Abstract
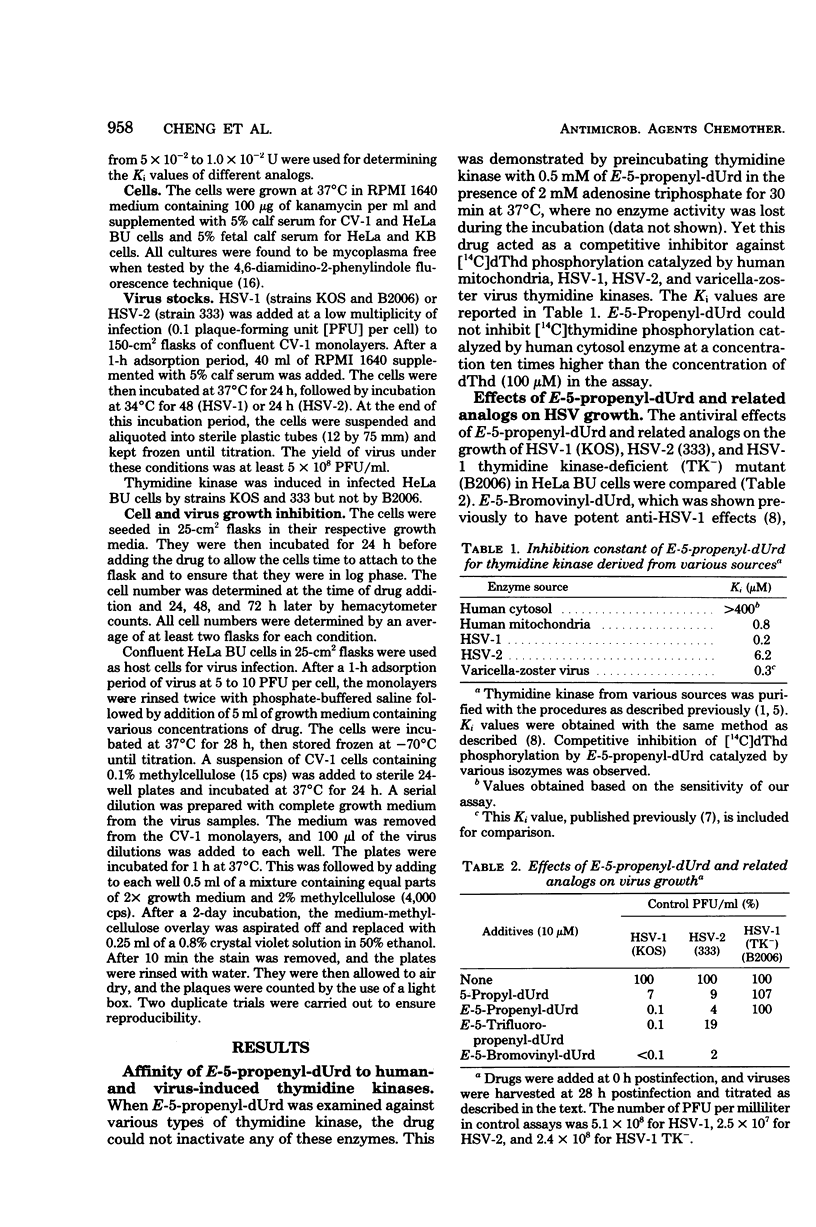
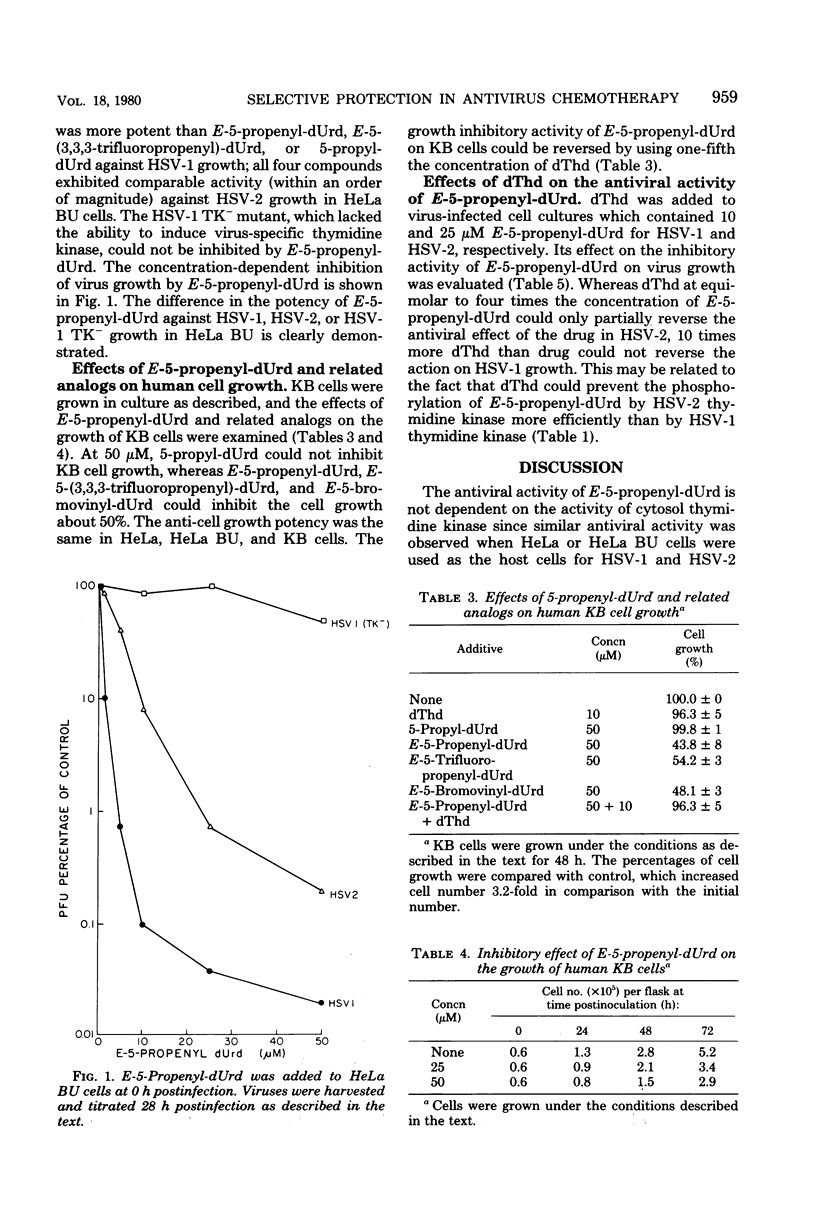
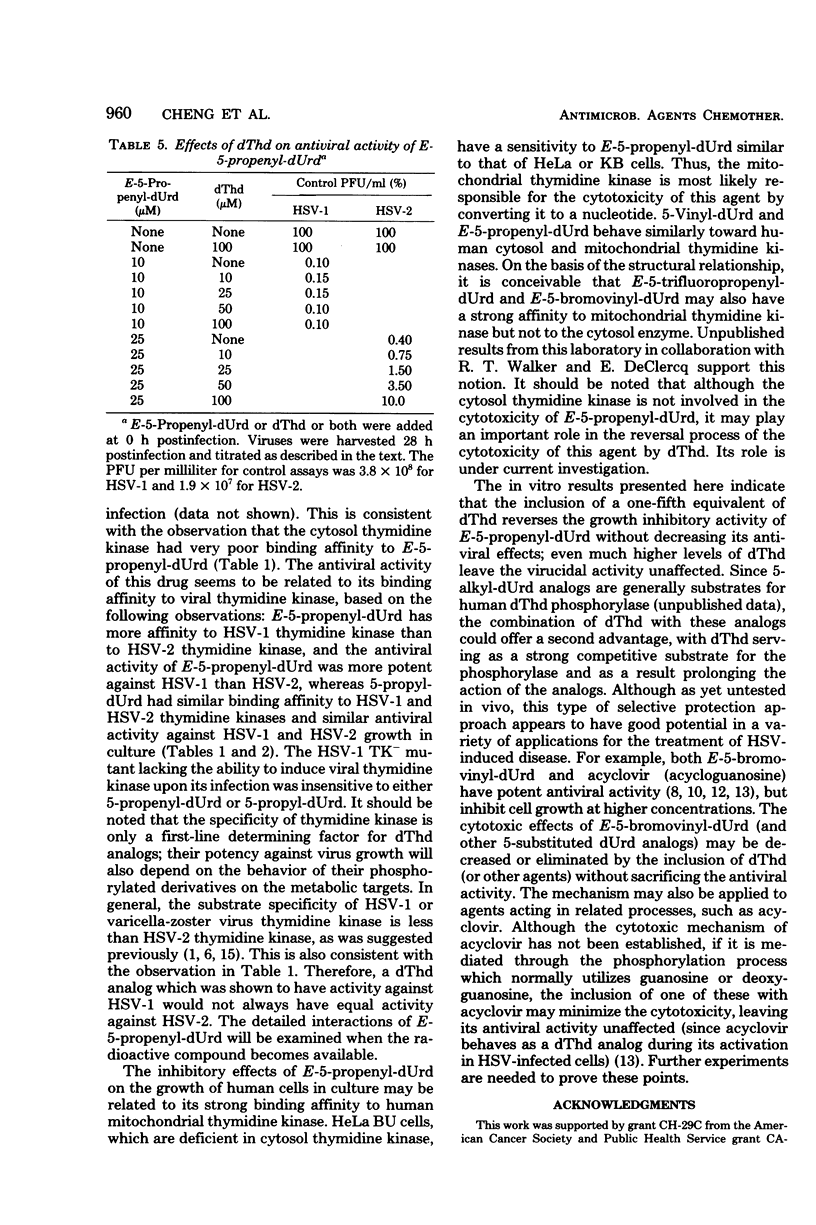
E-5-Propenyl-2'-deoxyuridine (E-5-propenyl-dUrd) inhibited the growth of herpes simplex virus (HSV) types 1 (HSV-1) and 2 in culture. The concentration of drug required to give a 2-log reduction in virus titer was 5 microM for HSV-1 and 23 microM for HSV-2. The anti-HSV-1 activity of this agent was more potent than 5-propyl-dUrd, equivalent to E-5(3,3,3-trifluoropropenyl)-dUrd, and less potent than E-5-bromovinyl-dUrd. The HSV-1 mutant (B2006) lacking the ability to induce virus-specific thymidine kinase could not be inhibited by E-5-propenyl-dUrd. The binding constants of E-5-propenyl-dUrd to HSV-1, HSV-2, varicella-zoster virus, and human mitochondrial thymidine kinases were established to be 0.2, 6.2, 0.3, and 0.8 microM, respectively. Thymidine phosphorylation catalyzed by human cytosol thymidine kinase could not be inhibited by E-5-propenyl-dUrd at a concentration 10-fold higher than the thymidine in the assay. When thymidine and E-5-propenyl-dUrd were added concomitantly at equal concentrations to virus-infected cells, the antiviral activity was not reversed in HSV-1 and only partially reversed in HSV-2. E-5-Propenyl-dUrd also inhibited the growth of human cells in culture with 50% inhibitory dose of 50 microM. Since this inhibition could be readily reversed by a lower concentration of thymidine, the idea of selective protection is proposed. This approach could avoid the cytotoxic effect of an antiviral agent with properties similar to E-5-propenyl-dUrd without sacrificing antiviral activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y. C. A rational approach to the development of antiviral chemotherapy: alternative substrates of herpes simplex virus Type 1 (HSV-1) and Type 2 (HSV-2) thymidine kinase (TK). Ann N Y Acad Sci. 1977 Mar 4;284:594–598. doi: 10.1111/j.1749-6632.1977.tb21992.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. Deoxythymidine kinase induced in the HELA TK- cells by herpes simplex virus type I and type II. Substrate specificity and kinetic behavior. Biochim Biophys Acta. 1976 Dec 8;452(2):370–381. doi: 10.1016/0005-2744(76)90186-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Domin B. A., Sharma R. A., Bobek M. Antiviral action and cellular toxicity of four thymidine analogues: 5-ethyl-,5-vinyl-, 5-propyl-, and 5-allyl-2'- deoxyuridine. Antimicrob Agents Chemother. 1976 Jul;10(1):119–122. doi: 10.1128/aac.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Cheng Y. C., Tsou T. Y., Hackstadt T., Mallavia L. P. Induction of thymidine kinase and DNase in varicella-zoster virus-infected cells and kinetic properties of the virus-induced thymidine kinase. J Virol. 1979 Jul;31(1):172–177. doi: 10.1128/jvi.31.1.172-177.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. c. Human deoxythymidine kinase II: substrate specificity and kinetic behavior of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. Biochemistry. 1976 Aug 24;15(17):3686–3690. doi: 10.1021/bi00662a007. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Watanabe K. A., Reichman U., Hirota K., Lopez C., Fox J. J. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2'-fluoro-2'-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979 Jan;22(1):21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]
- Wataya Y., Matsuda A., Santi D. V., Bergstrom D. E., Ruth J. L. trans-5-(3,3,3-Trifluoro-1-propenyl-)-2'-deoxyuridylate: a mechanism-based inhibitor of thymidylate synthetase. J Med Chem. 1979 Apr;22(4):339–340. doi: 10.1021/jm00190a001. [DOI] [PubMed] [Google Scholar]


