 = ((predicted peak V̇O2 in ml/min/kg)*predicted wt) + ((actual wt − predicted wt) * 6 ml/kg)] 37,54,55.
3.6. Oxygen Cost of Breathing and Breathing Mechanics
Oxygen cost of breathing was not significantly different in the obese men +DOE compared with −DOE (2.17±0.84 vs. 2.01± 0.75mL/L, respectively, p=0.67). There was no significant relationship between the oxygen cost of breathing and the rating of perceived breathlessness during steady state exercise at 90W. Also, there was no significant relationship between the oxygen cost of breathing and waist circumference or waist-to-hip ratio.
Ratings of perceived breathlessness, V̇E, VT, Fb, V̇O2, EELV, and ERV (%TLC) were not different between groups during the 60 and 90L/min bouts of EVH (Table 7). Note that EELV rises during EVH unlike during exercise when EELV falls. Only three men +DOE (19, 28, and 15%VT) and two men −DOE (29 and 16%VT) exhibited expiratory flow limitation.
Table 7.
Breathing Mechanics during Eucapnic Voluntary Hyperpnea
| V̇E (L/min) | VT (L) | Fb (bpm) | V̇O2 (L/min) | PETCO2 (torr) | EELV (%TLC) | EILV (%TLC) | RPB (0–10) | ||
|---|---|---|---|---|---|---|---|---|---|
| Rest EVH | Obese −DOE (n = 9) | 10 ± 1 | 0.8 ± 0.4 | 14 ± 6 | 0.29 ± 0.03 | 41 ± 3 | 41 ± 5 | 52 ± 4 | 0 ± 0 |
| Obese +DOE (n = 10) | 10 ± 2 | 0.9 ± 0.2 | 11 ± 4 | 0.32 ± 0.04 | 42 ± 3 | 38 ± 6 | 52 ± 7 | 1 ± 1 | |
| P value | 0.65 | 0.53 | 0.34 | 0.03 | 0.49 | 0.39 | 0.95 | 0.02 | |
| 60L/min EVH | Obese −DOE (n = 9) | 62 ± 1 | 1.7 ± 0.1 | 36 ± 2 | 0.38 ± 0.03 | 43 ± 1 | 45 ± 9 | 70 ± 8 | 2 ± 1 |
| Obese +DOE (n = 10) | 62 ± 2 | 1.7 ± 0.1 | 36 ± 1 | 0.41 ± 0.04 | 44 ± 1 | 41 ± 7 | 67 ± 6 | 2 ± 1 | |
| P value | 0.92 | 0.57 | 0.65 | 0.08 | 0.28 | 0.31 | 0.38 | 0.70 | |
| 90L/min EVH | Obese −DOE (n = 9) | 91 ± 3 | 2.3 ± 0.1 | 40 ± 0.7 | 0.47 ± 0.07 | 42 ± 1 | 46 ± 9 | 78 ± 8 | 3 ± 1 |
| Obese +DOE (n = 10) | 92 ± 2 | 2.3 ± 0.1 | 40 ± 0.8 | 0.49 ± 0.05 | 42 ± 1 | 41 ± 4 | 75 ± 4 | 4 ± 1 | |
| P value | 0.70 | 0.60 | 0.67 | 0.48 | 0.97 | 0.10 | 0.34 | 0.30 |
Values are mean ± SD. EVH, eucapnic voluntary hyperpnea; DOE, dyspnea on exertion; DOE, dyspnea on exertion; −DOE, without DOE; +DOE, with DOE; V̇E, minute ventilation; VT, tidal volume; Fb, breathing frequency; V̇O2, oxygen uptake; PETCO2, end-tidal CO2; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; RPB, ratings of perceived breathlessness; and ns, non-significant. O2 cost (mL/L), 2.17 ± 0.84 and 2.01 ± 0.75, respectively (p = 0.67); Pearson correlation coefficient r2, 0.97 ± 0.03 and 0.96 ± 0.03; O2 cost intercept (mL), 264 ± 33 and 299 ± 37, respectively (p = 0.05).
4. DISCUSSION
Of the obese men recruited, 37% had an elevated rating of perceived breathlessness during exercise at 90W. However, in contrast to our hypothesis, and our earlier findings in obese women (Babb et al. 2008a), the oxygen cost of breathing was not greater in obese men +DOE than in obese men −DOE (i.e., it was equally elevated in both groups compared with normal weight individuals), nor was it significantly associated with ratings of perceived breathlessness during exercise. Furthermore, the obese men +DOE and −DOE had only minor differences in subject characteristics, fat distribution, pulmonary function, submaximal cardio-respiratory exercise responses, or breathlessness descriptors. None of these measurements was significantly associated with ratings of perceived breathlessness during steady state exercise at 90W. Thus, differences in exercise intensity, ventilatory demand, cardiovascular conditioning and/or the quality of respiratory sensation did not appear to play a role in the development of dyspnea on exertion in the obese men we studied. However, ratings of perceived breathlessness was strongly correlated with RPE during steady state exercise at 90W, suggesting that not only was the perception of dyspnea on exertion increased in a large proportion of obese men, but also the perception of exercise intensity. The mechanism of the increased rating of perceived breathlessness is unclear.
4.1. Intensity of Respiratory Sensations in Obese Men
Dyspnea on exertion is a common complaint of obese adults (Whipp and Davis, 1984; Sahebjami, 1998; Gibson, 2000; Ferretti et al., 2001; Sin et al., 2002). We observed 37% of the otherwise healthy, obese men we studied to have a significantly elevated ratings of perceived breathlessness during exertion, which is similar to our previous finding in obese women (Babb et al. 2008a). Although we cannot address the true prevalence of dyspnea on exertion in obese men from this study, it is reasonable to infer that a large proportion of obese men experience significant dyspnea on exertion, which is in agreement with survey studies such as the National Health and Nutrition Examination Survey III (Sin et al. 2002).
We also found RPE to be increased in the obese men and a strong association between ratings of perceived breathlessness and RPE. RPE, unlike rating of perceived breathlessness, was moderately related (r≈0.50, p<0.05) to indicators of exercise intensity (i.e., HR, V̇O2, and V̇E as percent of peak or MVV). These findings would suggest that in a large proportion of obese men there is a related increase in both the perception of breathing and exertion.
4.2. Cardiovascular Conditioning in Obese Men
There was no difference in cardiovascular conditioning between obese men +DOE and −DOE. Furthermore, cardiovascular conditioning was within normal limits in both groups (Table 6). As in previous investigations, cardiovascular conditioning in otherwise healthy obese adults appears to be normal (Salvadori et al., 1992; Babb et al. 2002; DeLorey et al. 2005; Babb et al., 2011) and unrelated to dyspnea on exertion (Babb et al. 2008a), even though this remains a popular clinical convention. Nevertheless, participation in an exercise training program may help decrease both perceived exertion and breathlessness in obese men since they are related. In contrast, obese women with dyspnea on exertion may benefit more from a weight loss program to decrease the oxygen cost of breathing and rating of perceived breathlessness than an exercise program, but this has not been tested.
4.3. Oxygen Cost of Breathing and Lack of Association with Dyspnea On Exertion
Based on our findings in obese women (Babb et al. 2008a), we hypothesized that the oxygen cost of breathing would be increased in obese men +DOE compared with men −DOE, and that ratings of perceived breathlessness during 90W of cycling would be correlated with the increase in the oxygen cost of breathing; however, the current study failed to support these hypotheses in obese men. Not only was the oxygen cost of breathing not increased in the obese men +DOE, but the values in both groups of men were similar to the increased values observed in obese women −DOE (obese men +DOE: 2.2mL/L, obese men −DOE: 2.0mL/L, obese women +DOE: 3.0mL/L, obese women −DOE: 1.8mL/L) (Babb et al. 2008a). However, the oxygen cost of breathing was elevated (≈80% more) in all the obese men studied compared with previously published data on nonobese men (Lorenzo and Babb, 2011). Although the obese women weighed less than the obese men, the fat load on the chest wall (i.e., anterior subcutaneous fat + posterior subcutaneous fat + visceral fat) was very similar between the genders. The mechanism of the increased oxygen cost of breathing in the women with dyspnea on exertion is currently unclear, although an increase in mechanical work of breathing (Milic-Emili and D’angelo, 1997; Milic-Emili and Orzalesi, 1998), a decreased efficiency of the respiratory muscles (Cherniack and Guentter, 1961; Kress et al. 1999), or a increased level of ventilation (Kaufman et al. 1959) could be factors. In the obese men, subject characteristics, fat distribution, pulmonary function, submaximal cardio-respiratory responses, quality of respiratory sensations, peak exercise performance, and oxygen cost of breathing were all similar. Thus, it is unknown why ratings of perceived breathlessness during exercise would be increased in some obese men and not others but it does not appear to be related to an increased oxygen cost of breathing as in the women +DOE. Rather it appears to be related more to an increase in perception of breathing and exercise. It is unclear whether exercise training and/or weight loss would reduce breathlessness in obese men +DOE.
4.4. Quality of Respiratory Sensations in Obese Men
Surprisingly, although the intensity of breathlessness is different between obese men +DOE and −DOE, they still describe their respiratory sensations similarly during submaximal exercise. Thus, it seems that the intensity or quantity of the sensations, not the quality, is the factor that distinguishes the two groups. This is an important novel finding and substantiates the notion that the intensity and quality of breathlessness are independently perceived. The separation between intensity and quality of respiratory sensations is in line with previous work by Simon and coworkers (Simon et al. 1989; Simon et al. 1990) who demonstrated that different qualitative breathlessness clusters could nevertheless have the same intensity ratings. This refutes our hypothesis that the difference in intensity ratings originates in differing qualities of breathlessness.
Simon’s group developed 8 clusters from the 15 descriptors based on their studies in healthy adults and patients with shortness of breath, including pulmonary vascular, neuromuscular and interstitial lung disease, congestive heart failure, asthma, chronic obstructive pulmonary disease, and pregnancy. The experimentally induced respiratory stimulus condition ‘exercise’ in healthy volunteers elicited the sensation clusters “heavy” and “rapid”. These two clusters were found to be two of the highest represented in the current study as well. In fact, the cluster “heavy” was unique to the “exercise” condition. None of the other conditions, such as breathholding, CO2 inhalation, resistive or elastic loading, elicited the same combination of clusters (Simon et al. 1989). Furthermore, none of the patients with shortness of breath and/or cardiorespiratory disease chose this particular set of clusters to describe their respiratory sensation (Simon et al. 1990; Mahler et al. 1996) indicating that the obese individuals in the present study describe their breathlessness during exercise using the same words as normal healthy volunteers.
Based on Mahler et al.’s study (Mahler et al. 1996) the descriptors “my breathing is heavy” and “I feel that I am breathing more” did not cluster together as they did in Simon et al’s (Simon et al. 1989) analysis. The top four clusters found in the present study included the single-descriptor clusters “more”, “heavy”, and “rapid” as well as the four-descriptor cluster “work/effort”. These clusters were found in Mahler et al.’s study to be unique to a group they called “deconditioned” based on a lower than predicted peak V̇O2. However, the results of the current study clearly showed that our participants did not have lower than predicted values. This sharing of the same clusters by Mahler’s deconditioned patients and our non-deconditioned obese individuals suggests that the sensation of breathlessness may be mediated by similar receptors or neural pathways (Simon et al. 1990). This does not seem that surprising in terms of the mechanism by which ventilation rises from rest to exercise in deconditioned and obese subjects. Deconditioned individuals have to increase minute ventilation with lower exercise work rates than their normal counterparts and this may be sensed as an increased respiratory drive leading to dyspnea on exertion. In contrast, obese subjects do not have an increased V̇E compared with lean individuals, however, they also have an increased drive to the respiratory muscles in order to inflate the lungs against the load of the chest wall and abdominal mass resulting in dyspnea on exertion (El Gamal et al., 2005; Steier et al., 2009; Jensen et al., 2009). Thus, the sensation of breathing discomfort may be perceived via the increased respiratory drive necessary to maintain V̇E with additional respiratory work.
4.5. Conclusion
In conclusion, the oxygen cost of breathing was not increased in obese men +DOE, nor was it associated with ratings of perceived breathlessness during cycling. However, obese men +DOE do experience an increase in ratings of perceived breathlessness and RPE during exercise. The mechanism of this increased perception deserves further study in obese men. Also, these findings in obese men support the contention that women +DOE may experience gender-specific changes in the oxygen cost of breathing that also suggests the need for further study. However, oxygen cost of breathing is increased in obese men over normal weight men and many obese men have an increased intensity of breathlessness, which should be taken into account when prescribing exercise to treat obesity or prevent further obese levels in men.
Highlights.
Otherwise healthy, obese men with or without dyspnea on exertion (DOE) were studied.
37% of men studied had elevated ratings of perceived breathlessness during exertion.
Oxygen cost of breathing was not increased in men with DOE vs men without.
Fat distribution and pulmonary function were not different between groups.
Cardiovascular conditioning and quality of respiratory sensations and were not different.
Acknowledgments
The authors wish to thank T. Bassett, Dr. S. Lorenzo, S. Haller, E. Knoll, C. Rowell, C. Hollas, Dr. S.L. Davidoff, M. Klocko, and C. Storms, for their assistance in various stages of this project. The authors also wish to express their appreciation to the many staff members of the Rogers Center at UT Southwestern for their assistance with the MRI data, especially Dr. P.T. Weatherall. Drs. R.B. Banzett and A.P. Binks contributed substantially to the conceptual and theoretical development of the manuscript.
Funding Sources
American Lung Association, King Charitable Foundation Trust, The Research and Education Institute at Texas Health Resources, Cain Foundation, and Texas Health Presbyterian Hospital Dallas, and NIH HL096782. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
A portion of the data contained in this manuscript have been published in abstract form previously (Babb et al., 2008b; Davidoff et al., 2009).
Disclosure Statement
Dr. Bernhardt has no conflicts of interest to disclose. Dr. Wood has no conflicts of interest to disclose. Mrs. Moran has no conflicts of interest to disclose. Dr. Babb has not conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vipa Bernhardt, Email: VipaBernhardt@TexasHealth.org.
Helen E. Wood, Email: helen.wood@kcl.ac.uk.
Raksa B. Moran, Email: RaksaMoran@TexasHealth.org.
Tony G. Babb, Email: TonyBabb@TexasHealth.org.
References
- Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. Journal of Lipid Research. 1994;35:1490–1496. [PubMed] [Google Scholar]
- Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society. Standardization of spirometry (1994 update) Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Babb TG. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol. 1997a;109:15–28. doi: 10.1016/s0034-5687(97)84026-1. [DOI] [PubMed] [Google Scholar]
- Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol. 1997b;82:746–754. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol. 2002;92:2483–2490. doi: 10.1152/japplphysiol.00235.2001. [DOI] [PubMed] [Google Scholar]
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008a;178:116–123. doi: 10.1164/rccm.200706-875OC. [DOI] [PubMed] [Google Scholar]
- Babb TG, Rodarte JR. Estimation of ventilatory capacity during submaximal exercise. J Appl Physiol. 1993;74:2016–2022. doi: 10.1152/jappl.1993.74.4.2016. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, Chase PJ, DeLorey DS, Rodder SG, Feng MY, Ranasinghe KG. Weight Loss via Diet and Exercise Improves Exercise Breathing Mechanics in Obese Men. Chest. 2011;140:454–460. doi: 10.1378/chest.10-1088. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008b;134:704–711. doi: 10.1378/chest.07-1728. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am Rev Respir Dis. 1961;84:789–806. doi: 10.1164/arrd.1961.84.6.789. [DOI] [PubMed] [Google Scholar]
- Cherniack RM. Respiratory effects of obesity. Can Med Assoc J. 1959a;80:613–616. [PMC free article] [PubMed] [Google Scholar]
- Cherniack RM. The oxygen consumption and efficiency of the respiratory muscles in health and emphysema. J Clin Invest. 1959b;38:494–499. doi: 10.1172/JCI103826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack RM, Guentter CA. The efficiency of the respiratory muscles in obesity. Can J Biochem Physiol. 1961;39:1215–1222. doi: 10.1139/o61-127. [DOI] [PubMed] [Google Scholar]
- Coast JR, Rasmussen SA, Krause KM, O’Kroy JA, Loy RA, Rhodes J. Ventilatory work and oxygen consumption during exercise and hyperventilation. J Appl Physiol. 1993;74:793–798. doi: 10.1152/jappl.1993.74.2.793. [DOI] [PubMed] [Google Scholar]
- Davidoff SL, Rowell CJ, Wood HE, Storms CD, Hollas CN, Ranasinghe KG, Moran RB, Klocko MN, Babb TG. Exertional dyspnea in obese men is associated with low lung volume breathing. 179. 2009. p. A6053. [Google Scholar]
- DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes. 2005;29:1039–1047. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- El Gamal H, Khayat A, Shikora S, Unterborn JN. Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest. 2005;128:3870–3874. doi: 10.1378/chest.128.6.3870. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119:1401–1408. doi: 10.1378/chest.119.5.1401. [DOI] [PubMed] [Google Scholar]
- Gibson GJ. Obesity, respiratory function and breathlessness. Thorax. 2000;55(Suppl 1):S41–S44. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Sipple JH, Auchincloss JH. Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961;16:21–26. doi: 10.1152/jappl.1961.16.1.21. [DOI] [PubMed] [Google Scholar]
- Goldman HI, Becklake MR. Respiratory function tests. Normal values at median altitudes and the prediction of normal results. Am Rev Tuberc. 1959;79:457–467. doi: 10.1164/artpd.1959.79.4.457. [DOI] [PubMed] [Google Scholar]
- Jensen D, Ofir D, O’Donnell DE. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir Physiol Neurobiol. 2009;167:87–100. doi: 10.1016/j.resp.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Kamel EG, McNeill G, Van Wijk MCW. Usefulness of anthropmetry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res. 2000;8:36–42. doi: 10.1038/oby.2000.6. [DOI] [PubMed] [Google Scholar]
- Kaufman BJ, Ferguson GT, Cherniack RM. Hypoventilation in obesity. J Clin Invest. 1959;38:500–507. doi: 10.1172/JCI103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg J, Burrows B. Changes in the Normal Maximal Expiratory Flow-Volume Curve with Growth and Aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve: Normal standards, variability and effects of age. Am Rev Respir Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO2RESP) at rest. Am J Respir Crit Care Med. 1999;160:883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- Lorenzo S, Babb TG. Oxygen Cost of Breathing and Breathlessness during Exercise in Nonobese Women and Men. Med Sci Sports Exerc. 2011;44:1043–1048. doi: 10.1249/MSS.0b013e3182444c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Harver A, Lentine T, Scott JA, Beck KC, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med. 1996;154:1357–1363. doi: 10.1164/ajrccm.154.5.8912748. [DOI] [PubMed] [Google Scholar]
- Meek PM, Schwartzstein RM, Adams L, Altose MD, Breslin EH, Carrieri-Kohlman V, Gift A, Hanley MV, Harver A, Jones PW, Killian K, Knebel A, Lareau SC, Mahler DA, O’Donnell DE, Steele B, Stuhlbarg M, Titler M. Dyspnea mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J, D’angelo E. Work of Breathing. In: Crystal RG, West JB, et al., editors. The Lung: Scientific Foundations. Lippincott-Raven; Philadelphia: 1997. pp. 1437–1446. [Google Scholar]
- Milic-Emili J, Orzalesi MM. Mechanical work of breathing during maximal voluntary ventilation. J Appl Physiol. 1998;85:254–258. doi: 10.1152/jappl.1998.85.1.254. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Bertley JC, Chau LKL, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- Perry AC, Applegate EB, Jackson ML, Deprima S, Goldberg RB, Ross R, Kempner L, Feldman BB. Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol. 2000;89:636–643. doi: 10.1152/jappl.2000.89.2.636. [DOI] [PubMed] [Google Scholar]
- Ross R, Leger L, Morris D, DeGuise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–795. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest. 2004;125:909–915. doi: 10.1378/chest.125.3.909. [DOI] [PubMed] [Google Scholar]
- Sahebjami H. Dyspnea in obese healthy men. Chest. 1998;114:1373–1377. doi: 10.1378/chest.114.5.1373. [DOI] [PubMed] [Google Scholar]
- Salvadori A, Fanari P, Mazza P, Agosti R, Longhini E. Work capacity and cardiopulmonary adaptation of the obese subject during exercise testing. Chest. 1992;101:674–679. doi: 10.1378/chest.101.3.674. [DOI] [PubMed] [Google Scholar]
- Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990;142:1009–1014. doi: 10.1164/ajrccm/142.5.1009. [DOI] [PubMed] [Google Scholar]
- Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis. 1989;140:1021–1027. doi: 10.1164/ajrccm/140.4.1021. [DOI] [PubMed] [Google Scholar]
- Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64:719–725. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD. Magnetic resonance imaging of total body fat. J Appl Physiol. 1998;85:1778–1785. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- U.S.Department of Health and Human Services - National Institutes of Health. NIH publication no. 04–5493. U.S Department of Health and Human Services - National Institutes of Health; 2004. Strategic plan for NIH obesity research: A report of the NIH obesity research task force; pp. 1–90. [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Lippincott Williams and Wilkins; Philadelphia, PA: 2005. Measurements during integrative cardiopulmonary exercise testing; pp. 76–110. [Google Scholar]
- Whipp BJ, Davis JA. The ventilatory stress of exercise in obesity. Am Rev Respir Dis. 1984;129:S90–S92. doi: 10.1164/arrd.1984.129.2P2.S90. [DOI] [PubMed] [Google Scholar]