Abstract
Within the postero-lateral hypothalamus neurons that utilize hypocretin or melanin-concentrating hormone (MCH) as neuromodulators are co-distributed. These neurons have been involved in the control of behavioral states, and a deficit in the hypocretinergic system is the pathogenic basis of narcolespy with cataplexy.
In this report, utilizing immunohistochemistry and retrograde tracing techniques, we examined the hypocretinergic innervation of the nucleus pontis oralis (NPO), which is the executive site that is responsible for the generation of REM sleep in the cat.
The retrograde tracer cholera toxin subunit B (CTb) was administered in pontine regions where carbachol microinjections induced REM sleep. Utilizing immunohistochemical techniques, we found that approximately 1% of hypocretinergic neurons in the tuberal area of the hypothalamus project to the NPO. In addition, approximately 6% of all CTb+ neurons in this region were hypocretinergic. The hypocretinergic innervation of the NPO was also compared with the innervation of the same site by MCH-containing neurons. More than three times as many MCHergic neurons were found to project to the NPO compared with hypocretinergic cells; both neuronal types exhibited bilateral projections. We also identified a group of non-hypocretinergic non-MCHergic neuronal group of neurons that were intermingled with both hypocretinergic and MCHergic neurons that also projected to this same brainstem region. These neurons were grater in number that either hypocretin or MCH containing neurons; their soma size was also smaller and their projections were mainly ipsilateral.
The present anatomical data suggest that hypocretinergic, MCHergic and an unidentified companion group of neurons of the postero-lateral hypothalamus participate in the regulation of the neuronal activity of NPO neurons, and therefore, are likely to participate in the control of wakefulness and REM sleep.
Keywords: orexin, melanin-concentrating hormone, reticular formation, REM sleep
Introduction
The nucleus pontis oralis (NPO) in the cat (that comprises the peri-locus coeruleus α and a part of the medial pontine reticular formation) or its corresponding nucleus in the rat, which is called sub-laterodorsal nucleus, is considered to exert executive control over the initiation and maintenance of REM (rapid eyes movements) sleep, and to be involved in the control of wakefulness as a part of the reticular activating system (Reinoso-Suarez et al., 2001; Xi et al., 2001; Chase and Morales, 2005; Siegel, 2005; Fuller et al., 2007; Luppi et al., 2007; McCarley, 2007). A single injection of a cholinergic agonist, such as carbachol, results in the generation of a state with all the behavioral and electrographic signs of REM sleep with a very short latency (30 seconds to a few minutes); this state could last up to two hours (George et al., 1964; Baghdoyan et al., 1987). In addition, the microinjection of GABA or its agonists, result in a sustained period of arousal (Reinoso-Suarez et al., 2001; Xi et al., 2001; Chase and Morales, 2005; Siegel, 2005; Fuller et al., 2007; Luppi et al., 2007; McCarley, 2007).
In 1998, a group of phenotypically distinct neurons were discovered in the postero-lateral hypothalamus which contained the neuropeptides hypocretin 1 and hypocretin 2 (also called orexin A and orexin B), which are excitatory neuromodulators (de Lecea et al., 1998; Sakurai et al., 1998; van den Pol et al., 1998). The hypocretinergic neurons have been shown to be involved in the generation and maintenance of wakefulness (Del Cid-Pellitero and Garzon, 2007; Sakurai et al., 2010). These neurons are active during wakefulness (Torterolo et al., 2001; Torterolo et al., 2003; Lee et al., 2005; Mileykovskiy et al., 2005; Torterolo et al., 2011b), specially in conjunction with the presence of motor activity that occur during highly motivated goal-oriented behaviors (Torterolo et al., 2001; Torterolo et al., 2003; Lee et al., 2005; Mileykovskiy et al., 2005; Torterolo et al., 2011b). The sleep disorder Narcolepsy, characterized by hypersomnia and cataplexy, is produced by a degeneration of these neurons (Peyron et al., 2000; Thannickal et al., 2000).
Unit recording in rats have shown that the hypocretinergic neurons decrease their firing rate during the tonic periods of REM sleep (Lee et al., 2005; Mileykovskiy et al., 2005). However, there is an increase in the release of hypocretin-1 in the hypothalamus and basal forebrain of the cat during REM sleep (Kiyashchenko et al., 2002a). In addition, when hypocretin is microinjected into the NPO of the cat during non-REM (NREM) sleep, the animals immediately enter into prolonged periods of REM sleep (Xi et al., 2002; Xi and Chase, 2010).
Intermingled with hypocretinergic neurons are neurons that utilize as a neuromodulator the 19-aminoacid cyclic neuropeptide melanin-concentrating hormone (MCH) (Kawauchi et al., 1983; Torterolo et al., 2006). We have reported that MCHergic neurons project to the NPO, and that the microinjection of MCH into the NPO decreases the latency to REM sleep and increase the time spent in this state (Torterolo et al., 2009b). These data suggest that, through projections to the NPO, hypocretinergic and MCHergic neurons are involved in the control of sleep and waking states.
As a continuation of our previous report (Torterolo et al., 2009b), in the present study we employed the cholera toxin subunit b (CTb) as a retrograde tracer to examine hypocretinergic projections to the NPO in the cat. In addition, we compared these neuronal projections with MCHergic projections to the same pontine nucleus.
Results
Hypocretin and MCH immunoreactive fibers are present within the NPO
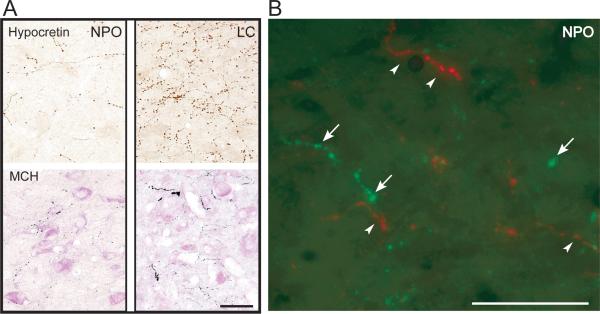
Neuronal somata in the hypothalamus as well as fibers and varicose terminals throughout the brain were observed after immunostaining against either hypocretin or MCH. As shown in Figure 1, hypocretinergic as well as MCHergic fibers and terminals were intermingled in the NPO; these neuropeptides were not co-localized. For purpose of comparison, a photomicrograph of immunostained fibers in a nearby nucleus, the locus coeruleus (LC), is shown in Figure 1.
Figure 1.
Hypocretinergic and MCHergic fiber in the nucleus pontis oralis. A. Photomicrographs showing hypocretin-2 (upper photos) and MCH (lower photos) immunostained varicose fibers and terminals in the NPO and LC. These photomicrographs were obtained from 14 μm-thick sections that were processed with the ABC-DAB method. MCH immunostaining was enhanced by nickel and the sections were lightly counterstained with Pyronin-Y. B. Hypocretin-1 (in green) and MCH-containing (in red) fibers and terminals are intermingled in the NPO. Arrows and arrowheads indicate hypocretinergic and MCHergic fibers, respectively. Photomicrographs were obtained from sections that were processed for the detection of immunoflourescence. FITC (for Hcrt-1) and Rhodamine (for MCH) were used as fluorescent agents. This figure was produced by superimposing photomicrographs that were obtained using green and red filters. Calibration bars: A, 100 μm; B, 50 μm.
Retrograde Tracing Studies
In seven cats, CTb was applied in sites where carbachol microinjections produced REM sleep; the average REM sleep latency was 3.3 ± 1.7 minutes (0.5 to 8 minutes, Table 1). As previously described, deposits of CTb consisted in a central zone of dark reaction product surrounded by a lighter-stained zone (Morales et al., 1999; McGregor et al., 2005; Torterolo et al., 2009b).
Table 1.
Numbers and percentages of single and double-labeled neurons.
| Animal | REM-Lat (min) | Injection site | Hcrt+ (n) | CTb+ (n) | Hcrt+CTb+ (n) | Hcrt+CTb+/Hcrt+ (%) | Hcrt+CTb+/CTb+ (%) |
|---|---|---|---|---|---|---|---|
| PA5 | 7 | NPO, M5 | 457 | 18 | 1 | 0.2 | 5.3 |
| PA15 | 8 | NPO, LCp | 429 | 18 | 1 | 0.2 | 5.3 |
| PA16 | 0.5 | NPO | 387 | 30 | 3 | 0.8 | 9.1 |
| PA17 | 2 | NPO, M5 | 344 | 14 | 1 | 0.3 | 6.7 |
| PA8 | 0.7 | LCp, NPO | 338 | 100 | 10 | 2.9 | 9.1 |
| PA14 | 2 | NPO, LDT | 444 | 114 | 6 | 1.3 | 5.0 |
| PA20 | 3 | LCp, NPO | 371 | 166 | 7 | 1.9 | 4.0 |
This table displays the animal name, REM-carbachol latency (REM-Lat), microinjection site, the number of single-labeled Hcrt+ neurons, the number of single-labeled CTb+ neurons, the number of Hcrt+CTb+ neurons, the percentage of Hcrt+CTb+ of the Hcrt+ neurons, and, the percentage of Hcrt+CTb+ of the total number of CTb+ neurons (single plus double-labeled). The neurons were counted from 2 sections (4 hemisections) per animal located at the tuberal level of the hypothalamus. n, number of neurons. LCp, locus coeruleus proper; M5, motor trigeminal nucleus.
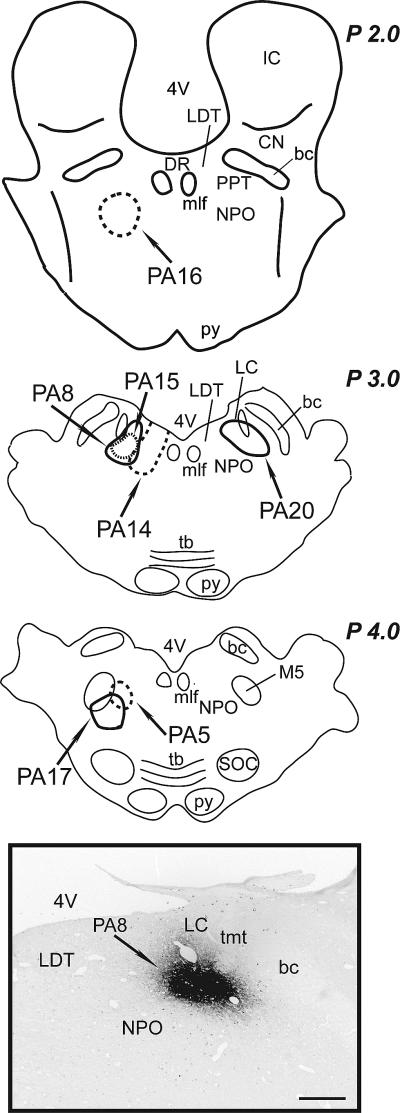
Although REM sleep was induced by carbachol in all the animals, the location of the CTb deposits was slightly different as shown in Figure 2; a representative CTb deposit (PA8 cat) is also shown in this Figure. The CTb deposit in all animals included the NPO, but in some animals the extension of the CTb deposit included nearby structures such as the LC (Table 1 and Figure 2). CTb deposits were located exclusively within the boundaries of the NPO in the animal in which the latency to REM sleep was the shortest (PA16).
Figure 2.
Sites of CTb application. Schematic summarizing the location of CTb deposits in the different animals. The boundaries of each CTb deposit are marked and the identification number for each cat is indicated. Inset. Photomicrograph showing the area of the CTb deposit in PA8 at P 3.0 (according to Berman, 1968). All CTb deposits were within the NPO according to the atlas and nomenclature of Reinoso-Suarez, (1961). bc, brachium conjunctivum; CN, cuneiform nucleus; DNLL, dorsal nucleus of the lateral lemniscus; IC, inferior colliculus; ll, lateral lemniscus; M5, motor trigeminal nucleus; mlf, medial longitudinal fasciculus; PB, parabrachial nucleus; PPT, pedunculopontine tegmental nucleus; py, pyramidal tract; SOC, superior olivary complex; tb, trapezoid body; tmt, mesencephalic tract of the trigeminal nerve; VT, ventral tegmental nucleus; 4V, forth ventricle. Calibration bar: 1 mm.
Retrograde CTb-labeled neurons (CTb+) were observed in the hypothalamus (Figure 3). These neurons contained punctuate black CTb granules within their cytoplasm and cell processes.
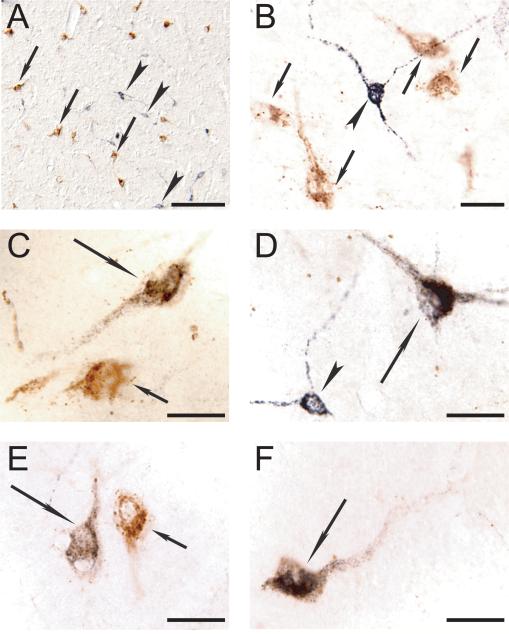
Figure 3.
Representative photomicrographs showing double immunolabeling for Hcrt and CTb in the postero-lateral hypothalamus. A. Photomicrograph of the perifornical region of the hypothalamus of PA8. Hcrt (stained in brown) and CTb (stained in black) labeled neurons are intermingled; examples are indicated by arrows and arrowheads, respectively. B to F. High magnification photomicrographs reveals double-labeled Hcrt+CTb+ neurons (long arrows) that are located in close relation with single labeled Hcrt+ neurons (small arrows) and single-labeled CTb+ neurons (arrowheads). Calibration bars: A, 150 μm; B to F, 30 μm.
Hcrt+CTb+ neurons
Single-labeled hypocretinergic neurons were identified on the basis of their brown immunostaining, while CTb single labeled neurons exhibited black staining (Figure 3). Double immunostained neurons (Hcrt+CTb+ neurons) were found in all animals; these double-labeled neurons contained black CTb granules within a brown-stained cytoplasm (Figure 3).
Eighty-four percent of the double-labeled Hcrt+CTb+ neurons were located at the tuberal (A 10-11; according to Berman and Jones, 1982) and tuberomamillary (A 9-10) levels of the hypothalamus. This distribution was expected because most of the hypocretinergic neurons in the cat are known to be concentrated in the tuberal level of the hypothalamus (Torterolo et al., 2006). Therefore, Hcrt+, CTb+ and Hcrt+CTb+ neurons were analyzed in the hypocretinergic field of the tuberal level of the hypothalamus.
First, the data from all cats were pooled in order to provide an overview of the projections from the hypocretinergic field to the pontine area where REM sleep was induced by microinjections of carbachol. Within the hypocretinergic field, the mean number of Hcrt+ neurons per section was 395.7 ± 18.2; in addition, there were 65.7 ± 23.0 single labeled CTb+ neurons and 4.1 ± 1.4 Hcrt+CTb+ neurons. Then, the number of Hcrt+CTb+ neurons per section was small; it corresponded to 1.1 ± 0.4 % of the total number of hypocretinergic neurons and 6.3 ± 0.8 % of the total number of CTb+ neurons. There was no significant difference between the numbers of Hcrt+CTb+ neurons projecting ipsilaterally (2.6 ± 1.0) or contralaterally (1.6 ± 0.4) (two-tailed paired Student's t-test).
Secondly, we analyzed the number and distribution of Hcrt+CTb+ neurons in each animal. The results for each cat are summarized in Table 1 and the approximate distribution of the Hcrt+CTb+ neurons is shown in Figure 4. In cat PA16, as note above, the microinjection of carbachol resulted in the generation of REM sleep with the shortest latency (Table 1), and the CTb deposit was located exclusively in the rostral NPO. Hcrt+CTb+ neurons in this animal were located in the perifornical and dorso-lateral region of the tuberal hypothalamic area (Figure 4B). Of the entire population of neurons that projected to the NPO (CTb+ neurons) from the hypocretinergic field, 9.1% of them were hypocretinergic (Hcrt+CTb+ neurons).
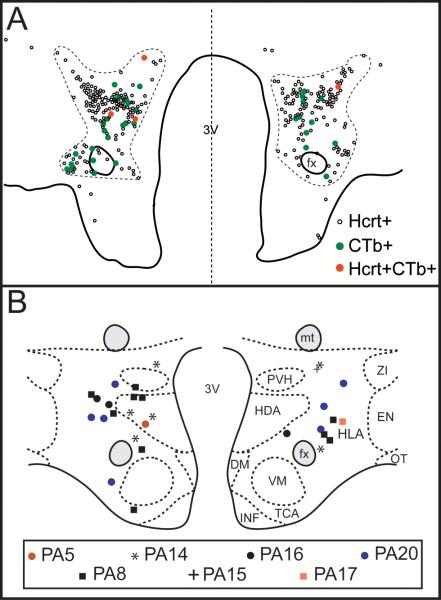
Figure 4.
Distribution of double-labeled Hcrt+CTb+ neurons. A. Camera lucida drawings of single-labeled Hcrt+ (small empty circles), single-labeled CTb+ neurons (green circles) and Hcrt+CTb+ neurons (red circles) at the tuberal level of the hypothalamus in cat PA14. Each dot represents one neuron. The dashed line demarcates the hypocretinergic field. B. Schematic drawing that depicts at the same hypothalamic level, the location of double-labeled Hcrt+CTb+ neurons that project to the NPO executive site for the generation of REM sleep. The neuronal distribution of 2 sections for each animal is presented. Different labels represent different cats; each label represents one neuron. DM, dorsomedial nucleus; EN, entopeduncular nucleus; fx, fornix; HDA, dorsal hypothalamic area; HLA, lateral hypothalamic area; INF, infundibular nucleus; mt, mamillothalamic tract; OT, optic tract; PVH, parvocellular nucleus; TCA, area of the tuber cinereum; VM, ventromedial nucleus; ZI, zona incerta; 3V, third ventricle.
The site of CTb application in cat PA14 included the caudal part of the laterodorsal tegmental nucleus (LDT); in this animal there was also a short latency to REM sleep. Figure 4A is a camera lucida drawing of a representative hypothalamic section from PA14 that was immunostained for Hcrt and CTb. Note that the number of single-labeled CTb+ neurons was greater than the number of double-labeled neurons.
A large number of both CTb+ and Hcrt+CTb+ cells was observed in cats PA8 and PA20 in which a portion of the CTb deposit included the LC proper (according to (Sakai, 1991)) (Figure 2). The greatest number of single CTb+ neurons was found in PA20; PA8 exhibited the largest number of Hcrt+CTb+ neurons. As shown in Figure 4B, relatively large numbers of Hcrt+CTb+ neurons dorsal to the fornix were found in PA20 and PA8.
The latency to REM sleep was relatively longer in PA15 than in the other animals (Table 1) and the CTb deposit was comparably smaller (Figure 2). The location of the injection site in PA5 was more caudal, and medially to the motor V nucleus and included only a portion of the NPO (Figure 2). The injection site for PA17 was located more caudally and laterally compared with the sites in other animals, although a portion of the CTb deposit was within the boundaries of the NPO. In all of these animals, the number of Hcrt+CTb+ neurons was relatively minor.
Comparison with the MCHergic neurons
The preceding CTb data involving hypocretinergic neurons was compared with the distribution of CTb-labeled MCHergic neurons which were described previously from the same animals (Torterolo et al., 2009b).
At the tuberal level of the hypothalamus, the number of single-labeled of hypocretinergic neurons (395.7 ± 18.2) was approximately one third the number of single labeled MCHergic neurons (1182.6 ± 59.3). This ratio was maintained when we compared the number of Hcrt+CTb+ neurons (4.1 ± 1.4) with the number of MCH+CTb+ neurons (13.1 ± 6.6). The number of MCH+CTb+ neurons was larger than the number of Hcrt+CTb+ neurons in all animals.
Non hypocretinergic, non MCHergic CTb+ neurons
A discrete population of single-labeled CTb+ neurons was found in a close relationship with hypocretinergic neurons (Figure 3 and 4A) and MCHergic neurons (Torterolo et al., 2009b). The number of single-labeled CTb+ neurons in the hypocretinergic and MCHergic fields (85.9 ± 39.4) was almost 5 times larger than the sum of the MCH+CTb+ neurons plus the Hcrt+CTb+ neurons (17.3 ± 7.4). Therefore, the majority of single-labeled CTb+ neurons were neither MCHergic nor hypocretinergic. The number of single-labeled CTb+ neurons was greater on the side ipsilateral to CTb deposit (67.4 ± 32.8) than on the contralateral side (18.4 ± 7.0).
Single-labeled CTb+ neurons exhibited a smaller soma size (major diameter, 22.2 ± 0.8 μm) than either hypocretinergic neurons (30.3 ± 1.0 μm, P < 0.001), or MCHergic neurons (30.7 ± 1.3 μm, respectively; P < 0.001, ANOVA and Fisher post hoc test). The small-sized single-labeled CTb+ neurons are shown in Figure 3.
Discussion
In the present report we confirmed that hypocretinergic and MCHergic neurons that originate from the hypothalamus project to the NPO of the cat, as we previously reported (Zhang et al., 2004; Torterolo et al., 2009b). We also determined that hypocretin and MCH are not co-localized in fibers or terminals within this or nearby mesopontine areas. This result was anticipated because neither of these peptides were co-localized in their respective cell bodies (Torterolo et al., 2006). Furthermore, after the application of CTb into the NPO, in the site wherein carbachol microinjections generated REM sleep, we found that approximately 6% of the retrograde-labeled neurons from the tuberal hypothalamic area were hypocretinergic and that their projections were bilateral. We also found that the number of Hcrt+CTb+ neurons was approximately one third of the number of MCH+CTb+ neurons. Finally, we observed a large population of non-hypocretinergic, non-MCHergic neurons in the postero-lateral hypothalamic area that project to the NPO; these neurons were smaller in size than either hypocretinergic or MCHergic neurons, and their projections were predominantly ipsilateral.
Hypocretinergic and MCHergic projections to the NPO
CTb was applied in the pontine area where microinjections of cholinergic agonists produce REM sleep with long duration and short latency (an example of a typical REM-carbachol state is presented in (Torterolo et al., 2006)). This is the same pontine area where GABA agonists suppress REM sleep and generate wakefulness (Xi et al., 2001). In this regards, Vanini et al., (2011) have recently confirmed that extracellular GABA within the NPO decreases during natural REM sleep and during neostigmine-induced REM sleep compared to wakefulness (Vanini et al., 2011).
CTb deposits were present in the NPO in all animals; however, the location and extent of the deposits were not exactly the same. Consequently, there were differences in the retrograde labeling of hypothalamic neurons, which was reflected by the presence of a larger number of CTb+ and Hcrt+CTb+ neurons in cats (PA20 and PA8) in which the LC proper was included in the injection site (Sakai, 1991). This finding is consistent with the large innervation of the LC in the cat by hypocretin-containing fibers and the relatively weak innervation of the REM sleep executive area (Figure 1 and (Zhang et al., 2004)).
In PA16, the latency to REM was the shortest and the CTb deposit was centered exclusively in the NPO, which is considered the “REM sleep executive area” (Garzon et al., 1998; Reinoso-Suarez et al., 2001). In this “exemplary” experiment, a relatively small number of bilateral hypocretinergic projections to the NPO were observed; the percentage of Hcrt+CTb+ of the entire number of CTb+ neurons was 9.1 %. Comparing these results with those which we previously presented (Torterolo et al., 2009b), MCH+CTb+ neurons in PA16 was three times larger than the number of Hcrt+CTb+ neurons; these double-labeled neurons represented less than 1% of the total number of tuberal MCHergic and hypocretinergic neurons.
In the remaining animals there were minor variations in the number of single and double-labeled cells, which is likely due to differences in the site or extent of the distribution of the CTb deposit and/or the microinjection procedure utilized (iontophoresis or pressure). Although there were differences in the distribution of the double-labeled neurons in the hypothalamus in relation to the injection sites, a larger sample size would be required to confirm that these projections are topographically organized.
Our results confirm those presented in a previous study in rat wherein hypocretinergic neurons were found to project bilaterally to the NPO (Nunez et al., 2006); in this study, approximately 17% of retrograde labeled neurons that projected to the NPO, were hypocretinergic.
Hypocretin in the NPO: waking or REM sleep promoting action?
Hypocretinergic neurons are active during wakefulness, in conjunction with motor activity that occurs in conjunction with highly motivated states such as those that occur during the exploration of a new environment (Torterolo et al., 2001; Torterolo et al., 2003; Lee et al., 2005; Mileykovskiy et al., 2005; Torterolo et al., 2011b). In the cat, the release of hypocretin increase during natural REM sleep, and one third of the hypocretinergic neurons are active during REM sleep induced by carbachol (Torterolo et al., 2001; Kiyashchenko et al., 2002b). Unit recordings in rats demonstrated that hypocretinergic neurons are active during wakefulness, decrease their firing rate during NREM and tonic REM sleep, but seems to be a burst discharge during phasic REM sleep (Lee et al., 2005; Mileykovskiy et al., 2005).
Several studies have demonstrate the presence of hypocretinergic fibers in the NPO of rats and cats (Peyron et al., 1998; Zhang et al., 2004), as well as hypocretinergic receptor mRNA and proteins in the NPO of the rat (Greco and Shiromani, 2001; Marcus et al., 2001; D'Almeida et al., 2005; Brischoux et al., 2008). Xi et al., (2002) demonstrated that microinjections of hypocretin-1 or hypocretin-2 into the NPO of the cat increases time spent in REM sleep and results in a decrease in the latency to the generation of this state. Furthermore, the juxtacellular application of hypocretin-1 results in an increase in the excitability of NPO neurons, which is associated with the induction of REM sleep (Xi et al., 2003). In addition, hypocretin-1 increases acetylcholine release in the NPO of the rat (Bernard et al., 2003, 2006); it is known that acetylcholine levels within this region increase during REM sleep (Kodama et al., 1990). However, Moreno-Balandran et al., (2008) have shown a decrease in REM sleep when hypocretin-1 was microinjected into the NPO of the cat. Furthermore, the iontophoretic application of hypocretin-1 into the NPO of the rat produce an inhibition of the NPO neurons, which was blocked by a previous iontophoretic application of bicuculline, a GABAA receptors antagonist (Nunez et al., 2006). In fact, it has been shown that hypocretin increases GABA levels in the NPO of the rat, and hypocretin and GABA interact within this nucleus to promote wakefulness (Watson et al., 2008; Brevig et al., 2011). The presence of hypocretin-2 receptor in GABAergic neurons within the NPO may be the cellular basis for this effect (Brischoux et al., 2008).
The paradoxical or contradictory findings involving the REM sleep and wakefulness promoting actions of hypocretin within the NPO were reconciled by Xi and Chase, (2010). They demonstrated that microinjections of hypocretin-1 within the NPO generates REM sleep when applied during NREM sleep, but promotes wakefulness when applied during this behavioral state. Thus, the behavioral state of the animal at the time of the application of hypocretin determines whether REM sleep or wakefulness occurs.
Anatomical and functional interactions between hypocretinergic and MCHergic neurons
We have shown that hypocretinergic and MCHergic neurons project to the NPO (and adjacent areas). In addition, fibers and terminals of both systems are highly intermingled, which suggests the presence of important interactions between these systems within their mesopontine targets, similar to the anatomical and functional interactions that have been described within the hypothalamus (Backberg et al., 2002; Bayer et al., 2002; Guan et al., 2002; van den Pol et al., 2004; Khorooshi and Klingenspor, 2005; Torterolo et al., 2006).
Several studies suggest that MCHergic neurons are involved in the generation of sleep, especially REM sleep (recently reviewed by Peyron et al., 2009 and Torterolo et al., 2011a). When MCH is microinjected in the area where REM-carbachol was generated, it produces an increase in the time that the animals spend in REM sleep together with a decrease in the latency to this behavioral state (Torterolo et al., 2009b). Thus, there are important interactions between the hypocretinergic and MCHergic system with respect to the generation of REM sleep.
Non-hypocretinergic, non-MCHergic neurons of the postero-lateral hypothalamus project to the NPO
We observed that a large population of non-hypocretinergic and non-MCHergic neurons in the lateral hypothalamic area projects ipsilaterally to the executive REM sleep site within the NPO. A similar group of neurons was found in the rat by Nunez et al., (2006). Therefore, in both species the postero-lateral hypothalamus is capable of directly influencing the activity of NPO neurons through MCHergic, hypocretinergic, and a still unidentified cell population. Hassani et al., (2009) identified five non-hypocretinergic, non-MCHergic neuronal types within the postero-lateral hypothalamic area according to their pattern of discharge during wakefulness and sleep. These neuronal types include neurons that fire at maximal rate during wakefulness, REM sleep, NREM sleep, wakefulness and REM sleep, as well as neurons that have equivalent firing rates across the preceding states. Alam et al., (2005) have shown that a group of non-hypocretinergic and non-MCHergic neurons express c-fos during wakefulness in the rat. In the cat, we demonstrated that non-hypocretinergic and non-MCHergic neurons within the postero-lateral hypothalamic region express c-fos during active wakefulness in conjunction with motor-exploratory activity (Torterolo et al., 2006).
One group of these unidentified neurons may be GABAergic. In fact, Rodrigo-Angulo et al., (2008) demonstrated GABAergic projection from the postero-lateral hypothalamus towards the NPO of the cat. Sapin et al., (2010) found that a large number of GABAergic neurons in the tuberal region of the rat hypothalamus express c-fos during the rebound of REM sleep that follows a long-term (3 days) REM sleep deprivation protocol. This finding accords with the electrophysiological study in rats of Hassani et al., (2010) that found GABAergic neurons which are intermingled with hypocretinergic and MCHergic neurons , are active during REM sleep. It would be important to determine if part of these REM sleep “ON” GABAergic neurons project to the NPO.
Conclusions
Utilizing CTb as a retrograde tracer in the cat, we found that hypocretinergic, MCHergic as well as non-hypocretinergic, non-MCHergic neurons project to the NPO, a site that exercises executive control of REM sleep in this species. These neuronal systems, we hypothesize, interact with locally situated GABAergic neurons, in a state dependent fashion, to determine the presence of wakefulness or REM sleep.
Experimental procedures
Experimental animals
Seven adult male cats, that were determined to be in good health by veterinarians, were used in this study; these animals were part of the group of animals that we used in a previous report (Torterolo et al., 2009b). All experimental procedures were conducted in accordance with the “Guide to the care and use of laboratory animals” (8th edition, National Academy Press, Washington D. C., 2010). Adequate measures were taken to minimize pain, discomfort or stress of the animals. In addition, we used the minimum number of animals that were necessary to produce reliable scientific data.
Surgical procedures
Details of the surgical procedures that were employed have been previously presented (Torterolo et al., 2009b). Briefly, the animals were chronically implanted with electrodes to monitor the states of sleep and wakefulness. Under anesthesia the head was positioned in a stereotaxic frame and the skull was exposed. Stainless steel screw electrodes were placed in the frontal and parietal bones to record the electroencephalogram (EEG) and in the orbital portion of the frontal bone to record eye movements (electro-oculogram, EOG). Bipolar electrodes were implanted in both lateral geniculate nuclei in order to monitor ponto-geniculo-occipital waves (PGO). A Winchester plug (connected to the electrodes) and a chronic head-restraining device were bonded to the skull with acrylic cement. A hole, 5 mm in diameter, which was drilled in the skull overlying the cerebellar cortex; it was then filled with bone-wax; this hole was subsequently used to provide access to the pons for drug administration. After the animals had recovered from the preceding surgical procedures, they were adapted to the recording environment for a period of at least two weeks.
Polysomnographic recordings
During experimental sessions, the EEG, EOG, PGO and neck electromyogram (EMG, which was monitored with acutely placed pin electrodes) were recorded. Data were stored using an AC-amplifier and Superscope® software running on a Macintosh® computer. All animals had free access to water and food until one hour prior to the beginning of each recording session.
During recording sessions, the head of the animal was held in a stereotaxic position by a chronic head-holder while the body was confined in a sleeping bag. Due to the long adaptation period, the animals did not exhibit any signs of stress during these sessions, as evidenced by their quiescent behavior and the fact that they fell rapidly asleep with a very short latency. All experiments were performed during a controlled ambient temperature of 21-23°C.
Microinjections of carbachol
In order to locate the optimal site for the induction of REM sleep, carbachol (0.8 μg in 0.2 μl of saline) was microinjected unilaterally for a period of one minute into the NPO with a 1 μl-Hamilton syringe at stereotaxic coordinates AP -2 to -3, L 1.5 to 2.5, H -3.5 to -5, according to (Berman, 1968). Carbachol microinjections were performed during NREM sleep (Lopez-Rodriguez et al., 1994).
Retrograde tracing experiments
CTb (1.5% dissolved in phosphate buffer 0.1 M at pH 6) was applied to the same stereotaxic site in the NPO where the microinjection of carbachol produced REM sleep. CTb was applied 30-60 minutes after the end of a REM sleep induced by carbachol episode of at least 30 minutes in duration.
In four cats, CTb was applied by iontophoresis; the diameter of the electrode tip was 15-20 μm and the current parameters were +2 μA, 7s on and 7s off for a period of 40 minutes (Morales et al., 1999; McGregor et al., 2005; Torterolo et al., 2009b). CTb was also applied by microinjections in three cats. In these animals, 0.1 μl of a solution of CTb was injected for a period of 3 minutes with a 1 μl-Hamilton syringe which was left in place for 20 minutes to avoid possible leakage during the withdrawal procedure (Sakai, 1991; Fort et al., 1998; Nunez et al., 2006). The animals were euthanized at least five days following CTb application in order to allow ample time for the retrograde transport of CTb to take place.
Immunohistochemical procedures
The antibodies and immunohistochemical procedures that were used have been employed in previous studies from our laboratory (Torterolo et al., 2001; Torterolo et al., 2003; McGregor et al., 2005; Torterolo et al., 2009a); for details on the procedures see (Torterolo et al., 2009b). After euthanasia, the animals were perfused with fixatives and the brain was removed and cryoprotected. Frontal sections of the forebrain and brainstem of 20 μm and 14 μm thickness, respectively, were obtained with a cryostat.
Single immunohistochemical techniques were used to identify CTb, hypocretin or MCH in sections of the brainstem. In forebrain sections double immunohistochemistry procedures were undertaken in order to identify hypocretin and CTb using the DAB method. Accordingly, the sections were initially processed to detect CTb (with nickel enhancement in order to obtain a black immunoreaction); subsequently, they were stained for hypocretin without nickel in order to produce a brown precipitate.
For CTb immunostaining, the sections were incubated in goat CTb antiserum (List Biological Laboratories, Campbell, CA, USA) 1:20,000 in phosphate-buffer-saline with 0.3% Triton X-100 (PBST) and exposed to biotinylated donkey goat antiserum (Jackson Immunoresearch, West Grove, PA, USA) 1:2000 in PBST, followed by incubation in ABC (Vector Laboratories, Burlingame, CA, USA) 1:400. They were then processed using the diaminobenzidine tetrahydrochloride (DAB) method which consisted of tissue immersion in 0.02% DAB and 0.015% hydrogen peroxide in a Tris buffer of 50 mM, pH 7.5, for 8–10 minutes; this reaction was enhanced by adding 0.6% nickel ammonium sulfate. In selected sections, CTb was also shown by immunofluorescent techniques as previously reported (McGregor et al., 2005).
Antibodies directed against hypocretin-2 were employed because hypocretin-1 is co-localized with hypocretin-2 in hypocretinergic neurons in the cat (Zhang et al., 2002). Accordingly, sections that were previously treated for CTb were subsequently incubated overnight with a hypocretin-2 antibody (1: 15000) and normal donkey serum (NDS; 3%). The sections were then rinsed and incubated for 90 minutes with biotinylated donkey anti-rabbit antibody (1: 300) plus NDS. After another rinse, the tissue was incubated with the ABC complex (1: 200) for 60 min and then exposed to diaminobenzidine and hydrogen peroxide (without nickel enhancement).
In order to identify MCH immunoreactivity we employed a rabbit polyclonal anti-MCH antibody (1:40000, Phoenix Pharmaceuticals Inc., Belmont, CA). In addition, the following double immunoflourescence procedures were performed to examine co-localization of MCH and Hcrt. First, free-floating sections were rinsed several times in PBST (0.1 M phosphate buffer saline with 0.25% Triton X-100). Thereafter, they were incubated simultaneously with goat anti-Hcrt-1 (Diagnostic systems laboratories, 1:500) and rabbit anti-MCH antibodies (Phoenix Pharmaceuticals; 1:2000) in PBST solutions for 3 days at 4°C. After the sections were rinsed four times in PBST for a total duration of 30 min, they were simultaneously incubated for 3 hs in PBST containing donkey anti-rabbit IgG conjugated with Rhodamine (Jackson laboratories; 1;300) and donkey anti-goat IgG conjugated with fluorescein isothiocyanate (FITC, Jackson laboratories, 1:200). We also employed the reverse procedure; donkey anti-rabbit IgG conjugated with FITC (Jackson Laboratories: 1:200) and donkey anti-goat IgG conjugated with Rhodamine (Jackson Laboratories: 1:200). Finally, the sections were rinsed for 45 minutes with PBST and coverslipped with Vectashield fluorescent mounting media (Vector laboratories).
Pyronin-Y counterstaining was utilized in immunolabeled or adjacent unstained sections in order to visualize the cytoarchitecture of the areas that were examined.
Analysis of the histological data
All sections were examined by conventional or fluorescent light microscopic techniques. Photomicrographs were obtained using a SPOT digital camera and the images were analyzed using Adobe PhotoShop software and a Power Macintosh G4 computer. The distribution of immunolabeled neurons and fibers was determined from drawings made with a camera lucida attachment.
In animals wherein CTb was applied (n = 7), single (Hcrt+ and CTb+) and double-labeled neurons (Hcrt+CTb+) were counted bilaterally in ten sections per animal. Two sections were selected at the mamillary (A 8-9), tuberomamillary (A 9-10), tuberal (A 10-11), supraoptic (A 13-14) and preoptic levels (A 14-15), according to (Berman and Jones, 1982).
In order to determine the presence of non-hypocretinergic non-MCHergic neurons, we counted the number of single labeled CTb+ neurons in the hypocretinergic and MCHergic neuronal fields, which were defined as the area at the tuberal level of the hypothalamus that contained most of the MCHergic and hypocretinergic neurons, respectively (a similar methodology was used previously (Torterolo et al., 2006; Torterolo et al., 2009b)). Thereafter, an average was obtained of the number of single-labeled CTb+ neurons that were present in the hypocretinergic and MCHergic fields. This number was then compared with the total number of the MCH+CTb+ neurons plus the Hcrt+CTb+ neurons.
The values presented in the Results are the mean ± S.E.M number of neurons per section. The statistical tests used to determine the statistical significance of cell counts are stated in the Results. The criterion chosen to discard the null hypothesis was P < 0.05.
Highlight.
CTb was administered in pontine regions where carbachol induced REM sleep
CTb-labeled hypothalamic hypocretinergic neurons were found bilaterally
The number of Hcrt+CTb+ neurons was about one third of the MCH+CTb+ neurons
A larger number of non-hypocretinergic non-MCHergic neurons also project to this area
These neurons were smaller and have mainly ipsilateral projections
Acknowledgments
This study was supported by USPHS grant NS09999.
Abbreviations
- CTb
cholera toxin subunit-b
- DAB
diaminobenzidine
- DR
dorsal raphe nucleus
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electro-oculogram
- FITC
flourescein isothiocyanate
- Hcrt
hypocretin
- LDT
laterodorsal tegmental nucleus
- LC
locus coeruleus
- MCH
melanin-concentrating hormone
- NPO
nucleus pontis oralis
- NREM
non-REM
- PGO
ponto-geniculo-occipital waves
- PBST
phosphate-buffer-saline with Triton
- REM
rapid eye movements
- REM-carbachol
REM sleep induced by carbachol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Bayer L, Mairet-Coello G, Risold PY, Griffond B. Orexin/hypocretin neurons: chemical phenotype and possible interactions with melanin-concentrating hormone neurons. Regul Pept. 2002;104:33–39. doi: 10.1016/s0167-0115(01)00320-2. [DOI] [PubMed] [Google Scholar]
- Berman AL. A citoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin; Madison: 1968. The brain stem of the cat. [Google Scholar]
- Berman AL, Jones EG. A citoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin; Madison: 1982. The thalamus and basal telencephalum of the cat. [Google Scholar]
- Bernard R, Lydic R, Baghdoyan HA. Hypocretin-1 causes G protein activation and increases ACh release in rat pons. Eur J Neurosci. 2003;18:1775–1785. doi: 10.1046/j.1460-9568.2003.02905.x. [DOI] [PubMed] [Google Scholar]
- Bernard R, Lydic R, Baghdoyan HA. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate g protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–171. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. Sleep. 2011;33:1285–1293. doi: 10.1093/sleep/33.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- Chase M, Morales FR. Control of motoneurons during sleep. In: Kryger MH, et al., editors. Principles and practices of sleep medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 154–168. [Google Scholar]
- D'Almeida V, Hipolide DC, Raymond R, Barlow KB, Parkes JH, Pedrazzoli M, Tufik S, Nobrega JN. Opposite effects of sleep rebound on orexin OX1 and OX2 receptor expression in rat brain. Brain Res Mol Brain Res. 2005;136:148–157. doi: 10.1016/j.molbrainres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E, Garzon M. Modulation by the hypocretinergic/orexinergic neurotransmission system in sleep-wakefulness cycle states. Rev Neurol. 2007;45:482–490. [PubMed] [Google Scholar]
- Fort P, Rampon C, Gervasoni D, Peyron C, Luppi P. Anatomical demonstration of a medullary enkephalinergic pathway potentially implicated in the oro-facial muscle atonia of paradoxical sleep. Sleep Res Online. 1998;1:102–108. [PubMed] [Google Scholar]
- Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007;584:735–741. doi: 10.1113/jphysiol.2007.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon M, De Andres I, Reinoso-Suarez F. Sleep patterns after carbachol delivery in the ventral oral pontine tegmentum of the cat. Neuroscience. 1998;83:1137–1144. doi: 10.1016/s0306-4522(97)00494-6. [DOI] [PubMed] [Google Scholar]
- George R, Haslett W, Jenden D. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagisawa M, Shioda S. Reciprocal synaptic relationship between orexin-and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–457. doi: 10.1111/j.1460-9568.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Khorooshi RM, Klingenspor M. Neuronal distribution of melanin-concentrating hormone, cocaine- and amphetamine-regulated transcript and orexin B in the brain of the Djungarian hamster (Phodopus sungorus). J Chem Neuroanat. 2005;29:137–148. doi: 10.1016/j.jchemneu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BI, Maidment N, Siegel JM. Hypocretin1/Orexin-A release across the sleep-wakefulness cycle. Sleep. 2002a;25:393.A. [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002b;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Kohlmeier K, Morales FR, Chase MH. State dependency of the effects of microinjection of cholinergic drugs into the nucleus pontis oralis. Brain Res. 1994;649:271–281. doi: 10.1016/0006-8993(94)91074-x. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: The switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. Journal of Physiology (Paris) 2007;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McGregor R, Damian A, Fabbiani G, Torterolo P, Pose I, Chase M, Morales FR. Direct hypothalamic innervation of the trigeminal motor nucleus: a retrograde tracer study. Neuroscience. 2005;136:1073–1081. doi: 10.1016/j.neuroscience.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR, Sampogna S, Yamuy J, Chase MH. c-fos expression in brainstem premotor interneurons during cholinergically induced active sleep in the cat. J Neurosci. 1999;19:9508–9518. doi: 10.1523/JNEUROSCI.19-21-09508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Balandran E, Garzon M, Bodalo C, Reinoso-Suarez F, de Andres I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–341. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- Nunez A, Moreno-Balandran ME, Rodrigo-Angulo ML, Garzon M, De Andres I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24:2834–2842. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Sapin E, Leger L, Luppi PH, Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–2059. doi: 10.1016/j.peptides.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso-Suarez F. Topographisher hirnatlas der katze. Merck; Darmstadt: 1961. [Google Scholar]
- Reinoso-Suarez F, de Andres I, Rodrigo-Angulo ML, Garzon M. Brain structures and mechanisms involved in the generation of REM sleep. Sleep Med Rev. 2001;5:63–77. doi: 10.1053/smrv.2000.0136. [DOI] [PubMed] [Google Scholar]
- Rodrigo-Angulo ML, Heredero S, Rodriguez-Veiga E, Reinoso-Suarez F. GABAergic and non-GABAergic thalamic, hypothalamic and basal forebrain projections to the ventral oral pontine reticular nucleus: their implication in REM sleep modulation. Brain Res. 2008;1210:116–125. doi: 10.1016/j.brainres.2008.02.095. [DOI] [PubMed] [Google Scholar]
- Sakai K. Physiological properties and afferents connections of the locus coeruleus and adjacent tegmental neurons involved in the generation of paradoxical sleep in the cat. In: Barnes CD, Pompeiano O, editors. Progress in Brain Res. Vol. 88. Elsevier Science Publisher B.V.; 1991. pp. 31–45. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- Sapin E, Berod A, Leger L, Herman PA, Luppi PH, Peyron C. A very large number of GABAergic neurons are activated in the tuberal hypothalamus during paradoxical (REM) sleep hypersomnia. PLoS One. 2010;5:e11766. doi: 10.1371/journal.pone.0011766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. REM Sleep. In: Kryger MH, et al., editors. Principles and practices of sleep medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 120–135. [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Benedetto L, Lagos P, Sampogna S, Chase MH. State-dependent pattern of Fos protein expression in regionally-specific sites within the preoptic area of the cat. Brain Res. 2009a;1267:44–56. doi: 10.1016/j.brainres.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Lagos P, Monti JM. Melanin-concentrating hormone (MCH): a new sleep factor? Frontiers in Neurology. 2011a;2:1–12. doi: 10.3389/fneur.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Ramos O, Sampogna S, Chase MH. Hypocretinergic neurons are selectively activated in conjunction with motor activities that involve survival behaviors. 2011b. submitted. [DOI] [PubMed]
- Torterolo P, Sampogna S, Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009b;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: Searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypothalamic neurons that contain hypocretin (orexin) express c-fos during active wakefulness and carbachol-induced active sleep. Sleep Res Online. 2001;4:25–32. http://www.sro.org/2001/Torterolo/2025. [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;1:25–28. [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Wathen BL, Lydic R, Baghdoyan HA. Endogenous GABA levels in the pontine reticular formation are greater during wakefulness than during rapid eye movement sleep. J Neurosci. 2011;31:2649–2656. doi: 10.1523/JNEUROSCI.5674-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi M, Chase MH. The injection of hypocretin-1 into the nucleus pontis oralis induces either active sleep or wakefulness depending on the behavioral state when it is administered. Sleep. 2010;33:1236–1243. doi: 10.1093/sleep/33.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976:253–258. doi: 10.1016/s0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Induction of wakefulness and inhibition of active (REM) sleep by GABAergic processes in the nucleus pontis oralis. Arch Ital Biol. 2001;139:125–145. [PubMed] [Google Scholar]
- Zhang J, Sampogna S, Morales F, Chase M. Co-localization of hypocretin-1 and hypocretin-2 in the cat hypothalamus and brainstem. Peptides. 2002;23:1479. doi: 10.1016/s0196-9781(02)00084-0. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res. 2004;995:205–217. doi: 10.1016/j.brainres.2003.10.004. [DOI] [PubMed] [Google Scholar]