Abstract
Background
Gene-environment interactions may contribute to bipolar disorder (BD) clinical course variability. We examined effects of brain-derived neurotrophic factor (BDNF) val66met genotype and early life stress (ELS) upon illness severity and chronicity in adult BD patients.
Methods
80 patients (43 BD I, 33 BD II, 4 BD not otherwise specified, mean±SD age 46.4±14.0 years, 63.7% female) receiving open evidence-based and measurement-based care in the Stanford Bipolar Disorders Clinic for at least 12 months underwent BDNF val66met genotyping and completed the Childhood Trauma Questionnaire. BDNF met allele carrier genotype and history of childhood sexual and physical abuse were evaluated in relation to mean prior-year Clinical Global Impressions-Bipolar Version-Overall Severity of Illness (MPY-CGI-BP-OS) score and clinical and demographic characteristics.
Results
BDNF met allele carriers (but not non-met allele carriers) with compared to without childhood sexual abuse had 21% higher MPY-CGI-BP-OS scores (3.5±0.7 versus 2.9±0.7, respectively, t=−2.4, df=28, p=0.025) and 35% earlier BD onset age (14.6±5.7 versus 22.8±7.9 years, respectively, t=3.0, df=27, p=0.006). Regression analysis, however, was non-significant for a BDNF-childhood sexual abuse interaction.
Limitations
small sample of predominantly female Caucasian insured outpatients taking complex medication regimens; only one gene polymorphism considered.
Conclusions
Between group comparisons suggested BDNF met allele carrier genotype might amplify negative effects of ELS upon BD illness severity/chronicity, although with regression analysis, there was not a significant gene-environment interaction. Further studies with larger samples are warranted to assess whether BDNF met allele carriers with ELS are at risk for more severe/chronic BD illness course.
Keywords: bipolar disorder, brain-derived neurotrophic factor, childhood abuse, early life stress, illness course, gene-environment interaction
Introduction
Bipolar disorder (BD) is a serious psychiatric illness affecting up to 4% of the population, characterized by recurrent debilitating episodes of depression and mood elevation (Merikangas et al., 2007). BD illness severity varies widely across affected individuals, ranging from relatively milder (fewer lifetime mood episodes with longer periods of interepisode affective and functional recovery) to more severe (more chronic course with more frequent severe episodes and at most brief partial interepisode improvement) (Suppes et al., 2000). Early identification of individuals prone to chronic severe illness trajectories may permit earlier more robust interventions (such as, perhaps, second-generation antipsychotics) for such individuals to minimize the longer-term neurobiological and functional sequelae of recurrent mood episodes (Post, 1992).
Unfortunately, to date, tools for predicting outcome in BD patients have been limited (Treuer et al., 2010), highlighting the need for further research to identify biomarkers and integrate them with clinical indicators to predict illness severity and chronicity. Both biological (e.g. genes) and environmental (e.g. early life stress, ELS) factors may contribute to bipolar illness severity and chronicity. For instance, familial clustering of aspects of bipolar illness course (e.g. age at onset, episode frequency, and suicidality) suggest a heritable component to more severe and chronic course (Craddock et al., 2009). Similarly, ELS (i.e. childhood physical and sexual abuse) has been associated with earlier BD onset age, higher cycling frequency, suicidality (Garno et al., 2005; Leverich et al., 2002), and greater prospective percent time ill (Leverich et al., 2002).
While both genes and environment thus appear to influence the course of BD, emerging evidence suggests that they act synergistically, with specific genetic factors rendering individuals more or less vulnerable to environmental stress (Caspi et al., 2010). To date, however, effects of gene-environment (G × E) interactions on BD outcomes have been relatively unexplored. Elucidating such interaction effects could facilitate early identification of individuals most at risk for severe, chronic bipolar illness course, and who are thus important candidates for earlier and more robust interventions to reduce episode recurrence and enhance functioning and quality of life.
A genetic factor of particular interest with respect to mood disorders is the functional polymorphism in the brain-derived neurotrophic factor (BDNF) gene, BDNF val66met, involving a valine-to-methionine substitution at the 66th codon. BDNF is a nerve growth factor important for neuronal survival (Ghosh et al., 1994), with evidence supporting its involvement in hippocampal long-term potentiation (Lu et al., 1999). In vitro expression of the BDNF met allele in hippocampal neurons results in impaired activity-dependent secretion of BDNF (Egan et al., 2003). In humans, BDNF met allele carrier genotype has been associated with hippocampal-dependent memory impairments (Egan et al., 2003; Hariri et al., 2003) and decreased hippocampal volumes (Bueller et al., 2006; Pezawas et al., 2004).
Despite its demonstrated associations with impaired neuronal development and functioning, BDNF met allele carrier genotype alone has not been clearly associated with increased risk of mood symptoms or syndromal mood disorders (Groves, 2007; Post, 2007); however, emerging data do support G × E interaction effects such that BDNF met allele carriers, when exposed to ELS, may experience greater affective disturbance. Specifically, a G × E interaction between BDNF met allele carrier genotype and ELS has been associated with violent suicide attempts (Perroud et al., 2008; Pregelj et al., 2011) and predisposes individuals in non-clinical samples to depressive symptomatology (Aguilera et al., 2009; Gatt et al., 2009; Juhasz et al., 2011; Wichers et al., 2008) and biomarkers associated with mood disorders, such as increased salivary cortisol and abnormal hippocampal, amygdala, and prefrontal cortex volumes (Casey et al., 2009; Gatt et al., 2009; Gerritsen et al., 2012).
We therefore conducted a pilot study examining whether the impact of childhood sexual or physical abuse on bipolar illness course is moderated by BDNF val66met genotype, such that BDNF met allele carriers compared to non-met allele carriers are more vulnerable to the negative affective sequelae of ELS.
Methods
Patients
This study was approved by the Stanford University Administrative Panel on Human Subjects, and patients provided verbal and written informed consent prior to participation. 80 outpatients 18 years of age and older, meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000) criteria for bipolar I disorder (BD I), bipolar II disorder (BD II), or bipolar disorder not otherwise specified (BD NOS), who had been followed in the clinic for at least 12 months were recruited from the Stanford Bipolar Disorders Clinic. Patients were excluded if they had a current primary Axis I disorder other than BD, or met DSM-IV-TR criteria for current manic or mixed episodes, or had current psychotic symptoms (delusions or hallucinations), as such clinical states could impair ability to complete study assessments.
Diagnostic and clinical assessments
Bipolar Disorders Clinic patients were initially assessed with the Systematic Treatment Enhancement for Bipolar Disorders (STEP-BD) Affective Disorders Evaluation (ADE) (Sachs et al., 2003) and monitored longitudinally for at least 12 months with the STEP-BD Clinical Monitoring Form (CMF) (Sachs et al., 2002). The ADE and CMF contain modified versions of the Structured Clinical Interview for DSM-IV Diagnosis (SCID) (First et al., 1997) mood disorders module. BD diagnoses ascertained by a Bipolar Disorders Clinic psychiatrist-administered ADE were confirmed by a Bipolar Disorders Clinic research coordinator-administered Mini-International Neuropsychiatric Interview-Plus (MINI-PLUS) (Sheehan et al., 1998). Administration of the ADE also entailed assessment of demographic information and clinical characteristics such as lifetime psychiatric comorbidities and history of psychosis, psychiatric hospitalization, or rapid cycling. For each clinic visit, a Clinical Global Impressions Bipolar Version-Overall Severity of Illness (CGI-BP-OS) (Spearing et al., 1997) score was determined based on the number and severity of depressive and/or mood elevation symptoms, in a standardized fashion. The CGI-BP-OS is an integrative measure that accounts for not only syndromal but also subsyndromal mood elevation and depressive symptoms, and ranges from 1 (normal, not at all ill) to 7 (extremely ill). CGI-BP-OS scores from the 12 months preceding study enrollment were averaged to yield the mean-prior-year (MPY-CGI-BP-OS) score, the primary metric of bipolar illness severity and chronicity utilized in the study.
DNA extraction and BDNF val66met genotyping
DNA was obtained from peripheral venous blood or saliva. All genotyping assays were conducted within the laboratory of Dr. Joachim Hallmayer at Stanford by the same technician blinded to CTQ, MPY-CGI-BP-OS, and all other clinical data. BDNF val66met genotyping was conducted as per the laboratory’s standard protocol, utilizing the following primers for G196A in the BDNF gene: forward 5′-ATC CGA GGA CAA GGT GGC-3′ and reverse 5′-CCT CAT GGA CAT GTT TGC AG-3′. This generated 300bp of polymerized chain reaction (PCR) products, subsequently digested by Pml I (New England Biolabs, Ipswich, MA) to yield either allele A (met; undigested, 300bp) or allele G (val; digested to 180bp + 120bp bands), which were visualized on 7% polyacrylamide gel using a 50bp marker. Patients were classified as either BDNF met allele carriers (val/met or met/met genotype, U.S. population combined prevalence approximately 27.1%) or BDNF non-met allele carriers (val/val genotype, U.S. population prevalence approximately 68.4%), as the prevalence of BDNF met/met homozygotes in our study was expected to be low (U.S. population prevalence approximately 4.5%) (Shimizu et al., 2004).
Early life stress assessment
At the time of study enrollment, patients completed the Childhood Trauma Questionnaire (CTQ), a validated self-report assessment of childhood trauma exposure (Bernstein et al., 2003). The CTQ contains 28 items and is divided into five subscales: physical abuse, emotional abuse, physical neglect, emotional neglect, and sexual abuse. CTQ subscales are scored continuously as well as categorically (“none”, “low”, “moderate”, or “severe” levels of abuse), with categories based on cutoff scores defined by the CTQ manual. The two metrics of ELS used in this study were presence or absence of childhood sexual abuse (CTQ-sexual) and physical abuse (CTQ-physical), based on established data linking these to adverse outcomes in adult BD patients (Garno et al., 2005; Leverich et al., 2002). CTQ-sexual/CTQ-physical presence was indicated by subscale scores consistent with at least “low” abuse levels.
Statistical analyses
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) Version 19, Release 19.0.0 (IBM Corporation, Somers, NY) software on an Apple MacBook Pro Computer (Apple Corporation, Cupertino, CA). Descriptive statistics were compiled for baseline clinical and demographic characteristics, CTQ-sexual, CTQ-physical, and MPY-CGI-BP-OS. Analytic statistics included unpaired t-tests for between-group comparisons of continuous variables, and Chi-Square tests or Fisher’s exact tests as indicated for between-group comparisons of categorical variables. Corresponding non-parametric tests were used when indicated. For this pilot study, statistical analyses used two-tailed tests with significance level set at p < 0.05, not adjusted for multiple comparisons.
To assess for a G × E interaction, analyses began with between-group comparisons of MPY-CGI-BP-OS in BDNF met allele carriers versus non-met allele carriers, and in patients with versus without CTQ-sexual and CTQ-physical, using unpaired t-tests. Preliminary assessments of G × E included comparing MPY-CGI-BP-OS in patients with versus without CTQ-sexual and CTQ-physical using unpaired t-tests, stratified by BDNF met allele carrier status. If preliminary analysis yielded a significant result, a more rigorous assessment of G × E was undertaken using multiple regression analysis to model MPY-CGI-BP-OS score as a function of BDNF met allele carrier genotype, CTQ-sexual/CTQ-physical, and the interaction between BDNF met allele carrier genotype and CTQ-sexual/CTQ-physical, including age and gender as covariates.
To evaluate the potential impacts of mood state at enrollment (which could confound ELS reporting) and race/ethnicity (which could confound BDNF genotype effects) on the G × E interaction, we performed additional regression analyses controlling for each of these factors (first separately and then combined) in addition to age and gender. The results of these more complex regression models were compared to those of the simpler model (that covaried only for age and gender), assessing for changes in the goodness of fit of the model and in the overall pattern of findings with respect to the G × E interaction.
Exploratory analyses included between-group comparisons of clinical and demographic characteristics in BDNF met allele carriers compared to non-met allele carriers, in patients with compared to without CTQ-sexual/CTQ-physical, and in patients with compared to without CTQ-sexual/CTQ-physical stratified by BDNF met allele carrier genotype.
Results
Sample Description
80 patients (43 BD I, 33 BD II, 4 BD NOS, mean±SD age 46±14 years, 63.7% female) were assessed. Demographic and clinical characteristics are shown in Table 1. Patients were seen in the clinic on average every 58±38 days, were taking 3.7±1.9 prescription psychotropic medications, and had MPY-CGI-BP-OS score of 3.1±0.9. Although approximately half (51.2%) of enrolled patients had experienced a syndromal mood episode in the prior year, at enrollment only 25.0% of patients had current syndromal or subsyndromal mood symptoms, consistent with efforts to enroll patients when euthymic in order to reduce the likelihood of mood disturbance confounding recollection of stressful life events.
Table 1.
Sample Description: Overall Group
| Mean ± SD or % | |
|---|---|
| Number of patients | 80 |
|
| |
| Age (years) | 46.4 ± 14.0a |
|
| |
| Gender (% female) | 63.7 |
|
| |
| Race/ethnicity (%) | |
| Asian | 11.3 |
| Black | 1.3 |
| Hispanic | 5.0 |
| White | 80.0 |
| Other/unspecified | 2.5 |
|
| |
| Marital status (%) | |
| Single | 28.7 |
| Married | 55.0 |
| Divorced/separated | 16.3 |
|
| |
| Education* (%) | |
| High school or less | 5.1 |
| Some college | 30.8a, b |
| College degree | 32.1a |
| Graduate degree | 32.1b |
|
| |
| Employment (%) | |
| Full-time | 40.0 |
| Part-time | 7.5 |
| Unemployed/student/homemaker | 35.0 |
| Disabled | 12.5 |
| Retired | 5.0 |
|
| |
| Diagnosis (%) | |
| Bipolar I Disorder | 53.8 |
| Bipolar II Disorder | 41.3 |
| Bipolar Disorder NOS | 5.0 |
|
| |
| Lifetime Comorbidity* (%) | |
| Any psychiatric disorder | 65.8 |
| Any anxiety disorder | 50.6 |
| Post-traumatic stress disorder ** | 11.7 |
| Alcohol use disorder | 19.0 |
| Substance use disorder | 20.3 |
| Personality disorder | 3.8a, c |
| Eating disorder | 17.7 |
|
| |
| Clinical Characteristics | |
| Onset age (years) * | 19.8 ± 9.4a |
| Illness duration (years) * | 26.8 ± 15.0a, b |
| Psychosis (lifetime)* (%) | 46.8 |
| Psychiatric Hospitalization* (lifetime) (%) | 59.0 |
| Rapid cycling (lifetime)* (%) | 46.8 |
| Rapid cycling (prior year) (%) | 2.5 |
| Visit frequency (prior year) (days) | 58 ± 38 |
| Number of psychotropic medications (prior year) | 3.7 ± 1.9 |
| MPY-CGI-BP-OS score (prior year) | 3.1 ± 0.9 |
| Syndromal mood episode (prior year) (%) | 51.2 |
| Syndromal/subsyndromal mood symptoms (at enrollment) (%) | 25.0 |
Data missing for one patient.
Data missing for 3 patients.
p < 0.05 CTQ-sexual versus no CTQ-sexual;
p < 0.05 CTQ-physical versus no CTQ-physical;
p < 0.05 BDNF met allele carriers versus non-met allele carriers.
BDNF val66met genotype
37.5% of patients were BDNF met allele carriers (7.5% met/met, 30.0% val/met), and 62.5% were non-met allele carriers (val/val). These genotype frequencies were all within 6% of those reported for the U.S. population (Shimizu et al., 2004), and were in Hardy-Weinberg equilibrium (Chi-Square=1.56, df=1, p>0.05).
Early life stress
31.3% of patients reported a history of CTQ-sexual, while 28.8% reported a history of CTQ-physical, and 13.8% reported a history of both types of abuse. Rates of CTQ-sexual and CTQ-physical were statistically similar in BDNF met allele carriers compared to non-met allele carriers.
Relationships between BDNF met allele carrier genotype, early life stress, and bipolar illness severity and chronicity
BDNF met allele carriers (but not non-met allele carriers) with compared to without CTQ-sexual had 21% higher MPY-CGI-BP-OS scores (Figure 1), whereas BDNF genotype and CTQ-sexual status considered individually had no significant effect upon MPY-CGI-BP-OS. However, a multiple regression model of MPY-CGI-BP-OS as a function of BDNF met allele carrier genotype, CTQ-sexual, and BDNF met allele carrier genotype × CTQ-sexual, including age and gender as covariates, yielded a non-significant G × E interaction term (β=0.69, s.e.=0.42, p=0.110). Nevertheless, in this model the effect of current age on MPY-CGI-BP-OS score was significant (β=0.02, s.e.=0.01, p=0.029). Analogous between-group comparisons using CTQ-physical (rather than CTQ-sexual) considered individually and stratified by BDNF met allele carrier genotype yielded all non-significant results.
Figure 1. Childhood Sexual Abuse Associated with Greater Bipolar Illness Severity and Chronicity in BDNF Met Allele Carriers.
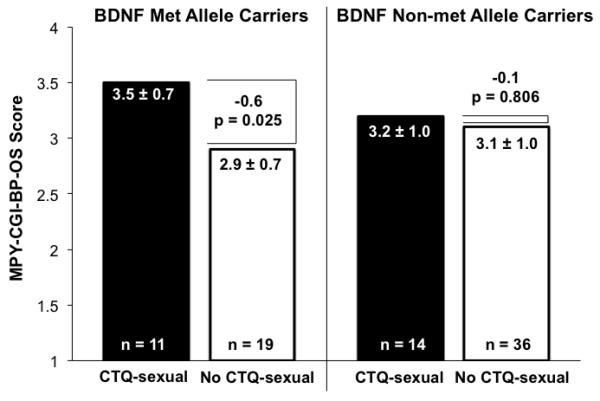
MPY-CGI-BP-OS indicates mean prior-year Clinical Global Impressions-Bipolar Version-Overall Severity of Illness, and results are shown as mean ± standard deviation. BDNF indicates brain-derived neurotrophic factor. CTQ-sexual indicates childhood sexual abuse history reported on Childhood Trauma Questionnaire.
Patients with (n=20) compared to without (n=60) syndromal/subsyndromal mood symptoms at study enrollment (at which time the CTQ was completed) reported statistically similar rates of CSA (40.0% in symptomatic compared to 28.3% in euthymic patients, p=0.406) and CPA (35.0% in symptomatic compared to 26.7% in euthymic patients, p=0.570), and had similar rates of BDNF met allele carrier genotype (40.0% in symptomatic compared to 36.7% in euthymic patients, p=0.796). Including mood state at enrollment (syndromal/subsyndromal mood symptoms=−0.5, euthymic=0.5) as a covariate in the regression analysis improved the fit of the overall model (F change=32.30, p<0.001). Mood state was a significant predictor of MPY-CGI-BP-OS (β=−1.08, s.e.=0.19, p<0.001), while age became non-significant (p = .087), and BDNF × CSA remained non-significant (p=.129).
Chi-square analysis demonstrated no statistically significant difference in proportion of met versus non-met allele carriers across racial/ethnic categories (p=0.113). Nevertheless, we included dummy variables for Asian versus non-Asian, black versus non-black, Hispanic versus non-Hispanic, and other/unspecified versus non-other/unspecified as covariates in the regression analysis (with Caucasian race serving as the reference category), which neither improved the fit of the model (F change=1.47, p=0.222) nor changed the overall pattern of findings: age remained a significant predictor of MPY-CGI-BP-OS (β=0.017, s.e.=0.007, p=0.021), and BDNF × CSA remained non-significant (p=0.124). Main effect of black versus non-black race (black=0.5, non-black=−0.5) was significant (β=1.79, s.e.=0.86, p=0.041), whereas effects of other racial/ethnic categories were non-significant in the regression model.
In a more comprehensive regression model evaluating MPY-CGI-BP-OS as a function of BDNF met allele carrier genotype, CSA, BDNF × CSA, age, gender, mood state at enrollment, and race/ethnicity, adding mood state at enrollment (F change=32.30, p<0.001) but not race/ethnicity (F change=0.76, p=0.556) improved the model fit. Mood state at enrollment was the only significant predictor of MPY-CGI-BP-OS (p<0.001), while all other terms were non-significant.
Relationships between BDNF met allele carrier genotype, early life stress, and clinical and demographic characteristics
BDNF met allele carriers (but not BDNF non-met allele carriers) with compared to without CTQ-sexual had 35% earlier bipolar onset age (Figure 2), and were more likely to have a personality disorder (27.3% versus 0.0%, Chi-Square=5.5, df=1, p=0.045), whereas analogous between-group comparisons using CTQ-physical (rather than CTQ-sexual) yielded non-significant results. Conversely, BDNF non-met allele carriers (but not BDNF met allele carriers) with compared to without CTQ-sexual had less education (64.3% versus 14.3% had only some college, Chi-square=12.3, df=1, Fisher’s exact p=0.001) and older current age (51.1±13.6 versus 42.6±11.2 years, t=−2.3, df=48, p=0.028), and with compared to without CTQ-physical had longer illness duration (30.9±16.0 versus 22.2±10.8 years, t=−2.3, df=48, p=0.027), higher rate of prior psychiatric hospitalization (88.2% versus 45.5%, Chi-square=8.6, df=1, Fisher’s exact p=0.005), and less education (52.9% versus 15.6% had only some college, Chi-square=7.6, df=1, Fisher’s exact p=0.009).
Figure 2. Childhood Sexual Abuse Associated with Earlier Bipolar Onset Age in BDNF Met Allele Carriers.
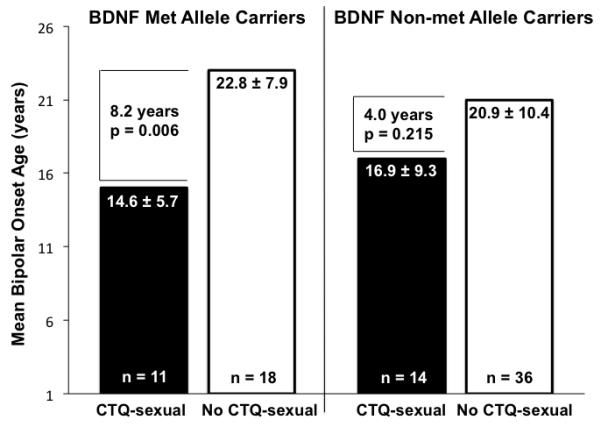
Onset age is shown as mean ± standard deviation. BDNF indicates brain-derived neurotrophic factor. CTQ-sexual indicates childhood sexual abuse history reported on Childhood Trauma Questionnaire.
CTQ-sexual more than CTQ-physical and BDNF genotype status, when considered individually, was associated with clinical characteristics and demographic features potentially related to poorer outcomes. Thus, patients with compared to without CTQ-sexual had a 30% earlier bipolar onset age (15.9±7.8 versus 21.6±9.6 years, t=2.6, df=77, p=0.01), longer bipolar illness duration (35.9±14.7 versus 22.5±13.3 years, t=−4.0, df=77, p<0.001), higher rate of personality disorder (12.0% versus 0.0%, Chi-square=6.7, df=1, Fisher’s exact p=0.03), older current age (51.8±13.5 versus 43.9±13.6 years, t=−2.4, df=78, p=0.02), and less education (60.0% versus 17.0% had only some college, Chi-square=14.8, df=1, Fisher’s exact p=0.0002; and 12.0% versus 41.5% had a college degree, Chi-square=6.8, df=1, Fisher’s exact p=0.01), but were otherwise similar with respect to the clinical and demographic characteristic parameters in Table 1. Patients with compared to without CTQ-physical also had longer bipolar illness duration (32.5±16.0 versus 24.5±14.2 years, t=−2.2, df=77, p=0.03), and less education (54.5% versus 21.4% had only some college, Chi-square=8.1, df=1, Fisher’s exact p=0.007; and 13.6% versus 39.3% had a graduate degree, Chi-square=4.8, df=1, Fisher’s exact p=0.03). BDNF met allele carriers compared to non-met allele carriers had a higher rate of personality disorder (10.3% versus 0.0%, Chi-Square=5.4, df=1, p<0.05).
Rates of current treatment with mood stabilizers, second-generation antipsychotics, sedative-hypnotics, antidepressants, or other psychotropic medications (including pramipexole, modafinil, and anticonvulsants not approved by the United States Food and Drug Administration for management of BD) were statistically similar in BDNF met allele carriers compared to non-met allele carriers, and in patients with compared to without CTQ-sexual/CTQ-physical. Just over half (52.5%) of the patients were not employed (i.e. unemployed/student/homemaker, disabled, or retired), and this group compared to employed individuals had a significantly higher MPY-CGI-BP-OS (3.4±0.9 versus 2.9±0.9, t=−2.8, df=78, p=0.007), but statistically similar rates of ELS and BDNF met allele carrier genotype.
Discussion
We found that among adult outpatients with BD, BDNF met allele carriers (but not non-met allele carriers) with history of childhood sexual abuse had greater bipolar illness severity and chronicity and earlier BD onset age, supporting the possibility that BDNF met allele carrier genotype might amplify the negative effects of ELS upon BD illness severity. These results are consistent with emerging literature suggesting that BDNF met allele carrier genotype moderates the impact of ELS on mood symptomatology (Aguilera et al., 2009; Gatt et al., 2009; Juhasz et al., 2011; Wichers et al., 2008). While previous studies have examined effects of BDNF met allele carrier genotype and ELS in non-clinical populations, this study represents the first to our knowledge to extend such investigation to BD patients. Nevertheless, our regression analysis yielded a non-significant BDNF-ELS interaction term, thus indicating that our results, while suggestive, are not robustly indicative of a G × E interaction and should be interpreted with caution.
Studies of G × E interactions in psychiatry have expanded rapidly in recent years, in part inspired by the important work of Caspi and associates, who found that a serotonin transporter gene promoter region (5-HTTLPR) polymorphism moderated the effect of stressful life events on risk for developing major depressive disorder (Caspi et al., 2003). While Caspi and associates demonstrated significant G × E interaction effects with both ELS and more proximal (adult) stress (Caspi et al., 2003), subsequent data suggested that ELS was the more relevant parameter for a G × E interaction effect on mood disorder risk (Brown, 2012). Moreover, such putative G × E interactions could differentially influence risk of more severe compared to less severe mood disorder phenotypes. For example, Uher and associates found that the 5-HTTLPR polymorphism amplified the negative effect of ELS upon risk of developing chronic/recurrent (rather than single-episode) unipolar major depressive disorder (Uher et al., 2011). Thus, we examined BDNF met allele carrier genotype and ELS (rather than proximal stress) as potential risk factors not for mood disorder onset, but rather for greater illness severity and chronicity in individuals already affected.
In our study, BDNF met allele carrier genotype amplified the negative effect of childhood sexual (but not physical) abuse upon BD illness severity and chronicity, consistent with evidence suggesting that childhood sexual abuse may have particularly profound effects on adult mood disturbance (Chen et al., 2010). Indeed, in adult patients with BD, childhood sexual abuse compared to other types of early adversity has been associated with increased rates of comorbid posttraumatic stress disorder (Goldberg et al., 2005) and lifetime suicide attempts (Garno et al., 2005), as well as lower serum BDNF protein concentrations (Kauer-Sant’Anna et al., 2007), the latter being a potential marker for acute mood disturbance in BD (Cunha et al., 2006; Machado-Vieira et al., 2007).
We also found that patients with compared to without childhood sexual (but not physical) abuse had an earlier BD illness onset age, although prior reports have associated both childhood sexual and physical abuse (not considering genetics) with earlier BD illness onset age (Garno et al., 2005; Leverich et al., 2002). Moreover, the overall negative effect of childhood sexual abuse upon BD illness onset age appeared to be driven by patients with the BDNF met allele carrier genotype. Given extensive evidence associating earlier BD illness onset age with worse longitudinal outcomes (Leverich et al., 2007; Perlis et al., 2009; Post et al., 2010), our finding suggest a potentially more complex mechanism (earlier BD illness onset triggered by childhood sexual abuse in BDNF met allele carriers) for childhood sexual abuse yielding greater illness severity and chronicity in BD.
This study had several noteworthy limitations, which indicate that our observations ought to be considered preliminary. Our small sample size limited power and therefore the likelihood of detecting a G × E interaction effect, perhaps contributing to the lack of statistical significance in the regression analysis. The 80.0% Caucasian, relatively affluent, highly educated, privately insured, low prevalence of alcohol/substance use disorder, tertiary teaching hospital clinic sample limits the generalizability of our findings to other BD samples. Not employed compared to employed patients had poorer clinical outcome (worse MPY-CGI-BP-OS), although these groups had statistically similar rates of ELS and BDNF met allele carrier genotype, reducing the likelihood that employment status confounded the observed genetic and environmental effects. Mood state at the time of completing the CTQ could introduce recall bias with respect to history of childhood trauma and is thus another potential confounder of the G × E regression analysis results. However, patients with compared to without mood symptoms at time of study enrollment reported statistically similar rates of ELS (and of BDNF met allele carrier genotype), consistent with mood state at enrollment not confounding the observed effects of ELS and BDNF met allele carrier genotype upon bipolar illness severity. Notably, the BDNF × CSA interaction term was non-significant with and without covarying for mood state at enrollment. Race/ethnicity could affect BDNF met allele frequencies, and although met allele carrier genotype frequencies were statistically similar across racial/ethnic categories in our sample, and including race/ethnicity as a covariate in our analysis did not change the overall pattern of results, the small number of non-Caucasian patients in this study limits the generalizability of our findings to non-Caucasian populations. Furthermore, we assessed effects of only one single nucleotide polymorphism (BDNF val66met), whereas BD is most likely a polygenic disorder with multiple genetic factors of relatively small effect sizes contributing to its onset and illness course.
Nevertheless, further studies in larger samples are warranted to assess whether BDNF met allele carriers with ELS are at risk for more severe/chronic BD illness course, and whether early utilization of more robust interventions can mitigate such risk.
Footnotes
Financial Disclosures: Dr. Miller reported having received an honorarium from Pamlab, Inc. and grant/research support from the National Institute of Mental Health. Dr. Hallmayer reported no biomedical financial interests or potential conflicts of interest. Dr. Wang reported having received grant/research support from Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Eisai Inc., Elan Pharmaceuticals Inc., Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceutica Products LP, Novartis Pharmaceuticals Corporation, Repligen Corporation, Shire Pharmaceuticals Group plc, Solvay Pharmaceuticals Inc., and Wyeth Pharmaceuticals; consulting fees from Abbott Laboratories, Inc., Corcept Therapeutics, Pfizer, and Sanofi-Aventis; and lecture honoraria from Abbott Laboratories, Inc, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline, Pfizer, and Sanofi-Aventis. Ms. Hill reported no biomedical financial interests or potential conflicts of interest. Dr. Johnson reported having received royalties from Wiley Publishing. Dr. Ketter reported having received grant/research support from the Agency for Healthcare Research and Quality, Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Cephalon Inc., Eli Lilly and Company, GlaxoSmithKline, the National Institute of Mental Health, Pfizer Inc., Repligen Corporation, Sunovion Pharmaceuticals, and Wyeth Pharmaceuticals; consulting fees from Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals LP, Astellas Pharmaceuticals, Bristol-Myers Squibb Company, Cephalon Inc., Dainippon Sumitomo Pharmaceuticals, Eli Lilly and Company, Forest Labs, GlaxoSmithKline, Janssen Pharmaceutica Products, LP, Jazz Pharmaceuticals, Inc, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Organon International Inc., a part of Schering-Plough Corp., Sunovion Pharmaceuticals, Solvay Pharmaceuticals, Inc., Valeant Pharmaceuticals, Vanda Pharmaceuticals, Wyeth Pharmaceuticals, and XenoPort, Inc.; and CME lecture honoraria from Abbott Laboratories, Inc, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline, and Noven Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–32. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision (DSM-IV-TR) American Psychiatric Association; Washington: 2000. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Brown GW. The promoter of the serotonin transporter genotype, environment and depression: A hypothesis supported? Journal of Affective Disorders. 2012;137:1–3. doi: 10.1016/j.jad.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met alleleis associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59:812–5. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–20. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen LP, Murad MH, Paras ML, Colbenson KM, Sattler AL, Goranson EN, et al. Sexual abuse and lifetime diagnosis of psychiatric disorders: systematic review and meta-analysis. Mayo Clinic Proceedings. 2010;85:618–29. doi: 10.4065/mcp.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends in Genetics. 2009;25:99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Goncalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neuroscience Letters. 2006;398:215–9. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Garno JL, Goldberg JF, Ramirez PM, Ritzler BA. Impact of childhood abuse on the clinical course of bipolar disorder. British Journal of Psychiatry. 2005;186:121–125. doi: 10.1192/bjp.186.2.121. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14:681–95. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, et al. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Molecular Psychiatry. 2012;17:597–603. doi: 10.1038/mp.2011.51. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Garno JL. Development of posttraumatic stress disorder in adult bipolar patients with histories of severe childhood abuse. Journal of Psychiatric Research. 2005;39:595–601. doi: 10.1016/j.jpsychires.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression? Molecular Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biological Psychiatry. 2011;69:762–71. doi: 10.1016/j.biopsych.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M, Tramontina J, Andreazza AC, Cereser K, da Costa S, Santin A, et al. Traumatic life events in bipolar disorder: impact on BDNF levels and psychopathology. Bipolar Disorders. 2007;9(Suppl 1):128–35. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Leverich GS, McElroy SL, Suppes T, Keck PE, Jr., Denicoff KD, Nolen WA, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry. 2002;51:288–97. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Post RM, Keck PE, Jr., Altshuler LL, Frye MA, Kupka RW, et al. The poor prognosis of childhood-onset bipolar disorder. The Journal of Pediatrics. 2007;150:485–90. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. Journal of Neuroscience Research. 1999;58:76–87. [PubMed] [Google Scholar]
- Machado-Vieira R, Dietrich MO, Leke R, Cereser VH, Zanatto V, Kapczinski F, et al. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode. Biological Psychiatry. 2007;61:142–4. doi: 10.1016/j.biopsych.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–52. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Dennehy EB, Miklowitz DJ, DelBello MP, Ostacher M, Calabrese JR, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: Results from the STEP-BD study. Bipolar Disorders. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Courtet P, Vincze I, Jaussent I, Jollant F, Bellivier F, et al. Interaction between BDNF Val66Met and childhood trauma on adult’s violent suicide attempt. Genes, Brain, and Behavior. 2008;7:314–322. doi: 10.1111/j.1601-183X.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. Journal of Neuroscience. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. Journal of Psychiatric Research. 2007;41:979–90. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Kupka RW, Keck PE, Jr., McElroy SL, Altshuler LL, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. Journal of Clinical Psychiatry. 2010;71:864–72. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- Pregelj P, Nedic G, Paska AV, Zupanc T, Nikolac M, Balazic J, et al. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. Journal of Affective Disorders. 2011;128:287–290. doi: 10.1016/j.jad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Guille C, McMurrich SL. A clinical monitoring form for mood disorders. Bipolar Disorders. 2002;4:323–327. doi: 10.1034/j.1399-5618.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biological Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2004;126B:122–3. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Research. 1997;73:159–71. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Suppes T, Dennehy EB, Gibbons EW. The longitudinal course of bipolar disorder. Journal of Clinical Psychiatry. 2000;61(Suppl 9):23–30. [PubMed] [Google Scholar]
- Treuer T, Tohen M. Predicting the course and outcome of bipolar disorder: a review. European Psychiatry. 2010;25:328–33. doi: 10.1016/j.eurpsy.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Uher R, Caspi A, Houts R, Sugden K, Williams B, Poulton R, et al. Serotonin transporter gene moderates childhood maltreatment’s effects on persistent but not single-episode depression: replications and implications for resolving inconsistent results. Journal of Affective Disorders. 2011;135:56–65. doi: 10.1016/j.jad.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, et al. The BDNF Val(66)Met × 5-HTTLPR × child adversity interaction and depressive symptoms: An attempt at replication. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147B:120–3. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]


