Abstract
Objectives
Determine the durability of anabolic effects and adverse events (AEs) after stopping testosterone and growth hormone supplementation in older men.
Design
Secondary analysis of a double-masked, randomized controlled trial of testosterone gel (5g or 10g/daily) plus rhGH (0, 3, or 5ug/kg/day) with follow-up of outcomes 3-months later.
Participants
108 community-dwelling 65-90 year-old-men.
Measurements
Testosterone and IGF-1 levels, body composition (DEXA), 1-repetition maximum (1-RM) strength, stair-climbing power, quality-of-life (QOL) and activity questionnaires, AEs.
Results
Despite improvements in body composition during treatment, residual benefits 3-months later (week-28) were variable. For participants with improvements exceeding their week-17 median changes, benefits were sustained at week 28 for lean body mass (LBM, 1.45±1.63kg, 45% of week-17 values, p<0.0001-vs-baseline), appendicular skeletal muscle mass (ASMM, 0.71±1.01kg, 42%, p<0.0001), total fat (-1.06±2.18kg, 40%, p<0.0001,), and trunk fat (-0.89±1.42kg, 50%, p<0.0001,); retention of ASMM was associated with greater week-16 protein intake (p=0.01). For 1-RM strength, 39%-43% of week-17 improvements (p≤0.05) were retained and associated with better week-17 strength (p<0.0001), change in testosterone from week-17-to-28 (p=0.004) and baseline PASE (p=0.04). Framingham 10-year cardiovascular risks were low (~14%), didn’t worsen, and improved by week-28 (p=0.0002). The hypothalamic-pituitary-gonadal axis recovered completely.
Conclusions
Durable improvements in muscle mass, strength, and fat mass were retained 3-months after discontinuing hormone supplementation in participants with greater than median body composition changes during treatment, but not in others with smaller gains. AEs largely resolved after intervention discontinuation. Additional strategies may be needed to sustain or augment muscle mass and strength gains achieved during short-term hormone therapy.
Keywords: Lean body mass, fat mass, muscle performance, quality of life, cardiovascular risks
INTRODUCTION
With advancing age, sarcopenia 1 contributes to decreased muscle strength and physical function 2, increased risk of falls with fractures, and diminished quality of life 3 Aging is also associated with accumulation of trunk fat, increases in blood pressure, insulin resistance, and abnormalities in lipid metabolism, which enhance cardiovascular disease (CVD) risks 4. These changes in body composition, physical function and substrate metabolism are related to decreased production of testosterone and growth hormone (GH) in older persons 5. Approximately 25-30% of men over 60 years-of-age have low levels of testosterone 6 that may be associated with sarcopenia, muscle weakness or frailty, central adiposity, increased CVD risks, and mortality 7-9. Declines in GH synthesis and release have also been associated with similar age-related co-morbidities including body adiposity and the metabolic syndrome 10-12 even in persons with normal testosterone levels 5. Levels of IGF-1, a mediator of several but not all anabolic effects of GH, continue to decline into the 8th and 9th decades 5 and are associated with loss of lean body mass (LBM) and increases in adiposity 10.
To better understand the combined effects of age-related changes in testosterone and GH/IGF-1 on body composition, muscle performance, and adiposity, we conducted a multicenter study (Hormonal Regulators of Muscle and Metabolism in Aging; the HORMA Trial) of testosterone supplementation alone and in combination with recombinant human GH (rhGH) at physiologic doses in older community dwelling men. We reported that these interventions produced significant gains in LBM, appendicular skeletal muscle mass (ASMM), maximum voluntary muscle strength, and aerobic endurance with significant decreases in fat mass according to assigned dosages 13. Although other studies have also reported the effects of testosterone and rhGH in older persons, either alone or in combination 10, 13-19, there is a paucity of data on the durability of the anabolic response to these interventions. A recent trial has heightened concerns about potential cardiovascular risks associated with testosterone administration, especially in older men with high burden of chronic diseases 20. Further, there are almost no data on recovery from expected adverse effects following discontinuation of testosterone administration. Further, suppression of the hypothalamic-pituitary-testicular axis that occurs as a result of exogenous androgen administration may persist for varying duration, could adversely affect body composition, and has been overlooked in prior androgen studies. Accordingly, we examined changes in hormone levels, LBM, ASMM, muscle performance, fat mass, safety measures, and determinants of cardiovascular risk during treatment and then 12-weeks after discontinuing hormonal interventions in the HORMA Trial. For participants with durable benefits after treatment discontinuation, we evaluated host factors that might contribute to these sustained anabolic responses.
METHODS
Study Design
HORMA was a controlled, double-masked investigation of testosterone and rhGH supplementation for 16 weeks in men 65-90 years old with age-related hormone levels 13. End of treatment measurements were collected at either week 16 (assessment for adverse events and hormone levels) or 17 (body composition and muscle performance). In the current secondary analysis, follow up evaluations were performed at week 28, 3-months after treatment discontinuation; this includes data not previously analyzed or reported.
Study Subjects
Participants provided written informed consent approved by the local IRBs. Eligible men had morning total testosterone levels in the lower half (5.2-19.1umol/L) of the adult male range and serum IGF-1 in the lower tertile for adults (<21.9nmol/L), typical of 65-90 year old men. Other inclusion criteria included PSA ≤4.0ug/L, hematocrit ≤50%, and fasting blood glucose <6.99mmol/L 13.
Treatment Regimens
Participants received a GnRH agonist (leuprolide acetate depot, 7.5mg intramuscularly; Tap Pharmaceutical Products Inc.) monthly for 12 weeks to suppress endogenous testosterone production (Leydig cell clamp). Participants were randomized to receive 5g or 10g of 1% testosterone transdermal gel (Solvay Pharmaceuticals Inc.) each morning and 0, 3 and 5μg/kg of rhGH (Nutropin, Genentech Inc.) by subcutaneous injection each evening for 16 weeks. The 3 and 5μg/kg doses of rhGH were chosen to increase whole body protein synthesis 21 but were expected to be low enough to minimize the adverse risks that occur with higher doses 22.
Outcome Measures
In this secondary analysis, the outcomes of interest include the durability of anabolic measures.
Body Composition
Lean tissue and fat mass were quantified by dual energy x-ray absorptiometry (DEXA). Scans were analyzed at the USC DEXA Reading Center by a single DEXA-certified technician and validated by a senior DEXA supervisor (CM). ASMM is the sum of appendicular LBM of the four extremities.
Muscle Performance
Upper and lower body maximal voluntary strength was determined by the one-repetition maximum (1-RM) method twice prior to randomization and at weeks 17 and 28 for the bilateral leg press, leg extension, leg flexion, latissimus pull-down, and chest press. Because 1-RM results were obtained on different equipment at the three clinical centers, changes in muscle strength are presented as percentage change from baseline for the composite sum of the five exercises. Aerobic endurance was the time to failure (unable to maintain 55rpm) during cycle ergometry at 80% of work at VO2peak. Margaria stair climbing power was calculated from body weight, force of gravity, and time (measured by photocells) to ascend the middle 4 steps of a 12-step staircase.
Hormone Assays
For screening, total testosterone was measured using standard platform immunoassays in the local clinical university laboratories and IGF-1 levels at Quest Diagnostics. After completion of the study, stored serum samples obtained at baseline, weeks 16 and 28 were batch tested. Testosterone levels were quantified using liquid chromatography-tandem mass spectrometry at Boston University (inter-assay CVs at 8.68 and 17.35 umol/L were 5% and 3% with intra-assay CV 3% and 2%, respectively). IGF-1 levels were determined in the USC GCRC Core Laboratory using an automated chemiluminescent analyzer (Immulite 1000, Siemans Healthcare Diagnostics, Deerfield, IL; sensitivity=2.62 nmol/L, inter-assay CV=3.6% and intra-assay CV=6.6%) 13.
Other Outcome Measures
Dietary Assessments
Entries in three-day food diaries were reviewed by study nutritionists with participants at baseline and weeks 16 and 28. Total energy and macronutrient intake were quantified using Nutritionist Pro (Axxya Systems, Stafford, Texas).
Safety Measures
Safety measures were obtained at monthly visits; individual tests were reported previously 13. Composite evaluations of Framingham cardiovascular risks were not assessed previously but are reported herein.
Physical Activity and Quality of Life Measures
Exercise and physical activity were assessed using the Physical Activity Scale for the Elderly (PASE) questionnaire. Quality of life (QOL) was assessed by the Geriatric Depression Scale (GDS) and Short-Form Health Survey (SF-12) consisting of physical component scores (PCS) and mental component scores (MCS). Questionnaires were administered by study coordinators in quiet rooms at baseline and study weeks 16 and 28.
Framingham Cardio-metabolic Risks
Framingham 10-year CVD risk scores were calculated (www.nhlbi.nih.gov) at baseline and weeks 16 and 28 using age, LDL-cholesterol, HDL-cholesterol, blood pressure, anti-hypertensive medications, smoking status, and diabetes as risk factors.
Statistical Considerations
Of 112 participants completing 16 weeks of study treatment, four participants were lost to follow up for their week 28 evaluations. Thus, 108 participants were included for analyses, since there were no differences in baseline characteristics or subsequent outcomes for N=112 versus N=108.
Change in body composition by DEXA was calculated as week 17 or 28 minus baseline, and hormone changes at week 16 was compared to baseline. The entire cohort was dichotomized into high versus low responders for week 17 change for total LBM, ASMM, total fat, and trunk fat. For LBM and ASMM, “high” responders were participants with increases above the respective body composition median change, where as “low” change responders were those below the respective medians. For total and trunk fat mass, “high” responders were participants with changes below the median change (i.e. larger improvements in fat mass) and “low” responders were those changes above the median (i.e. smaller improvement in fat mass). Baseline characteristics, hormone levels and their change at week 16 were compared between each pair of the high and low body composition subgroups by standard t-tests. Between subgroup comparisons of durability at week 28 (versus baseline) were assessed using the one-way ANCOVA adjusting for covariates age; baseline body composition, BMI, hormone levels, SF-12 PCS, SF-12 MCS and PASE score; and week 16 change of hormone levels, SF-12 PCS, SF-12 MCS, and PASE score. We were only interested in whether LBM, ASMM, and strength were higher, and whether total and trunk fat were lower at week 28 than baseline, thus, one-sided paired t-tests were conducted within each of the high and low subgroups. Since two durability components, LBM/ASMM and 1-RM strength, were tested for each subgroup (high/low responders), Bonferroni adjustment was conducted so that significance was reduced two fold for these analyses (i.e. p<0.025).
Stepwise linear regression was used to identify significant predictors in addition to the high/low responder variables for improvements in body composition and 1-RM parameters at week 28. Candidate predictors were based on known physiology and correlations reported in the literature; these included variables listed above (to adjust the ANCOVA) as well as protein intake, and change in testosterone and IGF-1 between the three time points (baseline, week 16 and week 28). We also conducted regression modeling to ascertain whether the original 2X3 factorial design for treatment assignment or changes in hormone levels during treatment affected the durability of responses. Since these factors did not affect outcomes, they are not presented or discussed further.
The incidence of adverse events and laboratory abnormalities at week 16 and those that persisted at week 28 were calculated. Paired t-tests were used to test for significance at weeks 16 and 28.
Statistical analyses were conducted using Statistical Analysis System 9.2 (SAS Institute Inc., Cary NC).
RESULTS
Study Cohort
Of 122 men randomized to study interventions, 112 completed study therapies 13. Of these, 108 returned for off-treatment evaluations 3-months later; three who did not return for final study visits were doing well and elected not to be evaluated further, and the fourth was unable to obtain transportation for the visit. Participants were ambulatory, community dwelling men with characteristics typical of older men (Table 1). There were no consistent baseline differences for participants who ultimately had improvements in LBM greater or lower than the median week 17 changes (Table 1). Some end of treatment outcomes at week 16 and 17 reported previously 13 are summarized here to demonstrate durability of their effects at week 28.
Table 1.
Baseline Characteristics Dichotomized for High and Low Responders by Change in Lean Body Mass
| Variable (N=108) | High responders* (N=56) | Low responders* (N=52) | P-value |
|---|---|---|---|
| Week 17 Δ ≥ 1.5 kg | Week 17 Δ < 1.5 kg | ||
| Age, yrs | 71±4 | 71±4 | 0.54 |
| BMI, kg/m2 | 28.0±3.6 | 27.2±2.9 | 0.22 |
| Non-Hispanic Caucasian | 50 (89%) | 43 (83%) | 0.32 |
| On treatment for hypertension | 14 (25%) | 14 (27%) | 0.82 |
| History of Smoking | 20 (36%) | 19 (37%) | 0.93 |
| History of elevated cholesterol† | 8 (14%) | 5 (10%) | 0.46 |
| History ischemic heart events | 18 (32%) | 19 (37%) | 0.63 |
| Daily food intake | |||
| Caloric intake kcal/day | 2166±503 | 2242±464 | 0.42 |
| Protein g/kg/day | 1.09±0.34 | 1.14±0.32 | 0.42 |
| Carbohydrate g/day | 263±86 | 255±73 | 0.59 |
| Fat g/day | 88±53 | 88±28 | 0.96 |
| Systolic blood pressure mmHg | 129±14 | 137±19 | 0.01 |
| Diastolic blood pressure mmHg | 75±9 | 79±8 | 0.02 |
| Activity and Quality of Life | |||
| PASE‡ Score | 145±62 | 145±55 | >0.99 |
| Geriatrics Depression Score | 3.1±3.4 | 2.5±2.9 | 0.31 |
| SF-12 Physical Component Score | 52±6 | 53±5 | 0.36 |
| SF-12 Mental Component Score | 56±6 | 56±6 | 0.62 |
| Serum creatinine umol/L | 76.3±15.3 | 76.3±7.6 | 0.53 |
| Albumin g/L | 41±3 | 41±3 | 0.71 |
| Hematocrit % | 43.2±2.7 | 43.2±2.8 | 0.90 |
| Prostate Specific Antigen ug/L | 1.6±0.9 | 1.3±0.8 | 0.08 |
| Blood Lipids | |||
| Triglycerides mmol/L | 1.47±0.75 | 1.39±0.63 | 0.55 |
| Total cholesterol mmol/L | 4.56±0.75 | 4.45±0.73 | 0.46 |
| LDL cholesterol mmol/L | 1.17±0.31 | 1.06±0.34 | 0.16 |
| HDL cholesterol mmol/L | 2.72±0.70 | 2.75±0.65 | 0.95 |
| Hormones: | |||
| Total testosterone§, ηmol/L | 11.9±3.6 | 13±3 | 0.09 |
| IGF-1, nmol/L | 14.1±4.3 | 25±3.3 | 0.54 |
| QUICKI | 0.161±0.017 | 0.161±0.015 | 0.87 |
| Body Composition: | |||
| Lean body mass, kg | 58±7 | 59±7 | 0.43 |
| Appendicular lean mass, kg | 25±3 | 26±3 | 0.26 |
| Total adipose tissue mass, kg | 23.5±7.3 | 20.7±5.9 | 0.03 |
| Trunk fat mass, kg | 13.2±4.3 | 11.9±3.7 | 0.09 |
| Cardiovascular characteristics: | |||
| VO2 peak ml/kg/min | 24.6±4.9 | 24.9±4.8 | 0.73 |
| Aerobic endurance time sec | 308±102 | 359±135 | 0.051 |
| Framingham 10 year risk score % | 13.7±1.3 | 14±1.2 | 0.27 |
Baseline characteristics were dichotomized by total lean body mass (LBM), appendicular skeletal muscle mass (ASMM), total fat and trunk fat. Because dichotomized results were only minimally different for the four body composition parameters due to slightly different sample sizes created by their respective median splits, the dichotomized groups are only shown for total LBM.
Based on past or current treatment for elevated cholesterol
Physical Activity Scale for the Elderly
Screening testosterone levels for eligibility were determined by immunoassay in the local university clinical laboratories
Conversion to non-SI units: divide triglycerides by 0.0113 (mg/dL); total cholesterol (C), LDL-C, and HDL-C by 0.0259 (mg/dL); testosterone by 0.0347 (ηg/dL); and IGF-1 by 0.131 (ηg/mL)
Changes in Hormones
Total morning testosterone levels by LC-MSMS rose to 28.7±16.1ηmol/L at study week 16 (p<0.0001 versus baseline) but by week 28 levels were similar to baseline (p=0.22, Figure 1). Luteinizing hormone (LH) declined from 4.2±3.5 at baseline to 0.2±0.1U/L at week 16 (p<0.0001) but returned to normal levels at week 28 in all participants and were slightly greater than baseline (4.8±3.9U/L, p<0.02). Serum IGF-1 levels rose to 24.2±8.8ηmol/L at week 16 (p<0.0001) and were similar to baseline at week 28 (p=0.69).
Figure 1. Change in Hormone Levels at Completion of Study Intervention and Late Follow Up.
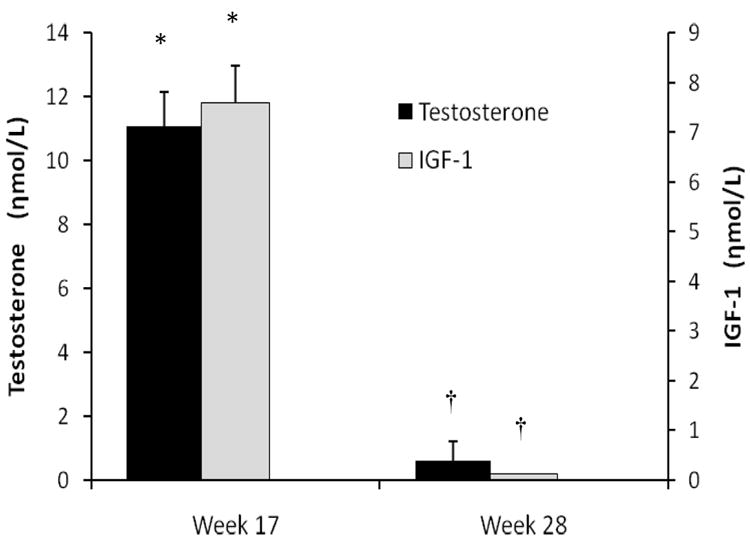
Changes in serum testosterone (black bars) and IGF-1 (gray bars) at week 16 compared to baseline and week 28 compared to baseline. Whiskers are standard error bars. * represents within group change of p<0.0001 compared to baseline and † represents differences (p<0.0001) between the two time points. At week 28, levels were not different from baseline (p>0.05). For conversion to non-SI units, divide testosterone by 0.0347 (ηg/dL) and IGF-1 by 0.131 (ηg/mL).
Changes in Body Composition
We herein report for the first time that total LBM increased by 1.84±1.89kg (p<0.0001) and ASMM by 0.88±1.15kg (p<0.0001), whereas total fat mass declined by -1.36±1.76kg (p<0.0001) and trunk fat by -0.90±1.23kg (p<0.0001) at week 17 for the entire cohort (N=108, Figure 2). At week 28, there were some, albeit small, residual benefits for LBM of 0.78±1.51kg (one-sided p<0.0001) and ASMM of 0.42±0.92kg (one sided p<0.0001) but not for total fat (-0.14±1.90kg, one-sided p=0.22) or trunk fat (-0.13±1.29kg, one-sided p=0.16) compared to baseline (Figure 2). Participants with durable effects at week 28 were more likely to have had greater improvements in body composition at week 17, namely for high responders (defined in statistical methods), 40-44% of lean mass gains and 44-47% of fat mass losses were retained (Table 2).
Figure 2. Changes in Body Composition and Muscle Performance at Study Intervention Completion and Late Follow Up.
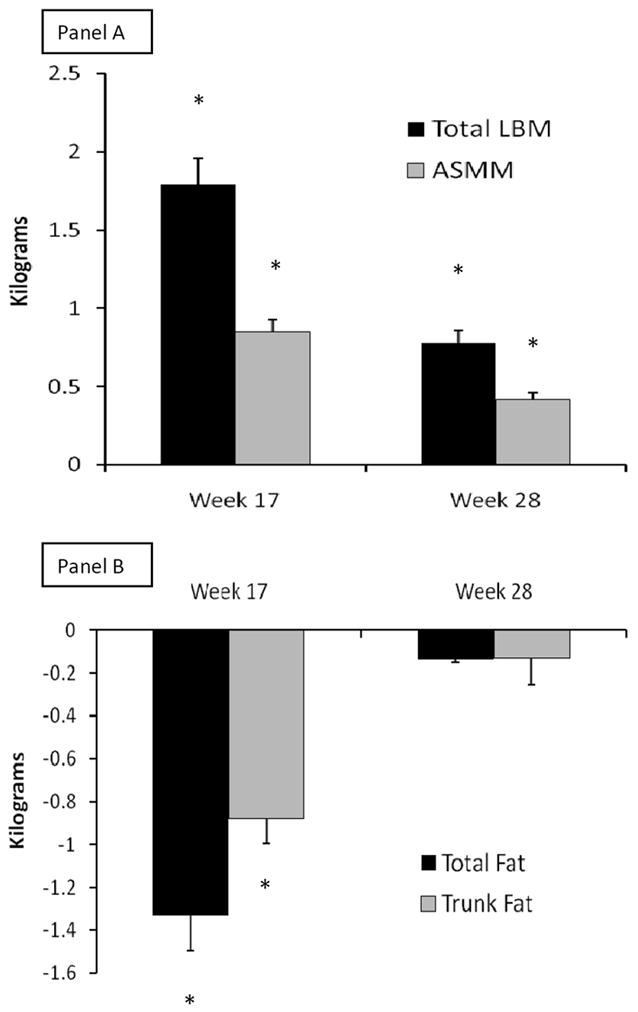
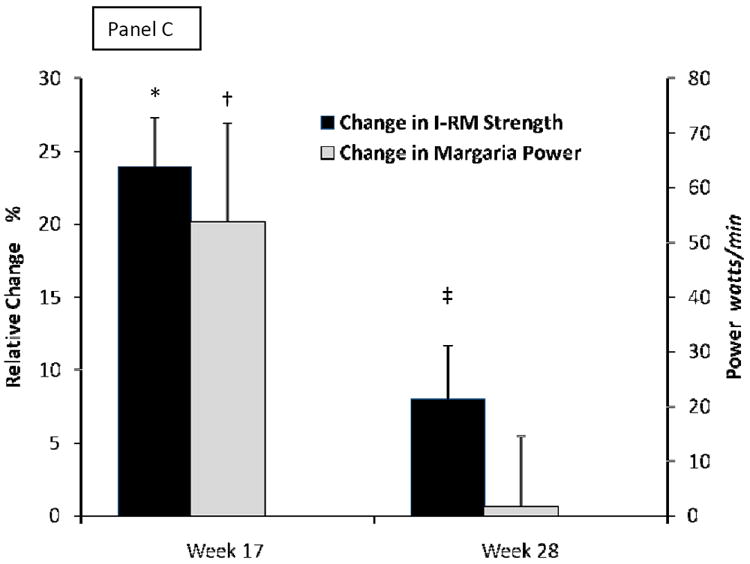
Changes from baseline to week 17 or week 28 for total lean body mass (LBM, black bars) and appendicular skeletal muscle mass (ASMM, gray bars) in panel A, for total fat mass (black bars) and trunck fat (gray bars) in panel B, and for maxium voluntary strength as composite of 1-repetition maximum (1-RM, black bars) and Margaria stair climbing power (gray bars) in panel C. Whiskers are standard errors bars. * is p<0.0001, † is p=0.002, and ‡ is p=0.03 for time points versus baseline. For conversion to non-SI units, divide testosterone by 0.0347 (ηg/dL) and IGF-1 by 0.131 (ηg/mL).
Table 2.
Residual Effects at Week 28 for High versus Low Responders
| Week 17 High and Low Responders* | N | Change in Body Composition | Change in 1-RM Muscle Strength | Hormone Levels at Week 17 | |||
|---|---|---|---|---|---|---|---|
| Δ at Week 28 vs baseline kg | P values§ | Δ at Week 28 vs baseline % | P values§ | Testosterone ηmol/L | IGF-1 ηmol/L | ||
| Δ in Total LBM | |||||||
| High gain (≥ 1.5kg) | 56 | 1.45±1.63‡ (45%)¶ | <0.0001 | 13.0±45.1 (43%) | 0.016 | 34.7±17.6 | 26.2±8.4 |
| Low gain (< 1.5kg) | 52 | 0.16±1.91 | 0.24 | -1.0±53.3 | 0.56 | 22.3±11.4 | 22.1±8.6 |
| P-value # | 0.0001 | 0.17 | <0.0001 | 0.02 | |||
| Δ in ASMM | |||||||
| High gain (≥ 0.85kg) | 54 | 0.71±1.01 (42%) | <0.0001 | 12.2±46.6 (39%) | 0.026 | __** | __** |
| Low gain (≤ 0.85 kg) | 54 | 0.21±1.08 | 0.06 | 1.2±49.7% | 0.43 | __ | __ |
| P-value # | 0.01 | 0.26 | |||||
| Δ in Total Fat Mass | |||||||
| High loss (≤ -1.1kg) | 55 | -1.06±2.18 (40%) | <0.0001 | N/A | N/A | 32.6±17.4 | 26.7±8.1 |
| Low loss (> -1.1kg) | 53 | +0.78±2.27 | >0.99 | N/A | N/A | 24.6±1..7 | 21.6±8.6 |
| P-value # | <0.0001 | 0.01 | 0.002 | ||||
| Δ in Trunk Fat | |||||||
| High loss (≤ -0.7kg) | 54 | -0.89±1.42 (50%) | <0.0001, | N/A | N/A | __** | __** |
| Low loss (> -0.7kg) | 54 | +0.65±1.42 | >0.99 | N/A | N/A | __ | __ |
| P-value # | <0.0001 | ||||||
Based on median changes in body composition as study week 17 minus baseline.
One-sided paired t-test within high and low responding subgroups at week 28 compared to baseline. For groups by LBM or ASMM, Bonferroni adjustment was (factor of 2) such that significance is p<0.025 instead of 0.05. For LBM, ASMM and 1-RM strength changes, the alternative hypotheses are mean value > 0. For fat components, the alternative hypotheses are mean value < 0.
Data are mean ± one standard deviation adjusted for age, baseline body composition, BMI, hormone levels, SF-12 PCS, SF-12 MCS and PASE score, and week 16 change of hormone levels, SF-12 PCS, SF-12 MCS, and PASE score.
% in parentheses are change in mean values at week 28 compared to mean values at week 17 for the same subsets.
Body composition and 1-RM strength: One-way ANCOVA for high versus low responders, adjusted for age, baseline body composition, BMI, hormone levels, SF-12 PCS, SF-12 MCS and PASE score, and week 16 change of hormone levels, SF-12 PCS, SF-12 MCS, and PASE score. For hormone level changes, standard t-tests were used for between group comparisons.
Blood levels and their differences were nearly identical for total LBM compared to ASMM as well as for total compared to trunk fat mass because of the slightly different sample size for each of the body composition parameters dichotomized at their median levels of change.
Changes in Muscle Performance
For 91 participants with strength testing at pretreatment, week 17 and 28, we herein report for the first time, composite 1-RM voluntary strength increased by 25±31% (p<0.0001; Figure 2C). There was a small retention of 1-RM strength at week 28 (8±35%, p=0.03). In men who were high responders for LBM and ASMM at week 17, there were more sizable retentions of 1-RM strength (31-36%) at week 28 (one-sided p=0.027 for LBM, and p=0.023 for ASMM, Table 2). Margaria stair climbing power increased from 663±210watts at baseline to 718±284watts at week 16 (one sided p=0.001; Figure 2C) but there was no durability of improvement at week 28 (665±199watts).
Predictors of Durable Change in Lean Tissue Mass and Muscle Performance
Participants with changes in total LBM and ASMM associated with residual strength benefits at week 28 had higher testosterone and IGF-1 levels compared to low responders at the end of treatment (week 16, Table 2). To further investigate the effects of hormone levels and other variables, stepwise linear regression was used to explore significant predictors of sustained responses at week 28 (data not shown). For LBM, the significant predictors of change at week 28 compared to baseline were the high/low responder variable, which accounted for 21% (partial R2) of change (p<0.0001), baseline SF-12 MCS (15%, p=0.001) and baseline protein intake (6%, p=0.04). Significant predictors for ASMM were baseline ASMM (13%, p=0.0002), week 16 protein intake (9%, p=0.01) and high/low responder variable (6%, p=0.04), For 1-RM strength, predictors of durability at week 28 included week 17 change from baseline (59%, p<0.0001), change in testosterone levels at week 28 from week 16 (15%, p=0.004) and baseline PASE score (8%, p=0.04, 8%); the two high/low responder variables for LBM and ASMM were not significant, (p>0.5 for both).
Physical Activity and Quality of Life Measures
There were no meaningful changes in PASE, GDS, SF-12 PCS, or SF-12 MCS from baseline to weeks 16 or 28 for the dichotomized groups (data not shown). However, for PASE, improvements at week 16 were correlated with change in testosterone (r=0.21, p=0.05) but were not durable at week 28. For the SF-12 MCS, scores were correlated with IGF-1 levels in participants receiving the 10g/day testosterone dose (r=0.32, p=0.03) during the 16 week course of treatment, but there were no apparent residual benefits at week 28.
Adverse Events and Cardiovascular Risks
Because changes in these measures were similar for high and low responders, outcomes at week 28 are not dichotomized by week 16 outcomes and are listed together for the 108 participants (Table 3). Of the safety tests that worsened during therapy, namely hematocrit, which increased by 2.0±3.2% and PSA by 0.2±0.7 (p=0.0006) by week 16 13, values at week 28 declined and did not differ from baseline. There were no serious prostate events. Because some cardio-metabolic risk factors worsened (e.g. blood pressure and LDL-cholesterol) and others improved (e.g. HDL-cholesterol and fasting triglycerides) 13, we calculated Framingham 10-year cardiovascular risk scores. At week 16, Framingham scores were not statistically different compared to baseline (13.8±1.2% versus 13.9±1.3, respectively, p=0.77) and were typical of men with “low” 10-year CVD risks. At week 28, the Framingham scores were lower than baseline by -0.34±0.97 (p=0.0004) and week 16 by -0.38±0.99 (p=0.0002).
Table 3.
Safety Measures and Cardiovascular Risk Scores
| Change from baseline | Week 16 | Week 28 | P value* |
|---|---|---|---|
| Δ in Systolic blood pressure mm Hg | 11.8±14.2§ | 7.9±13.9§ | 0.009 |
| Δ in Diastolic blood pressure mm Hg | 7.6±8.1§ | 6.2±9.8§ | 0.12 |
| Δ in Hematocrit % | 1.99±3.19§ | -0.39±2.77 | <0.0001 |
| Δ in Prostate specific antigen ug/L | 0.24±0.72¶ | 0.13±0.98 | 0.26 |
| Δ in Qualitative insulin check index | -0.004±0.016¶ | -0.005±0.013§ | 0.24 |
| Δ in Triglycerides mmol/L | -0.21±0.65 ¶ | -0.18±0.51¶ | 0.21 |
| Δ in LDL cholesterol mmol/L | 0.09±0.61 | 0.31±0.52§ | 0.0002 |
| Δ in HDL cholesterol mmol/L | 0.09±0.17§ | 0.20±0.20§ | <0.0001 |
|
| |||
| Framingham Risk Score | 0.03±0.97 | -0.34±0.97¶ | 0.0002 |
Paired t test comparing week 16 change versus week 28 change
p<0.0001 by paired t test for week 16 or 28 changes compared to baseline
p<0.01 by paired t-test for week 16 or 28 changes compared to baseline
DISCUSSION
We report for the first time the durability of effects resulting from supplemental therapy with transdermal testosterone alone and when combined with rhGH supplementation 3-months after treatment discontinuation in older community dwelling, ambulatory men. We previously described end-of-treatment benefits (week 17) related to specific dose combinations. In the current follow-up evaluation, those benefits were largely lost in many men 3-months after study therapies were stopped. However, for participants achieving improvements in LBM and ASMM greater than their respective medians at study week 17, there was significant retention of lean mass gains (~1.4 and 0. ~7kg, respectively) at week 28, which averaged 40-45% of week 17 values. Further, more than 39% of their maximum voluntary strength gains were maintained. Although residual improvements in strength were modest compared to baseline, week 28 values were compared to the higher of two pretreatment test results, thereby reducing the likelihood that these improvements were due to learning effects 23, and thus validating that accrual of functional myofibrillar proteins had occurred during the 4-months of hormone supplementation. These data help to define minimal clinically meaningful changes in lean tissue mass; namely changes in the range of ~1.5kg and ~0.7kg or greater for LBM and ASMM, respectively, at end of treatment appear to be necessary to maintain enhancements in skeletal muscle performance for at least 3-months. Improvements below the median threshold for changes in LBM and ASMM would presumably require continued therapy to sustain or to further enhance gains in muscle function.
These durable improvements in body composition and maximal voluntary muscle strength 3-months after discontinuing hormone supplementation were associated with higher testosterone and IGF-1 levels achieved during supplementation. Average testosterone levels at week 16 (~31umol/L) for those with durable benefits at week 28 were similar to levels expected in 20-30 year-old men and substantially higher than in participants without durable effects (~21umol/L), whose week 16 testosterone levels were only modestly higher than their baseline levels. Physical activity scores, quality of life measurements, and nutritional factors (protein intake) were also determinants of durable benefits. Although the contribution of these life-style factors was relatively modest (8-15% contributions), these factors should be controlled as potential confounding variables in future hormone intervention trials or possibly included as adjunctive strategies to maximize retention of anabolic effects.
The effects of testosterone supplementation on QOL have been inconsistent in previous trials 24, 25 whereas rhGH has been reported to improve mood, cognition and other components of QOL in GH-deficient adults 26, 27. We found no significant changes in GDS or SF-12 PCS, but changes in PASE were correlated with changes in testosterone during treatment. For the SF-12 mental component score, there were improvements for participants receiving the high dose of testosterone that were correlated with changes in IGF-1 levels. This may reflect that testosterone and IGF-1 levels affect blood flow to regions of the brain, which could affect different components of QOL 27-29. However, there were no durable effects of treatment for either PASE or SF-12 MCS at week 28, possibly due to lack of statistical power or the relatively healthy status of the test population.
We selected doses of testosterone (10g/day) and rhGH (3 or 5ug/kg/day) expected to produce physiologic levels similar to those found in younger men in their third-to-fourth decades of life. However, the occurrence of certain adverse events during treatment could adversely affect prostate or cardiovascular health. Thus, an important feature of this study was to demonstrate the potential reversibility of adverse events that we reported previously 13. For example, increases in hematocrit, one of the most frequent adverse events in clinical trials of testosterone, abated completely and minimal increases in PSA levels returned to baseline values by week 28.
The week 16 increases in blood pressure were unanticipated and reached hypertensive levels in 11 participants. In a large longitudinal study of older men, testosterone levels were inversely related to blood pressure 8 and thus higher levels of testosterone are expected to be associated with lower blood pressure. Indeed, blood pressure has been reported to decline in obese middle aged and older men treated with either testosterone 30 or rhGH 31. Blood pressure elevation in our participants may have been due to salt and water retention as reflected by the fact that 35% of participants had new lower extremity edema during the study interventions 13. Regardless, blood pressure levels were lower three months after completion of study interventions, but they were still statistically higher than at baseline.
The QUICKI index of insulin sensitivity, worsened minimally for the study population at week 16 and changes persisted at week 28. The effects of testosterone and rhGH on insulin sensitivity have been inconsistent across trials. Epidemiologic studies have generally reported an inverse relationship between testosterone levels and insulin resistance 8 but intervention trials have not consistently found improvements in insulin sensitivity 32, 33. Short term treatment with rhGH is often associated with impairments in insulin sensitivity, when measured by frequently sampled intravenous glucose tolerance and hyperinsulinemic euglycemic clamp methods 31, 34, although some longer term trials of rhGH have reported improvements in insulin sensitivity 31, 34, presumably because of later reductions in fat mass.
In our study, there were significant decreases in fat mass that persisted 3-months later with about ~1.2kg reductions in total and ~0.9kg of trunk fat (44% and 47%, respectively) for the high responders. We can only speculate whether longer therapy and further reductions in upper body obesity might have improved insulin sensitivity. Among the cardiovascular risk factors, blood pressure improved somewhat but total and LDL-cholesterol remained minimally elevated 3-months after stopping treatment. The explanation for these observations is not clear as the physical activity domain of PASE was not changed over the 28 weeks. Likewise, intake of energy, carbohydrate, and fat did not change. By contrast, improvements in HDL-cholesterol and triglycerides were sustained at week 28. These favorable changes in HDL-cholesterol and triglycerides are consistent with other studies showing improvements in these lipids in concert with favorable effects of testosterone and GH therapies on body composition 30, 31.
To assess the global effects of improvements in trunk fat, HDL-cholesterol, and triglycerides versus worsening blood pressure, total and LDL-cholesterol during treatment, we calculated Framingham 10-year CVD risks scores. The average baseline CVD risk of 14% was well below the 25-30% risk typical of men 65-74 years of age. In this relatively healthy population of older men, there was no worsening of Framingham scores at the completion of study therapies and there was a small reduction in risk scores 3-months later. Thus, the short term worsening of some cardiovascular risk factors were offset by improvements in other risk factors resulting in continued low overall CVD risk status over the 7-month period. Finally, testosterone, LH, and IGF-1 returned to baseline levels by week 28 despite the use of the long acting GnRH agonist and exogenous hormone supplementation expected to centrally suppress GnRH and LH. While the reversal of adverse events after discontinuation of short-term testosterone and rhGH is reassuring, additional studies are needed to determine the long-term risk of cardiovascular and prostate events.
In conclusion, our results demonstrate for the first time the reversibility of expected adverse events, magnitude of adverse events that persist, and sustained anabolic responses after replacement therapy using these two endogenous anabolic hormones in some participants if testosterone and IGF-1 levels are augmented to youthful values. These data also provide the basis for selecting such combination strategies (e.g. testosterone, selective androgen receptor modulator, or antimyostatin therapy) plus either rhGH or GH secretagogue (e.g. capromorelin or oral ghrelin mimetic) 35, 36 for more focused future studies in populations at increased risk of functional limitations and disability (e.g. those with sarcopenia, frailty, obesity and adverse metabolic profiles) to assess their effectiveness in improving health outcomes and quality of life. Additional strategies such as nutritional support or exercise may be needed to sustain the gains in muscle mass and strength achieved during short term administration of such combination therapies.
Acknowledgments
We express our gratitude for the dedication of the study volunteers and other members of the HORMA research team without whom the study would not have been possible
Funding support: Support for HORMA was provided from R01 AG18169, NCRR GCRC M0I RR00043 at USC, the U.S. Department of Agriculture (USDA) ARS Cooperative Agreement 58-1950-9-001, the NCRR GCRC grant M01 RR000054 at Tufts University, the Mass Spectrometry Research Resource at Washington University (RR000954, DK020579, and DK056341), and U01AG14369 and 1R01DK70534 at Boston Medical Center, Boston University of Medicine.
Footnotes
Conflict of interest: Study therapies were provided by Solvay Pharmaceuticals Inc, Genentech Inc, and Tap Pharmaceutical Products Inc; industry sponsors provided no monetary support and no input for the design, methods, subject recruitment, data collection or analysis, and did not review this manuscript. FRS, SB, and EB have received independent funding support from Solvay Pharmaceuticals for other clinical studies unrelated to the current investigation and manuscript.
BIBLIOGRAPHY
- 1.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 3.Dutta C, Hadley E, Lexell J. Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl. 1997;5:S5–S9. [PubMed] [Google Scholar]
- 4.Reaven GM. Syndrome X: 6 years later. J Intern Med Suppl. 1994;736:13–22. [PubMed] [Google Scholar]
- 5.Abbasi AA, Drinka PJ, Mattson DE, et al. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc. 1993;41:975–982. doi: 10.1111/j.1532-5415.1993.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 6.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupelian V, Page ST, Araujo AB, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 10.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [see comments] [DOI] [PubMed] [Google Scholar]
- 11.Marin P, Kvist H, Lindstedt G, et al. Low concentrations of insulin-like growth factor-I in abdominal obesity. Int J Obes Relat Metab Disord. 1993;17:83–89. [PubMed] [Google Scholar]
- 12.Snel YE, Doerga ME, Brummer RM, et al. Magnetic resonance imaging-assessed adipose tissue and serum lipid and insulin concentrations in growth hormone-deficient adults. Effect of growth hormone replacement. Arterioscler Thromb Vasc Biol. 1995;15:1543–1548. doi: 10.1161/01.atv.15.10.1543. [DOI] [PubMed] [Google Scholar]
- 13.Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 15.Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- 16.Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 17.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [see comments] [DOI] [PubMed] [Google Scholar]
- 18.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 19.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 20.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucidi P, Lauteri M, Laureti S, et al. A dose-response study of growth hormone (GH) replacement on whole body protein and lipid kinetics in GH-deficient adults. J Clin Endocrinol Metab. 1998;83:353–357. doi: 10.1210/jcem.83.2.4545. [DOI] [PubMed] [Google Scholar]
- 22.Toogood AA, Shalet SM. Growth hormone replacement therapy in the elderly with hypothalamic- pituitary disease: a dose-finding study. J Clin Endocrinol Metab. 1999;84:131–136. doi: 10.1210/jcem.84.1.5408. [DOI] [PubMed] [Google Scholar]
- 23.Frontera WR, Hughes VA, Dallal GE, et al. Reliability of isokinetic muscle strength testing in 45- to 78-year-old men and women. Arch Phys Med Rehabil. 1993;74:1181–1185. [PubMed] [Google Scholar]
- 24.Gray PB, Singh AB, Woodhouse LJ, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 26.Sathiavageeswaran M, Burman P, Lawrence D, et al. Effects of GH on cognitive function in elderly patients with adult-onset GH deficiency: a placebo-controlled 12-month study. Eur J Endocrinol. 2007;156:439–447. doi: 10.1530/eje.1.02346. [DOI] [PubMed] [Google Scholar]
- 27.Arwert LI, Deijen JB, Muller M, et al. Long-term growth hormone treatment preserves GH-induced memory and mood improvements: a 10-year follow-up study in GH-deficient adult men. Horm Behav. 2005;47:343–349. doi: 10.1016/j.yhbeh.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Azad N, Pitale S, Barnes WE, et al. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88:3064–3068. doi: 10.1210/jc.2002-020632. [DOI] [PubMed] [Google Scholar]
- 29.Arwert LI, Veltman DJ, Deijen JB, et al. Memory performance and the growth hormone/insulin-like growth factor axis in elderly: a positron emission tomography study. Neuroendocrinology. 2005;81:31–40. doi: 10.1159/000084872. [DOI] [PubMed] [Google Scholar]
- 30.Marin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl 4):609S–612S. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 31.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–734. doi: 10.1210/jcem.82.3.3809. [see comments] [DOI] [PubMed] [Google Scholar]
- 32.Marin P, Krotkiewski M, Bjorntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med. 1992;1:329–336. [PubMed] [Google Scholar]
- 33.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfalck AM, Fisker S, Hilsted J, et al. The effect of the deterioration of insulin sensitivity on beta-cell function in growth-hormone-deficient adults following 4-month growth hormone replacement therapy. Growth Horm IGF Res. 1999;9:96–105. doi: 10.1054/ghir.1999.0091. [DOI] [PubMed] [Google Scholar]
- 35.White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94:1198–1206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 36.Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


