Abstract
Both obesity and sleep reduce lung volume and limit deep breaths, possibly contributing to asthma. We hypothesize that increasing lung volume dynamically during sleep would reduce airway resistance in asthma. Asthma (n=10) and control (n=10) subjects were studied during sleep at baseline and with increased lung volume via bi-level positive airway pressure (BPAP). Using forced oscillations, respiratory system resistance (Rrs) and reactance (Xrs) were measured during sleep and Rrs was partitioned to upper and lower airway resistance (Rup, Rlow) using an epiglottic pressure catheter. Rrs and Rup increased with sleep (p<0.01) and Xrs was decreased in REM (p=0.02) as compared to wake. Rrs, Rup, and Rlow, were larger (p<0.01) and Xrs was decreased (p<0.02) in asthma. On BPAP, Rrs and Rup were decreased (p<0.001) and Xrs increased (p<0.01), but Rlow was unchanged. High Rup was observed in asthma, which reduced with BPAP. We conclude that the upper airway is a major component of Rrs and larger lung volume changes may be required to alter Rlow.
Keywords: BPAP, forced oscillation technique, airway resistance, lung
1. Introduction
Asthma and obesity frequently co-occur in part because both conditions are common, but evidence suggests that obesity may also impact asthma. For example, the incidence and prevalence of asthma increases as Body Mass Index (BMI) increases (Camargo et al., 1999; Ford, 2005; Von Behren et al., 2009). Furthermore, asthma symptoms improve with weight loss after gastric bypass surgery (Dixon et al., 2011; Macgregor and Greenberg, 1993). The clinical syndrome of asthma may be different in the obese vs. the non-obese; obese people with asthma tend to have less atopy, markers of allergic inflammation and respond less well to corticosteroids than lean asthma patients (Dixon et al., 2011; Moore et al., 2010; van Veen et al., 2008).
Several different mechanisms have been proposed for the increased asthma risk observed in the obese. Although inflammatory and hormonal factors are likely important, mechanical changes with obesity may play a role. Increased BMI is associated with decreased Functional Residual Capacity (FRC) (Ding et al., 1987) which may in turn unload the airway smooth muscle (ASM). Additionally, lower tidal volumes (Vt) during quiet breathing are reported, as well as atelectasis. (Sampson and Grassino, 1983) (Shore, 2008). These gross changes may impact airway smooth muscle function and properties. Decreased FRC and decreased Vt leads to increased force generation upon stimulation and hence more severe bronchoconstriction, and to a stiffer, less distensible muscle via actin-myosin cross-bridging (Fredberg et al., 1997).
Consistent with mechanical changes, worsening asthma is a long-noted phenomenon of nocturnal asthma (Floyer, 1698). After a night’s sleep, asthma is worse in the morning as compared to the evening as measured by peak expiratory flow (Ballard et al., 1989). Consistent with the hypothesis that lung volumes may modulate airway resistance and reactivity; lung volumes are lower during sleep than during wake, particularly in people with asthma (Ballard et al., 1990), and deep breaths are limited (Perez-Padilla et al., 1983). Combining the effects of obesity (low resting lung volumes, low tidal volumes) and sleep (further drop in lung volumes, tidal volume, and lack of deep breaths) may lead to hyper-reactive ASM and the worsening of asthma symptoms.
Thus, it has been proposed to attenuate asthma symptoms by increasing lung volumes during sleep. One method to induce lung volume changes is through continuous positive airway pressure (CPAP), a common treatment for obstructive sleep apnea (OSA). CPAP has been shown to increase FRC (Layon et al., 1986) and it has been hypothesized that the use of CPAP would lead to improvements in asthma symptoms and severity. However, several investigators have applied CPAP to patients with both OSA and asthma with mixed results. One study found improvements in peak expiratory flow rate (PEFR) after CPAP treatment (Chan et al., 1988), while other studies found no significant changes in the forced expiratory volume in 1 second (FEV1) or airway reactivity, but did find improvements in asthma symptom scores (Ciftci et al., 2005; Lafond et al., 2007). Conversely, Martin et al. gave CPAP to a group of subjects with asthma but without OSA and found a significant increase in time spent awake and no significant changes in FEV1 (Martin and Pak, 1991).
These studies have several limitations. First the primary outcome measure was FEV1, which is dependent on patient effort and requires a deep breath to perform; thus it may not capture important changes occurring during sleep. Furthermore, CPAP provides a tonic elevation in lung volume, yet does not provide larger tidal breaths. We believe that these dynamic changes in lung volume (i.e. deep breaths) may be necessary to break actin-myosin crossbridges and decrease ASM stiffness. Bi-level positive airway pressure (BPAP) contains two pressure settings, a low expiratory pressure (EPAP) and a higher inspiratory pressure (IPAP). By modifying IPAP, dynamic lung volume changes may be achieved.
We hypothesize that dynamic changes in lung volume will reduce mean airway resistance in people with asthma, as compared to baseline. The aim of this study was to examine the influence of dynamic lung stretching (via BPAP) on airway resistance and bronchoreactivity. We used the forced oscillation technique (FOT) to monitor continuous changes in airway resistance during sleep.
2. Methods
2.1 Patient Selection and Screening
The protocol was approved by the Partners Human Research Committee institutional review board for work done at Brigham and Women’s hospital. All subjects gave informed, written consent prior to participation. Overweight and obese subjects (BMI ≥25 kg/m2) with and without a physician diagnosis of asthma were recruited. All subjects were non-smokers (<10 pack-years) and non-habitual snorers. A complete history and physical exam was performed by a physician to exclude other respiratory disease or use of medications known to affect airway function or sleep. The diagnosis of asthma was confirmed by methacholine challenge. To proceed asthma subjects had a PC20 (concentration of methacholine required to elicit a 20% reduction in FEV1) <16mg/ml while healthy subjects had a PC20 >16mg/ml. If a subject had an FEV1 below 55% predicted or was exhibiting any wheezing a bronchodilator was given instead of methacholine and the subjects had to have a 12% improvement post bronchodilator (and at least a 200ml increase) to be included. Asthma subjects had no changes in asthma regimen in the 4 weeks prior to study.
2.2 Study Protocol
Subjects were scheduled for two overnight visits 2–14 days apart. During one of the nights (random order), subjects slept with BPAP applied via a nasal mask while on the other night they slept with a nasal mask without any positive airway pressure. The primary outcome measures were respiratory system resistance (Rrs) and reactance (Xrs) during sleep, assessed by the forced oscillation technique (FOT), and airway reactivity as measured by partial methacholine challenge the morning after each sleep study.
2.3 Overnight Visits
Those with asthma were asked to withhold their short acting medications (e.g. albuterol) for at least 6 hours prior to their arrival time and to withhold their long acting medications (e.g. salmeterol, fluticasone) 24 hours prior to their arrival time. At each visit, baseline spirometry was performed with subjects sitting and supine. Electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG) were used to measure brain, eye, and chin muscle activity respectively for accurate sleep staging using standard criteria (Iber C, 2007). Electrocardiogram (ECG) leads were used to monitor heart rate. Four magnetometers on the chest were used to calculate relative changes in end expiratory lung volume (EELV) (Banzett et al., 1995).
A pressure tipped catheter (model MCP-500, Millar) was placed through the nostril and lowered to 1–2 cm below the base of the tongue to measure pressure at the level of the epiglottis (Pepi). Prior to insertion, both nostrils were sprayed with 0.05% oxymetazoline hydrochloride, a decongestant, and the more patent nostril was then anesthetized with 4% lidocaine topical spray. Subjects breathed through a nasal mask ComfortGel, Philips-Respironics Inc) through which pressure (Pmask) (Validyne Corp) and flow (V̇) (heated pneumotachometer: model 3700A, Hans Rudolf Inc; differential pressure transducer: Validyne Corp) were measured. Pressure and flow measurements were sampled at 128 Hz (1401 plus, Cambridge Electronic Design Limited). Arterial oxygen saturation was measured through a finger probe and the exhaled partial pressure of carbon dioxide was monitored (Capnograph monitor, BCI).
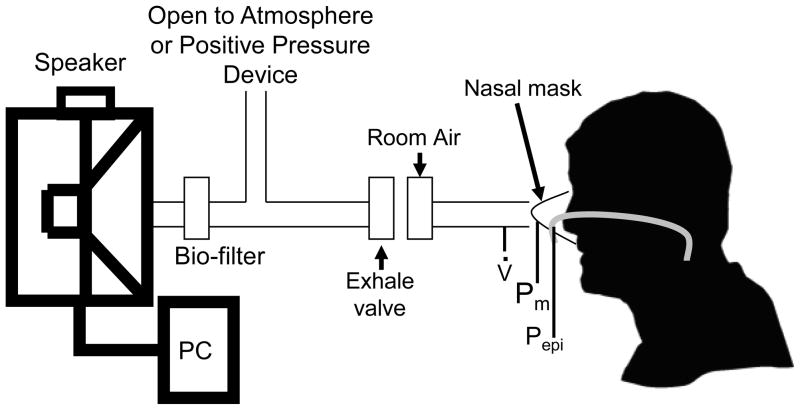
An in-house system was designed to deliver forced oscillations at 8Hz to the subject with or without positive airway pressure (Figure 1). The system consists of a 12in subwoofer speaker (Image Dynamics D4 V.3) housed in a Plexiglas box. A disposable bio-filter (nSpire KoKo Moe Filter) was placed at the outlet of the speaker box. A parallel dead space tube was used with a one-way exhalation valve was used, which allows exhaled gas to exit the system. Fresh room air was also bled into the system via a bias flow of medical air to avoid hypoxia/hypercapnia due to large dead space in the system. To induce oscillations over bi-level positive airway pressure, a BPAP device was used in parallel to the speaker rather than the dead space tube. The system has been validated with resistances of known value both with and without BPAP (Campana et al.).
Figure 1.
Forced oscillation system schematic. A speaker produces pressure oscillations which are supplied to the subject via a nasal mask. Flow and pressure are measured at the nasal mask (V̇, Pm) and pressure is measured at the level of the epiglottis (Pepi).
2.4 Measurements during Wakefulness
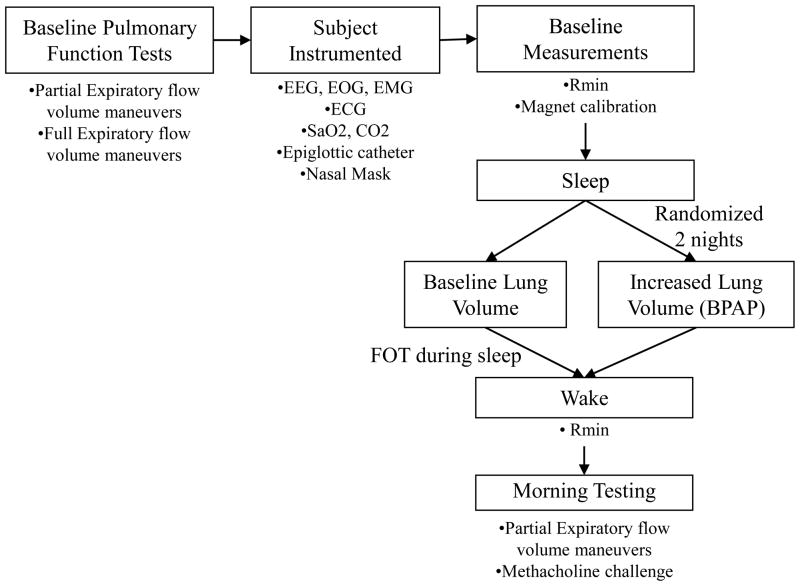
A schematic of the study protocol is shown in Figure 2. Before lights out several baseline measurements were performed. The magnetometers were calibrated by having the subject breathe 3–4 large breaths, 3–4 medium sized breaths, and 3–4 small breaths and correlating the tidal volumes with a change in the distance between the magnets. Baseline resistance data were assessed by turning FOT on and monitoring the subject for 2–3 minutes. Following baseline measurements lights were turned off and the subject was allowed to sleep. The FOT was kept on the remainder of the night.
Figure 2.
Study protocol schematic. Baseline pulmonary function tests were first performed. Next the subject is instrumented and baseline measurements were conducted in the supine position. Subjects were randomized to 2 nights (1–2 weeks apart) to either receive no positive airway pressure or BPAP. After a 6–8 hour sleep opportunity subjects were awakened and a methacholine challenge and pulmonary function tests were performed.
2.5 Intervention
Two study nights occurred in random order. On one night subjects slept at their baseline lung volumes (baseline night), while on a second night volumes were increased using a BPAP device (BiPAP synchrony, Philips-Respironics) (therapy night). On the therapy night the subject fell asleep without any positive pressure (0 PAP). After the subject was asleep for ~20 mins CPAP was turned on, at a level of 4cmH2O, for ~1hr. The mode of the device was then switched to BPAP with an inspiratory pressure (IPAP) set to 6–8 cmH2O while expiratory pressure (EPAP) is kept at 4 cmH2O. If the subject remained asleep the IPAP was pushed up incrementally to 8, 10, 12, or 14 cmH2O. If the subject had an arousal for longer then 30 seconds then IPAP was decreased to 4 cmH2O. From there the process began again of waiting for the subject to obtain stable sleep before IPAP was increased to 10–14 cmH2O.
2.6 Post-sleep measurements
In the morning after 6–8 hours of sleep, subjects were awakened and any positive pressure support was discontinued. Rmin was measured again using a TLC maneuver and the subject was then de-instrumented. Shortly after, a partial methacholine challenge was performed in the sitting position (Skloot et al., 1995). During this challenge partial expiratory flow maneuvers were used in order to avoid deep inspirations. The challenge was ended when the partial FEV1/FVC ratio dropped below 0.55 or if the subject exhibited wheezing and/or chest tightness. After the challenge ended, full expiratory flow volume maneuvers were performed and albuterol was given to reverse any airway obstruction.
2.7 Analysis
Flow and pressure measurements were used to calculate the 8Hz component of resistance and reactance using the cross power spectrum method, the full details have been reported elsewhere (Campana et al.). Respiratory system resistance (Rrs) and reactance (Xrs) are calculated by using the Pmask and V̇ signals. Upper airway resistance and reactance (Rup, Xup) is calculated by using Pmask-Pepi and V̇, and lower airway resistance and reactance (Rlow, Xlow) is found by using Pepi and V̇. The coherence function was calculated to confirm a robust signal to noise ratio and any impedance value with coherence<0.90 was discarded. Furthermore, any negative resistance values were also discarded. Resistance and reactance values were taken at end inspiration and grouped by sleep stage (wake, NREM, or REM) and pressure setting (baseline, 0 PAP, CPAP, BPAP) and then averaged for each subject.
2.8 Statistics
We first sought to determine if there were any differences in impedance between sleep stages and disease state at baseline, without any positive airway pressure. A 2 way ANOVA (factor 1: Wake vs. NREM vs. REM; Factor 2: Healthy vs. Asthma) was performed on end inspiration resistance and reactance values of the baseline night only. To determine if resistance varied between study visits we performed a paired t-test between Rrs during NREM sleep from the baseline night and 0 PAP Rrs during NREM sleep from the therapy night. Finally, we sought to determine the effect of BPAP on airway resistance. We performed a 2 way ANOVA to compare impedance between baseline and BPAP study nights. To minimize multiple comparisons we selected impedance values only from NREM sleep (Factor 1: Healthy vs. Asthma; Factor 2: Baseline vs. BPAP)
3. Results
3.1 Demographics
A total of 10 healthy subjects and 10 subjects with asthma were recruited and completed all study procedures. There were no significant differences in age or BMI between the two groups (Table 1). Those with asthma were hyper-reactive to methacholine and FEV1 percent predicted was lower compared to healthy controls (t-test, p<0.01). Two asthma subjects were not given methacholine due to wheezing (1 subject) and FEV1 <55% predicted (1 subject). Both subjects had a positive response to bronchodilator with a 13% (400 ml) and 40% (520 ml) improvement respectively in FEV1.
Table 1.
Subject demographics
| Healthy
| ||||||
|---|---|---|---|---|---|---|
| Subject # | Age (yrs) | Sex | BMI | PC20 (mg/ml) | FEV1 (L) | FEV1 (%) |
| 1 | 24 | M | 32.9 | >25 | 6.58 | 113 |
| 2 | 36 | M | 32.3 | >25 | 3.90 | 93 |
| 3 | 26 | M | 31.5 | >25 | 3.50 | 81 |
| 4 | 31 | F | 27.7 | >25 | 3.75 | 104 |
| 5 | 58 | M | 31.3 | >25 | 3.51 | 95 |
| 6 | 21 | F | 31.1 | >25 | 3.92 | 110 |
| 7 | 50 | M | 29.6 | >25 | 3.64 | 103 |
| 8 | 27 | M | 36.7 | >25 | 5.41 | 105 |
| 9 | 59 | F | 28.4 | >25 | 1.81 | 87 |
| 10 | 22 | F | 37.8 | >25 | 4.05 | 117 |
|
| ||||||
| Avg | 35.4 | 6 M, 4 F | 31.9 | 4.01 | 100.8 | |
| SD | 14.8 | 3.3 | 1.25 | 11.6 | ||
| Asthma
| |||||||
|---|---|---|---|---|---|---|---|
| Subject # | Age (yrs) | Sex | BMI | PC20 (mg/ml) | FEV1 (L) | FEV1 (%) | Asthma Medications |
| 1 | 26 | F | 35.2 | 0.25 | 2.22 | 73 | Fluticasone/salmeterol, Albuterol |
| 2* | 19 | F | 28.6 | - | 3.39 | 94* | Fluticasone/salmeterol, Montelukast, Albuterol, cetrizine |
| 3 | 48 | F | 36.4 | 0.747 | 2.35 | 82 | Fluticasone, Albuterol |
| 4 | 52 | F | 41.6 | - | 1.08 | 40 | Fluticasone, Albuterol |
| 5 | 56 | M | 28.1 | 16 | 2.36 | 77 | Fluticasone, Albuterol |
| 6 | 25 | F | 31.7 | 0.446 | 3.37 | 103 | Albuterol |
| 7 | 19 | F | 28.8 | 7.823 | 3.75 | 103 | Albuterol |
| 8 | 30 | M | 26.4 | 1.536 | 3.10 | 80 | Fluticasone, Albuterol, Loratadine |
| 9 | 31 | F | 46.4 | 0.683 | 2.06 | 62 | Albuterol |
| 10 | 20 | F | 33.1 | 1.49 | 2.83 | 85 | Albuterol, Loratadine |
|
|
|||||||
| Avg | 32.6 | 2 M, 8 F | 33.6 | 3.6 | 2.65 | 79.9 | |
| SD | 14.1 | 6.4 | 5.2 | 0.79 | 19.0 | ||
|
| |||||||
| P-Value | 0.67 | 0.47 | 0.01 | 0.01 | |||
First and best FEV1, subject bronchoconstricted with each subsequent FEV1 maneuver
3.2 Sleep architecture
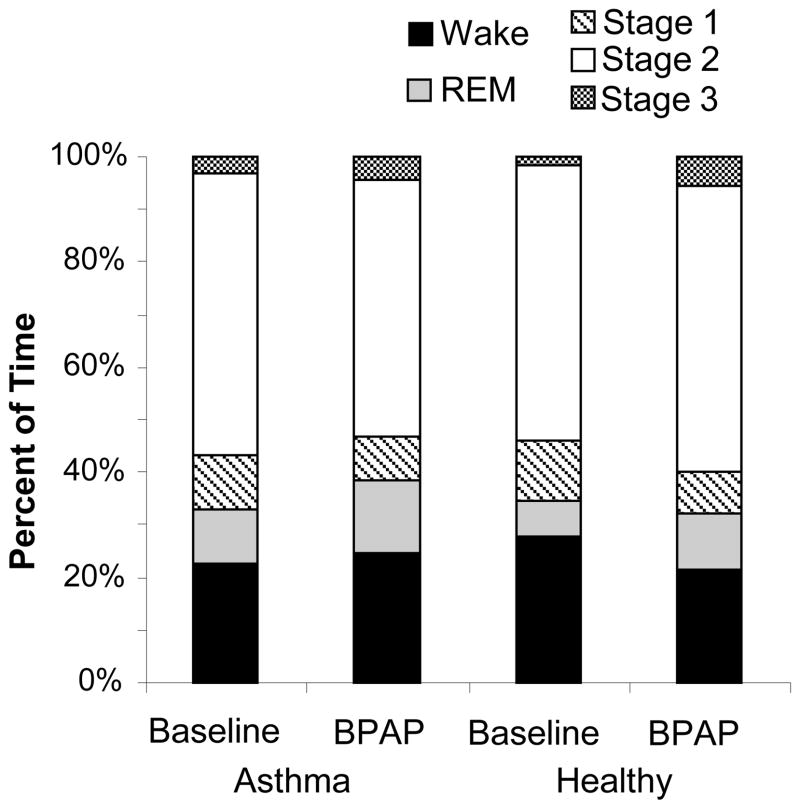
No differences in sleep quality were seen between healthy and asthma or between baseline and therapy nights in either group (Figure 3), as assessed by total sleep time, percent of time spent awake, and percent of time awake after sleep onset. Subjects spent roughly 80% of the night asleep. On the baseline night the average apnea hypopnea index (AHI) in the asthma group was 4.4±6.3 events/hr, with only 1 subject having an AHI>10events/hr. In the control group the average AHI was 14.3±15.5 events/hr, with 3 subjects having an AHI>10 events/hr. On the therapy night CPAP was applied for an average of 1.4 ± 0.9 hrs and 1.6 ± 0.9 hrs in healthy and asthma groups respectively, while BPAP was applied for an average of 4.2 ± 1.3 hrs in the healthy group and 3.9 ± 1.3 hrs in the asthma group.
Figure 3.
Sleep efficiency for asthma and healthy groups at baseline and on BPAP. No significant differences in sleep stages were found between groups.
3.3 Methacholine Challenge
Partial methacholine challenges were attempted on every subject; however some of the asthma subjects had an FEV1/FVC ratio below the minimum of 0.55. In these subjects if the full FEV1 >=55% predicted then a full methacholine challenge was performed according to ATS guidelines. Of the 10 subjects with asthma, only 3 had a partial FEV1/FVC ratio greater than 0.55 and were given a partial methacholine challenge. Of the remaining 7 subjects, a full methacholine challenge was performed on 4, while the remaining 3 subjects were unable to receive methacholine. Given that all the subjects with asthma were not able to complete the partial methacholine challenge we matched this in our control group and gave 4 of the healthy subjects a partial challenge, while 6 received full methacholine challenges. Listed in Table 2 are the pulmonary function results and methacholine dose given to the subjects with asthma. All healthy subjects were given the maximum dose of methacholine (25mg/ml) on both study nights with no reactions, except for 1 subject who had a PC20 of 12.27 mg/ml after the baseline night only. No differences were found in pulmonary function tests or methacholine reactivity between baseline and BPAP nights in either healthy or asthma groups.
Table 2.
Methacholine results
| Asthma | Baseline | BPAP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Subject # | Partial FEV1/FVC | Full FEV1 | Methacholine | Partial FEV1/FVC | Full FEV1 | Methacholine | ||||
| Type | Last Dose | PC 20 | Type | Last Dose | PC 20 | |||||
| 3 | 0.69 | x | Partial | 1 | 0.69 | x | partial | 4 | ||
| 6 | 0.68 | x | Partial | 1 | 0.66 | x | partial | 1 | ||
| 7 | 0.96 | x | Partial | 16 | 0.89 | x | partial | 16 | ||
|
| ||||||||||
| 1 | 66 | Full | 0.25 | 0.12 | 61 | Full | 0.25 | 0.10 | ||
| 8 | 0.51 | 62 | Full | 1 | 1.0 | 0.5 | 61 | full | 1 | 0.97 |
| 9 | 0.59 | 51 | Full | 1 | 0.60 | 0.54 | 52 | full | 1 | 0.63 |
| 10 | 0.57 | 77 | Full | 4 | 1.59 | 0.64 | 75 | full | 16 | 5.10 |
|
| ||||||||||
| 2 | 0.41 | 54 | None | 0.44 | 63 | None | ||||
| 4 | 0.74 | 53 | None | 34 | None | |||||
| 5 | 0.53 | 68 | None | 0.47 | 71 | None | ||||
3.3 Lung volume changes
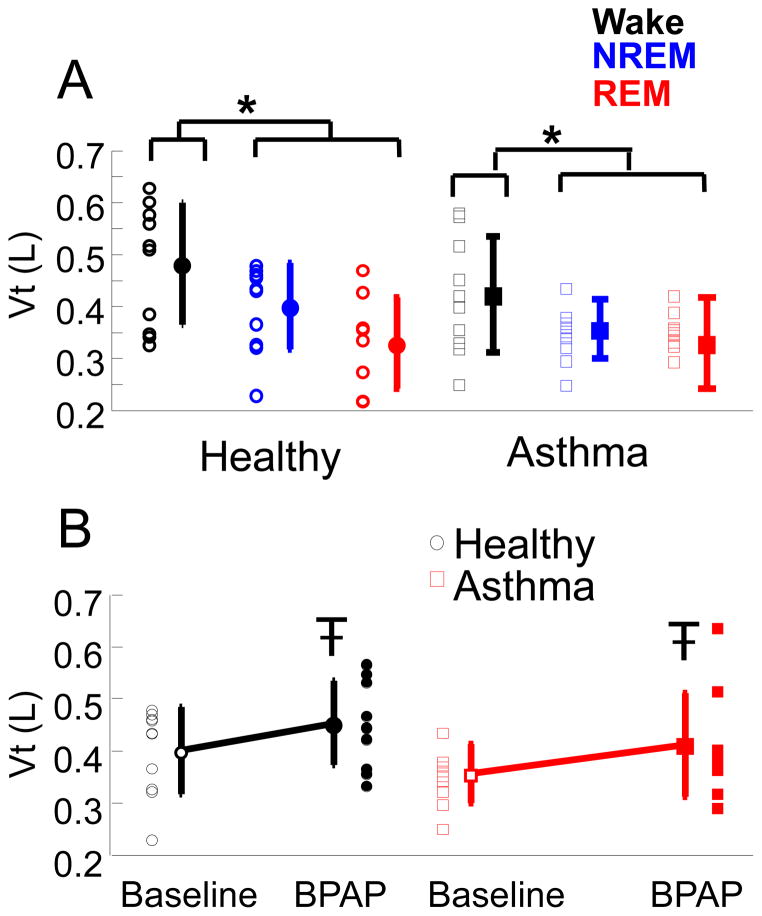
During the baseline night, EELV fell by 73±34 ml in healthy subjects and 115±137 ml in asthma subjects with sleep onset (p=0.36). Tidal volumes were also decreased in NREM and REM sleep as compared to wake, independent of disease state (p<0.001) (Figure 4A). When positive pressure was initially turned on (either to a CPAP level of 4cmH20 or a BPAP level of 8cmH20 IPAP and 4cmH2O EPAP) EELV increased on average by 148±139 ml in the healthy group and by 111±99 ml in the asthma group (p=0.52). Tidal volumes were significantly increased with BPAP (p <0.05). However, the change of Vt with BPAP was small, only a 15% increase relative to baseline (Figure 4B).
Figure 4.
A) Tidal volumes (Vt) for healthy (circles) and asthma (squares) subjects at baseline in wake (black), NREM (blue), and REM (red) sleep. Individual data and Mean ± SD are plotted. *p<0.001 ANOVA, tidal volumes during wake are larger than NREM and REM sleep, independent of disease.
B) Tidal volumes (Vt) for healthy (black circles) and asthma (red squares) during NREM sleep at baseline and on BPAP. Individual data and Mean ± SD are plotted. p<0.05 ANOVA, Vt on BPAP is larger than Baseline independent of disease.
3.4 Resistance and Reactance at Baseline
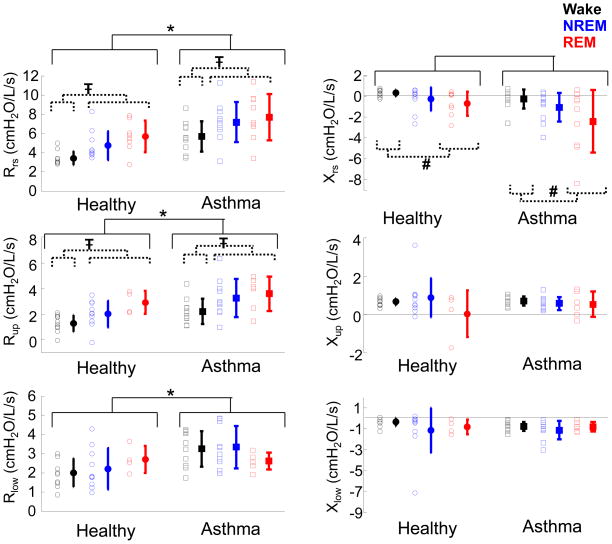
Over the course of the night, Rrs was increased during NREM and REM sleep as compared to wakefulness (p<0.01) independent of disease state (Figure 5). Similarly Xrs was more negative in REM sleep as compared to wake (p<0.05). Furthermore, as expected, Rrs was greater (p<0.001) and Xrs was more negative (p<0.02) in the asthma group as compared to the healthy. The sleep stage and disease effects seen in Rrs were mirrored in Rup. Rup was increased in NREM and REM sleep as compared to Wake (p<0.01) and was increased in the asthma group as compared to healthy (p<0.001). Rlow was also significantly higher in those with asthma as compared to healthy (p<0.001); however, there were no significant changes with sleep stage. No significant differences were found with Xup or Xlow, between sleep stages or disease at baseline.
Figure 5.
Baseline resistance (left) and reactance (right) during wake (black), NREM sleep (blue), and REM sleep (red). Individual data and means ± SD are plotted; healthy (circles) and asthma (squares). *p<0.001, Rrs, Rup, Rlow larger in asthma than healthy. Ŧ p<0.01, Rrs and Rup larger in NREM and REM sleep as compared to wake. § p<0.02, Xrs more negative in asthma. # p<0.05 Xrs is more negative in REM sleep as compared to wake.
3.5 Resistance and Reactance from Baseline to Therapy
We also tested to see whether impedance differed between the baseline night (baseline) and the therapy night before any positive pressure intervention was applied (0PAP). Since at baseline, resistance may be drastically changing over the course of the evening we compared the first 1/3 of the baseline night to the 0PAP condition, which also occurred during the first 1/3 of the night. We found no significant difference in Rrs between baseline and therapy nights for either healthy or asthma (p = 0.6 and p = 0.2 respectively, paired t-test).
3.6 Resistance and Reactance with BPAP
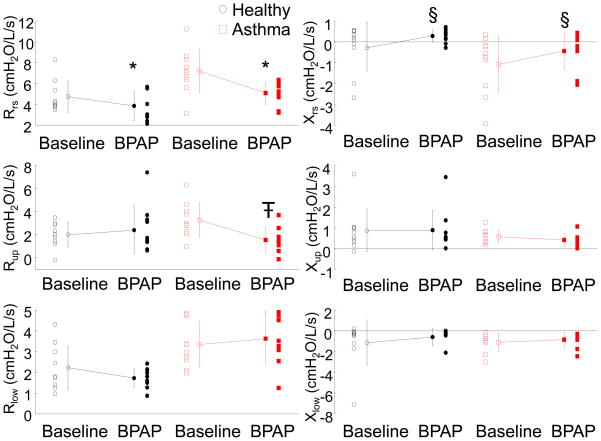
Since not all subjects achieved REM sleep we characterized changes in resistance from baseline to BPAP therapy during NREM sleep only. When subjects were on the BPAP device, Rrs decreased (p<0.001) compared to baseline, and there was an increase in Xrs (p<0.05), independent of disease state (Figure 6). No significant differences were found in resistance or reactance between CPAP and BPAP treatment periods. BPAP significantly lowered Rup as compared to baseline (p<0.01); however, there was an interaction effect with disease state (p<0.01). Rup wass significantly lower with BPAP in the asthma group but not in the healthy group. No differences were found in Xup or Xlow between healthy and asthma or baseline and BPAP.
Figure 6.
Baseline and BPAP resistance (left) and reactance (right) during NREM sleep. Individual data and means ± SD are plotted; healthy (circles) and asthma (squares). *p<0.001, Rrs is decreased with BPAP application independent of disease. Ŧ p<0.01, Rup is decreased with BPAP, only in asthma group. § p<0.05, Xrs is more positive with BPAP application.
4. Discussion
In this study, using the FOT technique to measure airway resistance and by altering lung volumes in subjects with and without asthma, we have made several important observations. 1) Overall sleep quality did not change significantly from baseline to therapy, indicating that BPAP was not detrimental to sleep in our positive pressure naïve subjects. 2) Our data confirm prior studies suggesting a drop in EELV in healthy and asthma subjects with sleep onset, though no differences were found between the two groups. 3) As expected, resistance was increased and reactance was decreased in the asthma group as compared to healthy, indicating airway disease and obstructive physiology. However, we found that the increased total resistance seen in the asthma group was not only due to increased lower airway resistance, but also increased upper airway resistance. 4) Changes in reactance during REM sleep suggest the lung is becoming stiffer with sleep, particularly in those with asthma. 5) When BPAP was applied, respiratory system resistance was decreased as compared to baseline in both the healthy and asthma group. However, this effect was driven by a decrease in upper airway resistance, particularly in those with asthma. 6) A single night of BPAP did not change airway reactivity in the morning as compared to baseline.
4.1 Sleep architecture
Sleep architecture was not different on BPAP as compared to baseline. This finding suggests that a clinical trial may be feasible to test whether BPAP therapy over a longer time period (i.e. successive nights for several weeks) would alter respiratory mechanics and asthma symptoms. One caveat is that since the subjects were heavily instrumented on both study nights, their sleep quality at baseline may have been impaired to begin with and no additional decrement was seen with BPAP application.
4.2 Lung Volumes
EELV and tidal volumes decreased with sleep and the application of BPAP led to increases in both EELV and tidal volume back to pre-sleep levels. With sleep onset the drop in lung volume was similar between asthma and healthy subjects and was smaller than previously reported (Ballard et al., 1990). Given that all the subjects were overweight or obese it is likely that their resting awake FRC was lower than in lean healthy subjects (Salome et al., 2010), limiting the degree to which lung volume can further drop with sleep onset. An analogy could be made to lung volume changes that occur in obese subjects with position change – the change is much smaller than that seen in lean subjects (Yap et al., 1995).
4.3 Resistance and Reactance
At baseline, those with asthma had not only increased Rrs and Rlow as compared to healthy, but also increased Rup. This finding suggests that asthma patients even without obstructive sleep apnea may have some upper airway anatomical changes that lead to increased resistance. We hypothesize that this finding is due to either baseline allergic inflammation in the nasopharynx, or an exuberant response to catheter placement in the nares. Furthermore, BPAP greatly reduced this upper airway resistance, particularly in the asthma group. In an overweight population with little to no obstructive sleep apnea CPAP or BPAP may be a valuable tool in reducing upper airway resistance and improving sleep and breathing quality.
We also found important changes in Xrs with sleep stage. Previous modeling studies have shown that lung elastance (EL), which is proportional to lung reactance by −2πf below the resonant frequency, is highly frequency dependent, and is altered in those with severe asthma (Kaczka et al., 1999). After bronchoconstriction, EL is increased in both healthy and asthma subjects at low (0.1 Hz) and high (8 Hz) frequencies (Lutchen et al., 2001). Based on modeling data, two factors may be involved in altering 8 Hz EL: heterogeneity of parallel airways and airway wall shunting (absorption of pressure waves into airway walls) due to substantial constriction of peripheral airways. Since we did not measure reactance over multiple frequencies we cannot separate out what factors are precisely causing a decrease in reactance with REM sleep, but hypothesize that it is due to airway closure and subsequent airway wall shunting. During REM sleep lung volumes may further decrease, causing airway closure. With this constriction airflow is shunted into the side of the airway causing apparent decreases in Xrs (increase in EL at 8 Hz). We did not find a significant decrease in Xlow with REM sleep, possible due to the fact that Xlow was inherently noisier than Xrs. The peak to peak pressure oscillations that were measured on the epiglottic catheter are reduced from what is measured at the airway opening due to pressure losses in the upper airway. This loss in pressure reduces the signal to noise ratio for the impedance calculation for the lower airways. Xup was largely positive in both groups, indicating that inertive forces are dominating the measurement.
A surprising finding was that BPAP did not importantly decrease lower airway resistance. If lung volume increases, it would lead one to believe that resistance would fall concurrently. There are several possibilities for why we did not find a drop in Rlow with BPAP application. This finding is similar to what was found in Irvin et al. that increasing lung volumes during sleep did not cause a drop in resistance (Irvin et al., 2000). The authors concluded that ‘uncoupling’ of the airway from the parenchyma was responsible for this change. While lung volume did increase somewhat in our study, it did not reach the degree of inflation achieved in the Irvin et al study {Irvin, 2000 #3064}. The most likely explanation for a lack of observed decrease in Rlow is related to signal/noise, such that changes would be expected to occur with greater increases in lung volume than those achieved in the present study. Heizner et al. found significant increases in end expiratory lung volume with application of CPAP, on the order of ~770ml with an average CPAP level of 13 cmH2O (Heinzer et al., 2006). We found only moderate increases in EELV with addition of BPAP, likely due to the fact that EPAP was only set to 4cmH2O. In addition, we found no significant differences in airway resistance between CPAP and BPAP, suggesting that reductions in airway resistance from baseline can be achieved with CPAP alone and the increased IPAP level on BPAP does little to alter tidal volumes and airway resistance. Further increases in EELV may be achieved with greater EPAP levels; however, we generally observe repetitive arousal in CPAP naïve patients during more marked hyperinflation. A higher EPAP level may also limit the amount of dynamic stretching we can achieve. Thus, larger stimuli than those applied in the present study may not be feasible during stable sleep in CPAP naïve patients.
Another possibility is that airway wall stiffness changed based on the reactance measurements. As wall stiffness increases for a given BPAP level there will only be a small increase in airway volume, resulting in little change in resistance. The small increase in tidal volume with BPAP application may be insufficient to test the hypothesis that dynamic lung stretch alters airway mechanics and caliber; however, our results are consistent with a recent publication from Laprad et al.(LaPrad et al.) demonstrating that in excised bovine airways, deep inspirations did little to alter the caliber of the airway after constriction with acetylcholine.
We did see high baseline Rrs in the asthma group (driven primarily by the upper airway) which reduced when BPAP was applied. Collett et al. have shown that glottal area is decreased after bronchoconstriction in those with asthma and that this finding was reversed temporarily with CPAP application during wakefulness (Collett et al., 1983). The effects of changes in airway resistance on airflow are complex, but in theory changes in upper airway resistance could importantly influence more distal airway function (Venegas et al., 2005).
Perhaps based on the modest stimuli feasible during a single night of sleep, we found no change in airway reactivity as measured by methacholine, or pulmonary function tests. Unfortunately, we could not complete all the methacholine challenges as originally intended due to poor lung function in our asthma group, but it is likely that even with more subjects the effect of BPAP therapy on airway reactivity would be similar to that observed, given the modest increase in lung volumes and short treatment period. In animal studies, CPAP has been shown to reduce airway reactivity as measured by resistance after 4 days of CPAP therapy as compared to no therapy(Xue et al.). We suspect that one night of BPAP application is not sufficient to alter these outcomes using our current paradigm.
4.4 Limitations
Despite our study’s strengths, we acknowledge a number of weaknesses. One limitation of this study is that we did not experiment with increasing EPAP levels which should increase EELV more significantly. Furthermore, IPAP levels were maximized to induce large tidal volumes in order to induce lung stretch. However, we found that interestingly, the tidal volumes did not increase by a large amount when on BPAP. When IPAP was turned up, tidal volumes were larger for the first few breaths, but then returned almost to baseline. With higher IPAP, inspiratory muscle activity (Pmus) likely decreased, with the machine taking over the work of inspiration (Meza et al., 1998). Hence, major tidal volume increases did not occur. Moreover, with large IPAP increases, recurrent arousal from sleep were observed suggesting the stimulus applied was the maximum feasible using the current experimental paradigm.
We also were not able to separate out the contributions of chest wall resistance to the total resistance measured. We placed an esophageal balloon in several subjects in order to try to calculate impedance of the chest wall. However, due to the logistics of the study setup no 8 Hz oscillations were captured due to damping of the tubing connecting the balloon to the pressure transducer. Larger peak to peak pressure oscillations may increase the signal seen on Pes but may also keep subjects awake. Finally, we selected a signal frequency of oscillation in order to track optimally changes in impedance over time. However, a multi-frequency oscillation would be better suited to interpret changes in Xrs.
There was a disparity in the gender of our control and asthma subjects. We attempted to recruit an equal number of men and women in our asthma and control arms; however, the majority of volunteers in the asthma cohort were women. Furthermore, the studies involved 1 daytime visit and 2 overnight visits with considerable instrumentation which limited overall recruitment. However, we do not feel the gender disparity limited our results, but would support further work in this area.
4.5 Conclusions
In summary we measured airway resistance over the course of a whole night in a group of healthy and asthma subjects. We found a large upper airway component of resistance in those with asthma, which was alleviated by BPAP application. There was no change in lower airway resistance or airway reactivity, implying that this level of positive airway pressure or the length of treatment period is not sufficient to induce mechanical changes within the lung. Further research will be required to determine the optimum feasible stimulus for mechanical lung stretching as a treatment for asthma.
Highlights.
Sleep and obesity cause reductions in lung volume, contributing to asthma
Increases in lung volume during sleep may reduce airway resistance
Resistance was quantified during sleep on and off positive airway pressure (PAP)
Upper airway resistance was reduced with Bi-level PAP
Upper airway resistance is a large component of total resistance, particularly in asthma
Acknowledgments
R01-HL-090897-2, F32HL097578, K24 HL093218, K23 105542, Ruth L. Kirschstein NRSA T-32, some research income from Philips Respironics, Harvard Catalyst funded by UL1 RR 025758-01.
Glossary
- ASM
Airway smooth muscle
- BPAP
Bi-level positive airway pressure
- EELV
End expiratory lung volume
- FEV1
Forced expiratory volume in 1 second
- FOT
Forced Oscillation Technique
- Pepi
Pressure measured at the epiglottis
- Pmask
Pressure measured at a nasal mask
- Rrs, Xrs
Respiratory system resistance and reactance
- Rup, Xup
Upper airway resistance and reactance
- Rlow, Xlow
Lower airway resistance and reactance
- V̇
Flow
- Vt
Tidal Volume
Footnotes
Disclosures
Dr. Malhotra has received consulting and/or research income from Philips Respironics, Pfizer, SHC, SGS, Apnex, Apnicure, but has no personal outside income following May 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol. 1990;68:2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- Ballard RD, Saathoff MC, Patel DK, Kelly PL, Martin RJ. Effect of sleep on nocturnal bronchoconstriction and ventilatory patterns in asthmatics. J Appl Physiol. 1989;67:243–249. doi: 10.1152/jappl.1989.67.1.243. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol. 1995;79:2169–2176. doi: 10.1152/jappl.1995.79.6.2169. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- Campana LM, Owens RL, Suki B, Malhotra A. Measuring Upper and Lower Airway Resistance During Sleep with the Forced Oscillation Technique. Ann Biomed Eng. 2012;40(4):925–933. doi: 10.1007/s10439-011-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137:1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99:529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Collett PW, Brancatisano T, Engel LA. Changes in the glottic aperture during bronchial asthma. Am Rev Respir Dis. 1983;128:719–723. doi: 10.1164/arrd.1983.128.4.719. [DOI] [PubMed] [Google Scholar]
- Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol. 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508-515.e501–502. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer SJ. A Treatise of the Asthma. 1698. [Google Scholar]
- Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910. [DOI] [PubMed] [Google Scholar]
- Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156:1752–1759. doi: 10.1164/ajrccm.156.6.9611016. [DOI] [PubMed] [Google Scholar]
- Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, A-IS, Chesson A, Quan SF. American Academy of Sleep Medicine. 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. [Google Scholar]
- Irvin CG, Pak J, Martin RJ. Airway-parenchyma uncoupling in nocturnal asthma. Am J Respir Crit Care Med. 2000;161:50–56. doi: 10.1164/ajrccm.161.1.9804053. [DOI] [PubMed] [Google Scholar]
- Kaczka DW, Ingenito EP, Israel E, Lutchen KR. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am J Respir Crit Care Med. 1999;159:169–178. doi: 10.1164/ajrccm.159.1.9709109. [DOI] [PubMed] [Google Scholar]
- Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J. 2007;29:307–311. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- LaPrad AS, Szabo TL, Suki B, Lutchen KR. Tidal stretches do not modulate responsiveness of intact airways in vitro. J Appl Physiol. 2010;109:295–304. doi: 10.1152/japplphysiol.00107.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layon J, Banner MJ, Jaeger MJ, Peterson CV, Gallagher TJ, Modell JH. Continuous positive airway pressure and expiratory positive airway pressure increase functional residual capacity equivalently. Chest. 1986;89:517–521. doi: 10.1378/chest.89.4.517. [DOI] [PubMed] [Google Scholar]
- Lutchen KR, Jensen A, Atileh H, Kaczka DW, Israel E, Suki B, Ingenito EP. Airway constriction pattern is a central component of asthma severity: the role of deep inspirations. Am J Respir Crit Care Med. 2001;164:207–215. doi: 10.1164/ajrccm.164.2.2008119. [DOI] [PubMed] [Google Scholar]
- Macgregor AM, Greenberg RA. Effect of Surgically Induced Weight Loss on Asthma in the Morbidly Obese. Obes Surg. 1993;3:15–21. doi: 10.1381/096089293765559700. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Pak J. Nasal CPAP in nonapneic nocturnal asthma. Chest. 1991;100:1024–1027. doi: 10.1378/chest.100.4.1024. [DOI] [PubMed] [Google Scholar]
- Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol. 1998;85:1929–1940. doi: 10.1152/jappl.1998.85.5.1929. [DOI] [PubMed] [Google Scholar]
- Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Padilla R, West P, Kryger MH. Sighs during sleep in adult humans. Sleep. 1983;6:234–243. doi: 10.1093/sleep/6.3.234. [DOI] [PubMed] [Google Scholar]
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. quiz 1094–1085. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434:777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- Von Behren J, Lipsett M, Horn-Ross PL, Delfino RJ, Gillilan F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, Waist Size, and Prevalence of Current Asthma in the California Teachers Study Cohort. Thorax. 2009 doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z, Yu Y, Gao H, Gunst SJ, Tepper RS. Chronic continuous positive airway pressure (CPAP) reduces airway reactivity in vivo in an allergen-induced rabbit model of asthma. J Appl Physiol. 2011;111:353–357. doi: 10.1152/japplphysiol.01345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79:1199–1205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]