Abstract
Synaptobrevin 2 (Syb2), Syntaxin (Sx1A), and SNAP-25, generate a force to induce fusion pore formation. The v-SNARE, Syb2, is anchored to the vesicle membrane by a single transmembrane domain. Here we show that 2 tryptophans (W89/W90) located in the juxtamembrane domain of Syb2, which stabilize the transmembrane (TM) domain position, control the ratio of spontaneous vs. stimulated membrane fusion events in chromaffin cells. Changing the 2 hydrophobic tryptophans to neutral alanines promotes spontaneous membrane fusion, faster transmitter release kinetics and complete release from individual vesicles. The results indicate that the two tryptophans act as a fusion clamp making fusion stimulus-dependent.
Keywords: exocytosis, membrane fusion, synaptobrevin, snare complex, amperometry
Introduction
Cells release pre-formed molecules stored in secretory vesicles by fusion of the vesicle membrane with the plasma membrane [1]. SNARE complexes, including Syntaxin-1 and SNAP-25 on the plasma membrane, and synaptobrevin 2 (Syb2) anchored in the vesicle membrane, provide energy to mediate the formation of the fusion pore, a channel connecting vesicle membrane and plasma membrane [2,3]. In Syb2, a short linker connects the SNARE motif to the C-terminal TM domain [4], the function of which is still debated. The linker may be the calmodulin and lipid binding site of synaptobrevin [5]. According to the post-fusion crystal structure of the SNARE complex [6], hydrophobic contacts exist not only between SNARE motifs but also between linkers and TM regions of Syb2 and syntaxin. An aromatic layer (Syntaxin: Y257; Syb2: Y88, W89, W90) in the linker region is surrounded predominantly by basic residues and appears to be crucial for the linker contacts [6].
Previous work suggested that complexin controls the coupling of SNARE-complexes to membrane fusion [7]. In mouse cortical neurons complexin knock-down increases spontaneous fusion but suppresses fast Ca2+-evoked fusion [8]. The Syb2 W89A W90A double mutation (named WA mutant) phenocopied the complexin knockdown, suggesting that these residues may be involved in the complexin-Syb2 interaction although the mutation had no effect on complexin-SNARE complex binding [8]. Residues W89 and W 90 are located in the membrane-water interface [9] and contribute to stabilization of the Syb2 TM domain position [10].
Chromaffin cells are a widely used model system to study fusion pore formation and transmitter release kinetics [11]. The kinetics of transmitter release from single vesicles has been characterized in great detail using electrochemical [12], electrophysiological [13] and imaging methods [14]. In amperometric recordings, single fusion events produce an amperometric spike [15], which may be preceded by a pre-spike foot signal [16], representing the catecholamine release kinetics through the early narrow fusion pore [17,18]. Using v-SNARE (synaptobrevin 2 and cellubrevin) double knock-out (dko) chromaffin cells, we characterized spontaneous and stimulated release events mediated by the syb2 WA mutant. The results indicate that the tryptophans W89/W90 act as a fusion clamp. We propose that this clamping function is released in the WA mutant by increased mobility of the Syb2 TM domain.
Materials and Methods
Cells and Viral Expression
Chromaffin cells from syb2/ceb dko E18 embryonic mice were isolated and cultured as described [19]. Virus production and infection was performed as described [19]. Syb2 W89AW90A (Syb2 WA) mutant constructs were generated by using the Quik-Change Site-directed Mutagenesis Kit (Stratagene CA) and verified by DNA sequencing. The Syb2 wt and mutants were cloned into a modified pSFV1 plasmid, where an internal ribosome entry site was inserted followed by the gene of enhanced GFP as described [20].
Syb2 expression level
Immunostaining to estimate the overexpression levels of wild type syb2 (WT) and syb2 WA, chromaffin cells from dko mouse embryos was performed as described ($ngatchou). Cells were cultured in glass bottom dishes (MatTek Corporation). Six hours after transfection, cells were fixed in 4% paraformaldehyde (PFA) solution for 30 min, permeabilized in 0.1% triton-100X for 10 min and blocked with 6% bovine albumin serum (Sigma-Aldrich) for 1 h. After removing the blocking buffer, the cells were incubated with the primary syb2 antibody (1:500 dilutions, Abcam ab70222, Cambridge) for 2 h, washed 5 times with PBS, incubated with Alexa 546-labeled secondary antibodies (Invitrogen A-11071,1:200 dilutions) for 1 h, washed and mounted. Fluorescence imaging was performed with a Zeiss Axiovert 135 microscope. Alexa 546 was excited with 546 ± 10nm and imaged through a 580 ± 30 nm emission filter with CCD camera (ANDOR™ E2V TECH CCD97) with fixed 100 ms exposure time. Images were analyzed using ImageJ.
Amperometry
Homemade Carbon fiber electrodes with a diameter of 5.4 μm were used for amperometric measurements [3]. Amperometric currents were recorded with an EPC-7 amplifier (HEKA) applying an electrode voltage of +700 mV, filtered at 3 kHz and analyzed by a customized macro for IGOR software [21]. The analysis was restricted to events with a peak amplitude >5 pA. Foot signal analysis was restricted to events with foot current >0.5 pA and foot duration >0.5 ms. The bath solution (BS) contained 140mM NaCl, 5mM KCl, 5mM CaCl2, 1mM MgCl2, 10mM Hepes/NaOH, and 20mM glucose (pH 7.3), and stimulation solution (SS) contained 45mM NaCl, 100mM KCl, 5mM CaCl2, 1mM MgCl2, 10mM Hepes/NaOH, and 20mM glucose (pH 7.3).
Capacitance measurement
Cell-attached patch-clamp capacitance measurements were performed with patch pipettes (1~2 MΩ) using an EPC-7 amplifier (HEKA) and a lock-in amplifier (SR 830; Stanford Research Systems) applying a 20-kHz, 50-mV (root-mean square) sine wave [22]. In the pipette solution 100 mM NaCl of BS was replaced with 100 mM TEA-Cl as described [3]. Vesicle sizes were quantified by measuring capacitance step sizes > 0.1 fF. All experiments were performed at room temperature.
Statistical Analysis
For each analyzed parameter the median value was determined for each cell and the mean of these values was calculated such that number of values equals the number of cells analyzed.
Results
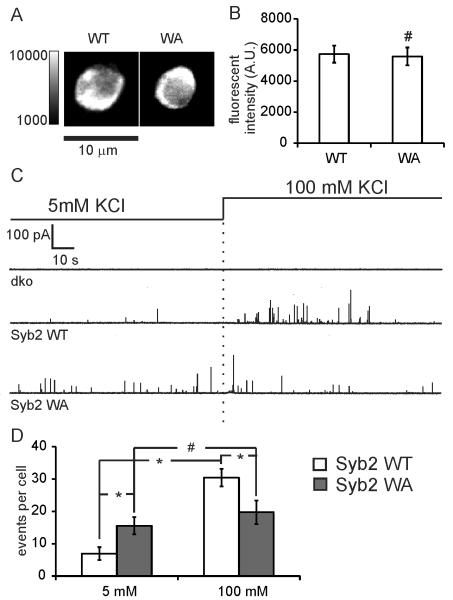
To investigate the functional role of the aromatic amino acid pair W89/W90 in the linker of Syb2 on exocytosis and fusion pore formation, wild-type Syb2 (Syb2 WT) or Syb2 WA (W89A/W90A) mutant were overexpressed in v-SNARE dko chromaffin cells. Spontaneous and stimulated exocytotic events were recorded by carbon fiber amperometry for 2 minutes in bath solution containing 5 mM KCl (spontaneous) followed by 2 minutes in 100 mM KCl (stimulated). The expression levels of the Syb2 WA mutant and Syb2 WT were similar (Fig. 1A,B).
Figure 1.
Syb2 WA mutant increases the frequency of spontaneous fusion events but decreased the frequency of stimulated events. (A) Syb2 immunofluorescence images of dko cells overexpressing either syb2 WT or syb2 WA mutant. (B) Averaged fluorescence intensity (A. U.) of dko cells overexpressing either syb2 WT (5731 ± 551, n=10) or syb2 WA (5585 ± 568, n=10). (C) Amperometric recording of catecholamine release from dko mouse chromaffin cells, and cell overexpressing Syb2 WT and Syb2 WA, in 5 mM or 100 mM KCl bath solution as indicated. (D) Average release frequency (events per cell) in cells overexpressing Syb2 WT (n=17) and Syb2 WA (n=12) in 5 mM KCl or 100 mM KCl (mimic spontaneous and stimulated release frequency, respectively). * p<0.05, # p>0.05 (student’s t test).
No spontaneous and stimulated events were detected in dko cells (Fig. 1C), consistent with previous reports in chromaffin cells [19], confirming that the v-SNARE is required for membrane fusion. Viral expression of Syb2 WT rescues fusion in response to stimulation as expected [2,19]. A few spontaneous exocytotic events were also detected in 5 mM KCl bath solution before stimulation. The number of stimulated exocytotic events was reduced (19.7 ± 3.7 events/cell) in Syb2 WA expressing cells compared to Syb2 WT expressing cells (35.3 ± 4.5 events/cell) (Fig. 1D). However, cells overexpressing the Syb2 WA mutant showed a marked increase in spontaneous fusion events (15.6 ± 2.7 events/cell), 2.3 fold more than cells expressing Syb2 WT (6.9 ± 2.0 events/cell). For Syb2 WA expressing cells, there was no statistically significant difference of amperometric spike frequency between spontaneous release and stimulated release.
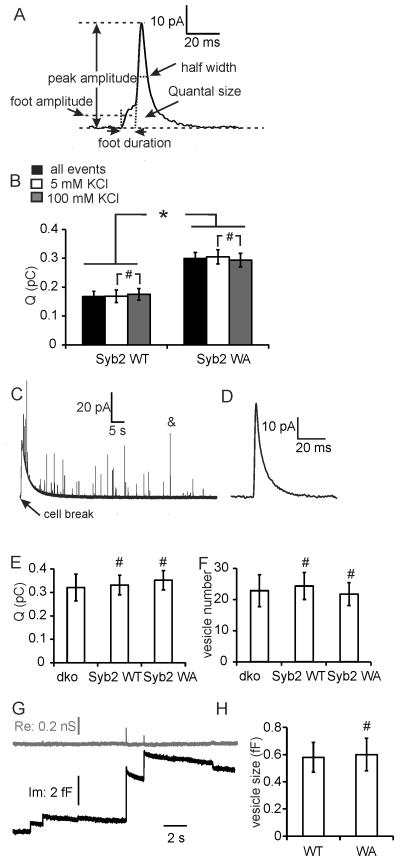
To determine whether the juxtamembrane tryptophans also modulate the kinetics of individual vesicle release events, the five parameters quantal size, spike half-width, peak amplitude, foot duration and foot current amplitude (Fig. 2A) were determined for each amperometric spike. Release events from Syb2 WA expressing cells had nearly twice the quantal size (0.30 ± 0.02 pC) than those of Syb2 WT expressing cells (0.17 ± 0.02 pC). Larger quantal size detected in Syb2 WA mutants may be due to either larger transmitter amount stored in each vesicle [23] or due to a larger extent of granule emptying [24].
Figure 2.
Syb2 WA mutant increases released vesicle quantal size but not the total vesicle transmitter content. (A) An amperometric spike example along with the five parameters: quantal size Q (pC), half-width (ms), peak amplitude (pA), foot duration (ms), and foot amplitude (pA). (B) Average quantal size of fusion events from cells overexpressing Syb2 WT (n=17) and Syb2 WA (n=12) in bath solution containing 5 mM or 100 mM KCl. (C-E) Total transmitter content of individual vesicles detected by carbon fiber pierce experiment. (C) Original amperometry trace of carbon fiber pierce experiment; (D) individual event on expanded scale (marked & in Fig. 2C); (E) mean quantal size of total transmitter stored per vesicle; (F) mean number of detected spikes/cell from dko (N=10), cells overexpressing Syb2 WT (N=10), and WA (N=10). (G) Recording from cell-attached capacitance measurement, real part (Re, gray line) and imaginary part (Im, black line). (H) Mean vesicle size determined form cell-attached capacitance steps from dko overexpressing syb2 WT (n=6) or syb2 WA mutants (n=6). * p<0.05, # p>0.05 (student’s t test).
To determine the total transmitter amount stored per vesicle, individual cells were pierced by the carbon fiber electrode. A large slow amperometric current wave (Fig. 2C) indicates release of oxidizable cytosolic transmitter from the pierced cell as previously shown by patch amperometry [25]. Superimposed on this slow wave are amperometric spikes (Fig. 2C,D) that are generated by intact chromaffin granules diffusing out of the cell, which burst as they encounter the surface of the CFE [25]. The total transmitter content recorded from such bursting vesicles (Fig. 2E) was indistinguishable between cells expressing the Syb2 WA mutant (0.35 ± 0.04 pC), cells expressing Syb2 WT (0.33 ± 0.04 pC) and dko cells devoid of both Syb2 and Cellubrevin (0.33 ± 0.06 pC).
The number of spikes (Fig. 2F) from cells expressing Syb2 WA mutant (21.6 ± 3.7 spikes/cell) was unchanged compared to Syb2 WT (24.2 ± 4.3 spikes/cell) and dko cells (22.7 ± 5.1 spikes/cell), consistent with unchanged number of granules in cells from dko and wild-type mouse embryos as determined by electron microscopy [19].
To determine whether syb2 WA changes vesicle size, cell-attached capacitance measurements were performed (Fig. 2G). Upward capacitance steps in the imaginary part (Im) indicate individual exocytotic events. Narrow, low conductance fusion pores lead to transient increases in the real part (Re) [22], which are evident for the larger events. Vesicle size of syb2 WA cells (0.60 ± 0.12 fF, diameter 148 ± 13 nm) is similar to that of syb2 WT cells (0.58 ± 0.11 fF; diameter 145 ± 12 nm), indicating that syb2 WA did not change vesicle size. The vesicle size detected by capacitance measurements is comparable to that observed by electron microscopy [19].
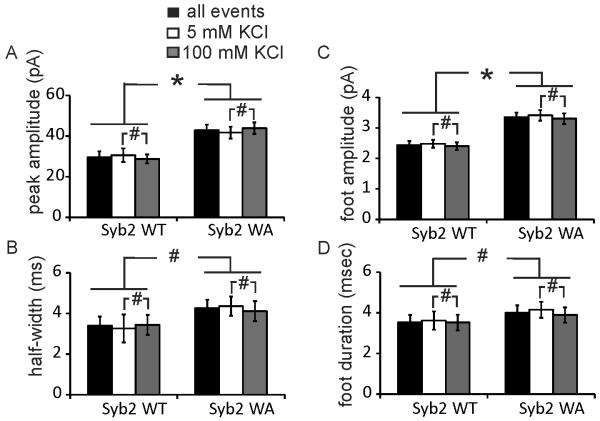
The amperometric spike peak amplitude (Fig. 3A) of Syb2 WA mutant cells (42.9 ± 2.8 pA) was nearly 50% larger than that of Syb2 WT cells (29.6 ± 2.9 pA). However, the amperometric spike half-width (Fig. 3B) of Syb2 WA expressing cells (4.26 ± 0.42 ms) was not significantly different from that of Syb2 WT expressing cells (3.40 ± 0.45 ms).
Figure 3.
Influence of Syb2 WA mutation on amperometric spike and amperometric foot kinetics quantified by mean peak amplitude (A), half-width (B), foot amplitude (C) and foot duration (D) for cells overexpressing Syb2 WT (n=17) or Syb2 WA (n=12) in bath solution containing 5 mM or 100 mM KCl. * p<0.05, # p>0.05 (student’s t test).
Amperometric spikes from chromaffin cells are frequently preceded by a foot signal that reflects transmitter leakage during the early narrow fusion pore stage [16,17]. The amperometric foot current amplitude indicates the flux of molecules through the early narrow fusion pore, and the duration of the foot signal represents the time interval from the initial fusion pore opening to the expanded fusion pore [3,18]. The mean foot amplitude of cells overexpressing Syb2 WA (3.36 ± 0.15 pA) was ~40 % larger than that of cells overexpressing Syb2 WT (2.43 ± 0.14 pA) (Fig. 3C). The amperometric foot signal duration (Fig. 3D) was not significantly different between the Syb2 WT (3.53 ± 0.36 ms) and Syb2 WA (4.01 ± 0.36 ms) cells.
To determine whether the quantal size and transmitter release kinetics differ between spontaneous and stimulated release events, the quantal size, spike peak amplitude and foot amplitude before and after stimulation were compared. As shown in Fig. 2B, 3A and 3C, the quantal size, spike peak amplitude and foot current amplitude of spontaneous release events were similar to those of stimulated events for both, Syb2 WT and Syb2 WA cells.
Discussion
Frequency of exocytotic events
Mutating W89 and W90 of Syb2 to alanine led to a nearly 50% decrease in the number of stimulated exocytotic spikes, which is consistent with a previous report on PC12 cells showing that the W89/W90 mutation strongly reduces Ca2+ dependent release [5]. We show here that the number of spontaneous exocytotic spikes from cells overexpressing Syb2 WA mutants were 2.3 fold increased and the number of spontaneous exocytotic spikes (15.6 ± 2.7 events/cell) was close to that of the stimulated spikes (19.7 ± 3.7 events/cell). The total number of vesicles present in the cell is unchanged in syb2 WT, syb2 WA and dko cells. This data suggests that transmitter release in chromaffin cells expressing the Syb2 WA mutant becomes largely independent of the stimulus, which increases the intracellular Ca2+ concentration. The results are consistent with those obtained in experiments using cultured neurons, which showed increased spontaneous and decreased stimulated release [8].
Properties of single vesicle release events
Single release events from Syb2 WA expressing chromaffin cells showed larger quantal size although vesicle size and total transmitter stored per vesicle was unchanged. Syb2 WA expression therefore does not change vesicle loading but the extent of release. The larger quantal size of exocytotic release events from Syb2 WA mutant expressing cells is consistent with complete release of the vesicular contents. In contrast, vesicles in Syb2 WT expressing cells release only approximately half of their transmitter content. This result is consistent with a previous report showing that large dense core vesicles in PC12 discharge only ~40 % of their total loaded transmitter [26]. Furthermore, the fact that quantal size measured in patch amperometry recordings (where full fusion is evident from capacitance measurements) is much larger than quantal size of conventional amperometry detection [27,28], suggests that full fusion is enhanced in patch amperometry due to tension in the membrane patch and may not be the generally preferred mode of fusion. Quantal size has also been reported to depend on the intensity of stimulation [24]. These reports suggested that fast release kinetics may lead to full release of vesicle transmitters.
In Syb2 WA expressing cells the larger quantal size was associated with larger amperometric spike peak amplitude, indicating that the WA mutant accelerates the rate of transmitter release from single granules, presumably due to a larger expansion of the fusion pore. If fusion pore opening is transient, a faster transmitter release rate may lead to more complete emptying of the granule if the duration of the fusion pore opening is limited and is unchanged between the mutant and wild type protein.
The mean amperometric foot amplitude represents the flux of transmitter released through the initial narrow fusion pore. Given the unchanged vesicular transmitter concentration, the change in flux of transmitter indicates a change in fusion pore structure [3]. The larger foot amplitude in Syb2 WA expressing cells suggests a wider or shorter initial fusion pore formed by the Syb2 WA mutant protein.
In hippocampal synapses, cells expressing the syb2 WA mutant exhibited similar mEPSC and mIPSC amplitudes as syb2 WT cells and knock-out cells [8]. This result supports the view that, in contrast to release from chromaffin granules, synaptic vesicle release mediated by wild type Syb2 is complete. This discrepancy may due to the higher curvature of synaptic vesicles that may promote full fusion [29]. Release through the fusion pore is an electrodiffusion process [18] and the time required for full release decreases with vesicle volume. It is thus also possible that for synaptic vesicles the time of transient fusion pore opening is usually sufficient to ensure complete release.
Role of the Syb2 juxtamembrane tryptophans in exocytosis
In its post-fusion structure, the helical SNARE complex continuously extends throughout the linkers and TM domains [6], Since the TM domains of syntaxin and Syb2 are located in different membranes before fusion, the conformation of the SNARE complex must change during the membrane fusion process. This conformational change may provide a force that helps to overcome the energy barrier for membrane fusion. The generation of such a force by SNARE complex zippering changes the location and orientation of the TM domain of Syb2 in the membrane [10]. The tryptophans are located in the membrane-water interface [9] and act as anchors. The generation of force by the SNARE complex may pull them towards the extravesicular side [10] to interact with Syntaxin Y257 and form an aromatic layer, which will help to extend the helical of SNARE complex continuity throughout the linkers and TM domain. The energy for dislocation of the Syb2 TM domain is considerably reduced in the WA mutant [10], which may explain the increased rate of spontaneous release events and indicates that the tryptophans contribute to the clamping mechanism that makes release stimulation-dependent. A recent report [30] shows that vesicles containing Syb2 exhibit stimulus-evoked release while vesicles containing the Syb2 homolog Vti1a prefer spontaneous release. Vti1a lacks the juxtamembrane aromatic amino acids, further supporting the conclusion that the tryptophans W89 and W90 play a key role in the clamping function that makes transmitter release dependent on stimulation by Ca2+.
Highlights.
Tryptophan pair (W89/W90) of synaptobrevin acts as a fusion clamp in chromaffin cells
W89AW90A mutant increases spontaneous release frequency
W89AW90A mutant produces increased quantal size of single release events
Acknowledgement
We thank Joan Lenz for excellent technical assistance, Drs. Eugene Mosharov and David Sulzer for the Igor macro used for amperometric data analysis. This work has been supported by NIH grant R01GM085808.
abbreviations used
- Syb2
synaptobrevin 2
- Syb2 WA
synaptobrevin W89AW90A mutants
- Syb2 WT
wild-type synaptobrevin
- SNARE
SNAP (Soluble NSF Attachment Protein) REceptor
- dko
synaptobrevin 2 and cellubrevin knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–7. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ngatchou AN, Kisler K, Fang Q, Walter AM, Zhao Y, Bruns D, Sorensen JB, Lindau M. Role of the synaptobrevin C terminus in fusion pore formation. Proc. Natl. Acad. Sci. U S A. 2010;107:18463–8. doi: 10.1073/pnas.1006727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fang Q, Berberian K, Gong LW, Hafez I, Sorensen JB, Lindau M. The role of the C terminus of the SNARE protein SNAP-25 in fusion pore opening and a model for fusion pore mechanics. Proc. Natl. Acad. Sci. U S A. 2008;105:15388–92. doi: 10.1073/pnas.0805377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca2+-triggered exocytosis. Cell. 2007;131:351–63. doi: 10.1016/j.cell.2007.09.025. [DOI] [PubMed] [Google Scholar]
- [5].Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–9. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–8. doi: 10.1038/nature08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reim K, Mansour M, Varoqueaux F, McMahon HT, Südhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- [8].Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–21. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bowen M, Brunger AT. Conformation of the synaptobrevin transmembrane domain. Proc. Natl. Acad. Sci. U S A. 2006;103:8378–83. doi: 10.1073/pnas.0602644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lindau M, Hall BA, Chetwynd A, Beckstein O, Sansom MSP. Coarse-Grain Simulations Reveal Movement of the Synaptobrevin C-Terminus in Response to Piconewton Forces. Biophys. J. 2012;103:959–969. doi: 10.1016/j.bpj.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pfluegers Arch./Eur. J. Physiol. 2006;453:261–8. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- [12].Chow RH, Rüden L.v. Electrochemical Detection of Secretion from Single Cells. In: Sakmann B, Neher E, editors. Single Channel Recording. Plenum Press; New York: 1995. pp. 245–275. [Google Scholar]
- [13].Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pfluegers Arch./Eur. J. Physiol. 1988;411:137–46. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- [14].Takahashi N, Hatakeyama H, Okado H, Noguchi J, Ohno M, Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- [15].Wightman RM, et al. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chow RH, Rüden L.v., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- [17].Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–12. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- [18].Gong LW, de Toledo GA, Lindau M. Exocytotic catecholamine release is not associated with cation flux through channels in the vesicle membrane but Na+ influx through the fusion pore. Nat. Cell Biol. 2007;9:915–922. doi: 10.1038/ncb1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Borisovska M, et al. v-SNAREs control exocytosis of vesicles from priming to fusion. EMBO J. 2005;24:2114–26. doi: 10.1038/sj.emboj.7600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sorensen JB, et al. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc. Natl. Acad. Sci. U S A. 2002;99:1627–32. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat. Methods. 2005;2:651–8. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- [22].Debus K, Lindau M. Resolution of patch capacitance recordings and of fusion pore conductances in small vesicles. Biophys. J. 2000;78:2983–97. doi: 10.1016/S0006-3495(00)76837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Z, Zhang Z, Jackson MB. Synaptotagmin IV modulation of vesicle size and fusion pores in PC12 cells. Biophys. J. 2010;98:968–78. doi: 10.1016/j.bpj.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J. Neurosci. 2005;25:7324–32. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mosharov EV, Gong LW, Khanna B, Sulzer D, Lindau M. Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells. J. Neurosci. 2003;23:5835–5845. doi: 10.1523/JNEUROSCI.23-13-05835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Omiatek DM, Dong Y, Heien ML, Ewing AG. Only a Fraction of Quantal Content is Released During Exocytosis as Revealed by Electrochemical Cytometry of Secretory Vesicles. Acs Chemical Neuroscience. 2010;1:234–245. doi: 10.1021/cn900040e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Segovia M, et al. Push-and-pull regulation of the fusion pore by synaptotagmin-7. Proc. Natl. Acad. Sci. U S A. 2010;107:19032–7. doi: 10.1073/pnas.1014070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Montesinos MS, et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J. Neurosci. 2008;28:3350–8. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Z, Jackson MB. Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis. Biophys. J. 2010;98:2524–34. doi: 10.1016/j.bpj.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73:121–34. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]