Abstract
The cerebral metabolic rate of oxygen (CMRO2) of small animals can be reliably imaged using the in vivo 17O MR approach at high field. However, a separate measurement is required for imaging the cerebral blood flow (CBF) in the same animal. In this study, we demonstrate that the 17O NMR signal of metabolically produced H217O in the rat brain following an 17O2 inhalation can serve as a perfusion tracer and its decay rate can be used to determine the absolute values of CBF across a wide range of animal conditions. This finding suggests that the in vivo 17O MR approach is capable of imaging both CMRO2 and CBF simultaneously and non-invasively; and it provides new utilities for studying the cerebral oxygen metabolism and perfusion commonly associated with brain function and diseases.
Keywords: in vivo17O NMR, cerebral blood flow (CBF), perfusion tracer, cerebral metabolic rate of oxygen (CMRO2), brain
INTRODUCTION
As a highly aerobic organ, the brain relies on the oxygen and nutrients supplied continuously via blood circulation to sustain its normal activities. Assessing the regional cerebral oxygen metabolism and blood flow in vivo has become a focal point for understanding both normal and diseased brains. While imaging the cerebral metabolic rate of oxygen (CMRO2) is still difficult, various neuroimaging techniques have been developed to quantify the cerebral blood flow (CBF) for biomedical research and clinical applications (1,2). Among them, the magnetic resonance (MR) modality is regularly used for its superior ability to image soft brain tissues and its increasing availability in the clinical setting. Generally, the MR-based CBF imaging methods can be divided into two categories based on the type of tracers used in the measurement: Exogenous and Endogenous tracers. Obtaining a quantitative CBF image via exogenous tracers involves introducing a diffusible, inert molecule with isotope labeling (e.g., D2O and H217O etc.) or an intravascular contrast agent (e.g., Gadolinium) into the blood stream and then monitoring its washout kinetics in the brain with optimal MR detection (3-5). The endogenous tracers method, utilizes the water signal of arterial blood as an internal tracer (e.g., Arterial spin labeling technique) to derive the CBF information (see the review articles of (6,7) and cited references therein). The latter approach is completely non-invasive and suitable for human applications, but is less sensitive when it comes to determining CBF and its change compared to the exogenous tracer method.
During the last three decades, significant efforts have been devoted to developing the in vivo 17O MRS and imaging techniques for assessing CMRO2 and CBF in animals and humans based on direct 17O-MR detection of brain H217O signals (8-30). The in vivo 17O MR imaging of CBF requires an invasive procedure for introducing the 17O-labeled water (H217O) as an exogenous tracer into the blood stream, and the CBF value is quantified based on the H217O tracer washout rate from the brain (8-10,14). In recent years, it has been shown that the similar 17O MR imaging approach can be applied for mapping CMRO2 in animals (11-13) and humans (16, 28) at high/ultrahigh fields. In the CMRO2 measurement, the metabolic H217O produced in the brain tissue via mitochondrial oxygen metabolism during the brief 17O2 gas inhalation (see the review article of (10) and cited references therein) will eventually be washed out from the brain tissue through blood circulation after the cessation of the 17O2 inhalation and thus may potentially serve as a perfusion tracer. Naturally, this washout process is expected to reflect the level of CBF in the brain. However, previous studies have shown that the metabolic H217O has a much slower washout rate than that of exogenous H217O tracer in the same animal(12), i.e. the expected CBF rate (18), suggesting possible permeability restrictions in the mitochondrial membranes (12).
The goal of this work was to quantitatively study the decay rate of the metabolically produced H217O during the post-17O2-inhalation period (defined as k) in the rat brain across a wide range of animal conditions; and to examine (i) whether the decay rate k correlates with the CBF rates when brain perfusion conditions are altered; and (ii) if the absolute CBF value can be simultaneously determined from the H217O decay rate k in the CMRO2 measurement.
MATERIAL AND METHOD
The experimental method has been described in detail elsewhere (12,13). Briefly, a 9.4T/31cm horizontal animal magnet (Magnex Scientific, Abingdon, U.K.) interfaced to a Varian INOVA console (Palo Alto, CA) and a multinuclear surface-coil probe consisting of an 17O coil (~1cm × 2cm) and a larger 1H coil were used. Scout images were acquired with repetition time TR=8ms; echo time TE=4ms; field of view FOV=3cm×3cm and 128×128 matrix size. The spatial localization of 17O signal was achieved either through the spatially well-defined radiofrequency-field (B1) profile of the 17O surface coil or the 3D 17O MR spectroscopic image (MRSI) approach. The single-pulse acquisition sequence was used to collect in vivo 17O spectra of the brain with the acquisition parameters: TR=10ms, 50μs hard pulse and nominal 90° flip angle, spectral width SW=30kHz, and 1 second temporal resolution per spectrum. The 3D 17O MRSI using the Fourier series window technique (12,13,31) was acquired with following parameters: TR=12ms, SW=30kHz, total scans=928, phase encodes=9×9×5 and FOV=25×25×22 mm3, voxel size=75μl, and 11.5 seconds temporal resolution per 3D MRSI volume.
Male Sprague-Dawley rats (250-350g body weight, n=10) were used. All animal surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. These rats underwent different preparations to alter CBF levels; and were divided into sub-groups according the preparation procedures described below:
Group A (n=3): the four-blood vessel occlusion (4BVO) model (32) was used for achieving 12 minutes acute forebrain ischemia in the rats anesthetized with α-chloralose (25mg/kg/hour). In vivo 17O measurements were performed under pre-ischemia, reperfusion or post-ischemia conditions.
Group B (n=3): normocapnia (fraction of inspired CO2 (FiCO2) less than 1%) and hypercapnia (FiCO2=3-7%) were applied for each rat under the same anesthesia condition using either isoflurane (~2%) or α-chloralose (25 mg/kg/hour). In vivo 17O measurements were performed at normal and elevated FiCO2 levels.
Group C (n=1): three anesthesia conditions were applied on this rat using isoflurane (~2%), low-dose pentobarbital (30 mg/kg/hour) and high-dose pentobarbital (70 mg/kg/hour), respectively; and in vivo 17O measurements were performed under each anesthesia condition.
Group D (n=3): the animals were anesthetized with α-chloralose and studied under normothermic (37°C) and hypothermic (32°C) conditions, respectively.
Multiple CMRO2 measurements, each consisting of 2-3 minutes 17O2 gas inhalation, were carried out on each animal at desired stages of the experiment. Simultaneous CBF measurements were conducted on six rats (three in Groups A, two in Groups B and one in Groups C) where the relative CBF were continuously monitored using Laser Doppler Flow (LDF) technique and Dual-channel OxyLab LDF/OxyFlo instrument (Oxford Optronix, UK), and the LDF probe(s) was inserted into the rat cortex.
Concurrent CBF measurements were carried out on three rats in Group D where the absolute CBF values at both 37°C and 32°C were determined using the 17O MRSI approach and the conventional tracer technique that involves a bolus injection of H217O (~40% 17O enrichment; 0.05 ml) into one internal carotid artery (12,13). The signal change of H217O washout as a function of time, S(t), can be used to calculate CBF according to Eq. [1]
| [1] |
where C1 and C2 are constants (10), and λ is the brain-blood water partition coefficient (=0.9 ml/g) (33).
The 17O MR signals were quantified from the amplitudes of the H217O resonance peak (a 100-Hz line broadening was applied for SNR enhancement), and were converted to H217O concentrations using the natural abundance H217O signal as an internal reference (12,13). Regression of the metabolic H217O washout time course following the cessation of the 17O2 inhalation to an exponential decay function deduced the decay rate constant k (min-1) using Eq. [2]
| [2] |
The absolute CBF values of Group D rats were quantified from the H217O signal decay rate following bolus injection of the H217O tracer according to Eq. [1] (12,13). The relative CBF values of Group A-C rats were determined in each animal based on the continuously recorded real time LDF signals in Blood Perfusion Unit (BPU) after correcting for the residual signal in the absence of perfusion after KCl injection, which were then time-averaged for each perfusion condition. Subsequently, the relative CBF values were converted to absolute CBF values based on the LDF readings and the corresponding k values measured at control conditions (i.e., pre-ischemia (Group A), normocapnia (Group B) or 2% isoflurane anesthesia (Group C)) as well as the relationship between the absolute CBF and the decay rate k obtained from the rats in Group D.
The reliability and sensitivity of the in vivo 17O MRS at 9.4T for measuring the dynamic change of the rat brain H217O during or after 17O2 inhalation and for detecting the CBF alteration caused by hypercapnia and vasodilatation were also evaluated using the data from another rat in Group B.
RESULTS
Figure 1A illustrates a typical in vivo 17O spectrum of natural abundance H217O obtained from a representative rat brain at 9.4T with only 1 second of acquisition time. The excellent 17O-MR detection sensitivity at high fields is evident. Figure 1B shows stack-plotted H217O spectra of the rat brain before (natural abundance), during and after an inhalation of 17O2. The progression of the H217O signal was characterized by three distinct phases: a constant signal (Phase I), a signal increase during the 17O2 inhalation (gray bar in Fig. 1B; Phase II), where the slope of the metabolic H217O accumulation determines the CMRO2 value (26), and the signal decay during the post-inhalation period (Phase III), where the decay rate is used to determine the CBF value in the present study. Figure 1C demonstrates the brain H217O time courses of the same rat under normocapnia and hypercapnia (6% CO2) conditions, respectively. Interestingly, these two time courses during Phase II were almost identical, which led to similar CMRO2 values (i.e., CMRO2,Normocapnia=1.66 μmol/g/min and CMRO2,Hypercapnia=1.65 μmol/g/min). In contrast, the H217O decay rate constant (k) during Phase III was 0.32 min-1 under hypercapnia conditions compared to 0.23 min-1 under normocapnia conditions, suggesting a 39% CBF increase due to vasodilation induced by a high CO2 level. These results are consistent with the corresponding literature (34). They also suggest that the measurements of CMRO2 during Phase II and CBF during Phase III are independent for small animals as studied herein.
Figure 1.
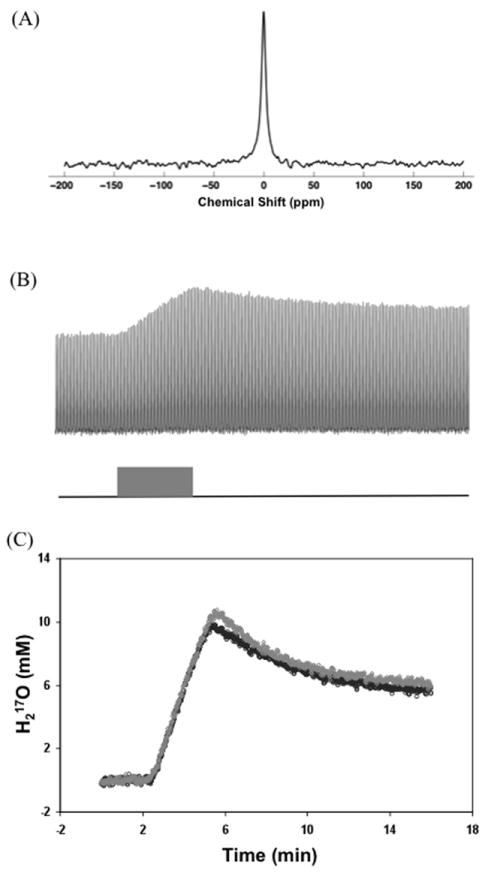
(A) A typical in vivo 17O spectrum of natural abundance H217O from a representative rat brain acquired within 1 second at 9.4T. (B) Stacked plot of H217O spectra acquired in the same rat brain before (natural abundance), during and after an inhalation of 17O2. One of every four spectra was plotted taken from a total of 960 spectra acquired with 1-second temporal resolution. The gray bar indicates the 17O2 inhalation duration. (C) Brain H217O time courses obtained from the same rat under normocapnia (black circle symbol) and hypercapnia (6% CO2, gray diamond symbol) conditions, respectively.
Figure 2 shows regional H217O time courses of a representative rat brain for imaging CMRO2 (Fig. 2A & 2B) and CBF (Fig. 2C & 2D) at a body temperature of 37°C (Fig. 2A & 2C) and 32°C (Fig. 2B & 2D), respectively. Similar to the previous observation (12), the metabolic H217O decay rates after the 17O2 inhalation at both temperatures (Fig. 2A & 2B) were much slower than the corresponding CBF values obtained directly using the H217O tracer method in the same brain (Fig. 2C & 2D). This finding suggests that the decay rate constant k does not directly represent the absolute CBF value. However, the trend of change in k in the same animal under different temperatures was consistent with that of CBF. Interestingly, both the rate of metabolic H217O production during the 17O2 inhalation (reflecting CMRO2) and the H217O decay rate were substantially reduced due to the lower enzyme activity at 32°C compared to 37°C, indicating a tightly coupled metabolism and perfusion relationship under varied brain temperature (12,13,20).
Figure 2.
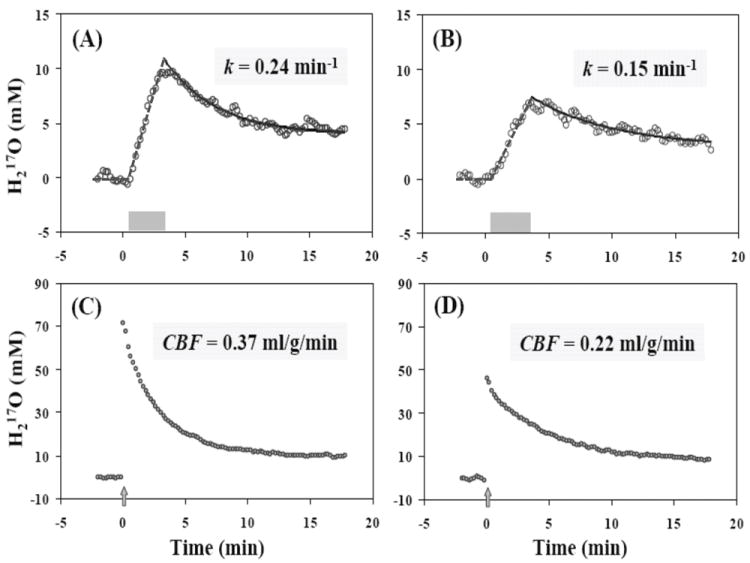
Regional H217O time courses of a representative rat brain obtained with in vivo 17O MR approaches for imaging CMRO2 (A-B) and CBF (C-D) at 37°C (A & C) and 32°C (B & D), respectively. The gray bars in (A) and (B) indicate the duration of a brief 17O2 gas inhalation, and the gray arrows in (C) and (D) indicate the bolus injection of H217O into an internal carotid artery.
The consistency in the trends of CBF and k was observed in all the animals studied. An example of the multiple CMRO2 and CBF (LDF) measurements conducted on a representative rat brain that underwent global forebrain ischemia (Group A) preparation is illustrated in Figure 3. The metabolic H217O decay rates obtained from the CMRO2 measurements during the baseline (i.e. pre-ischemia), reperfusion and post-ischemia periods (Fig. 3A) were closely correlated with the relative CBF levels determined with the LDF probe (Fig. 3B). Similar measurements were carried out for each animal with at least two conditions or perfusion levels. Figure 4 summarizes the relationships between the relative CBF levels measured with LDF probes and the metabolic H217O decay rates k.
Figure 3.
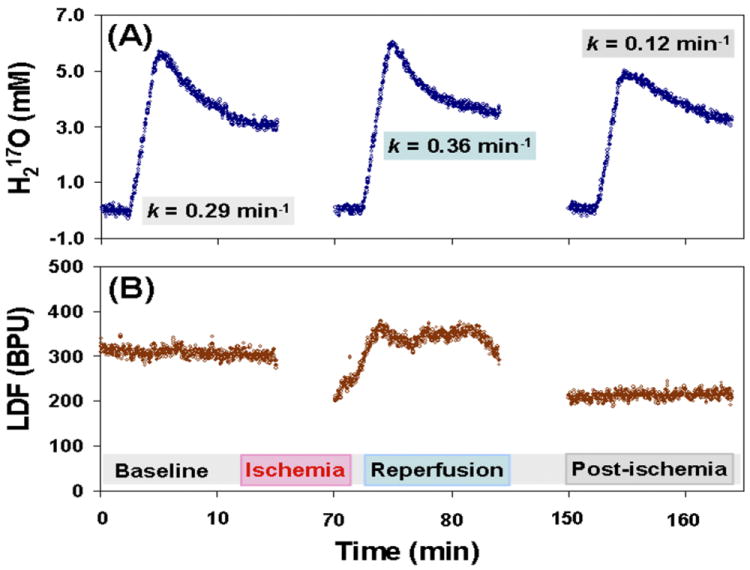
(A) Multiple CMRO2 and (B) CBF (LDF) measurements in a representative rat brain with global forebrain ischemia preparation. The H217O decay rate constants (k) during the baseline, reperfusion and post-ischemia periods were quantified in (A) and they correlate well with the relative CBF changes as shown in (B).
Figure 4.
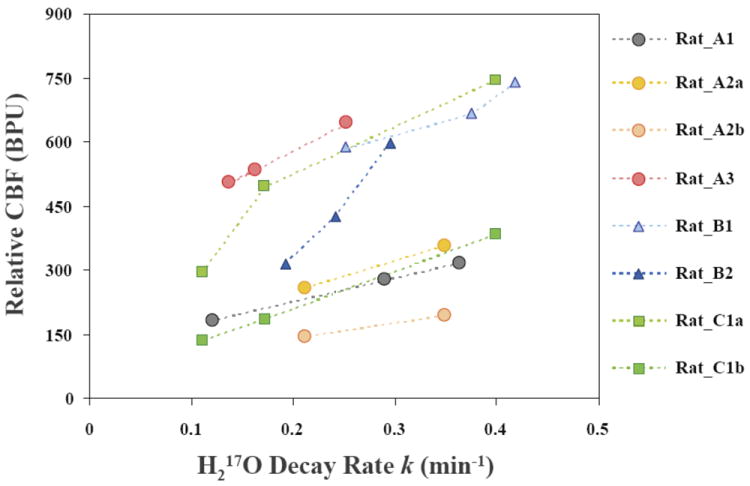
Summary of the relations between relative CBF levels measured with LDF probes and the metabolic H217O decay rate constants (k) following brief 17O2 inhalations in different rats under varied physiological conditions.
Since the H217O tracer technique can determine the absolute CBF values while the LDF measurements only provide relative CBF information, the absolute CBF and the decay rate k obtained from rats in Group D were used to calibrate the baseline CBF values, in the absolute perfusion unit, for rats in Groups A-C. The CBF values under other conditions (i.e., reperfusion or post-ischemia for Group A, hypercapnia for Group B or pentobarbital anesthesia for Group C) were determined based on the LDF measurements and the calibrated baseline CBF in each animal. Figure 5 summarizes all paired CBF and k data obtained from the animals in the experimental Groups A-D. The figure clearly shows a linear correlation between the absolute CBF values and the metabolic H217O decay rates following a brief 17O2 inhalation in the rat brain under various animal conditions.
Figure 5.
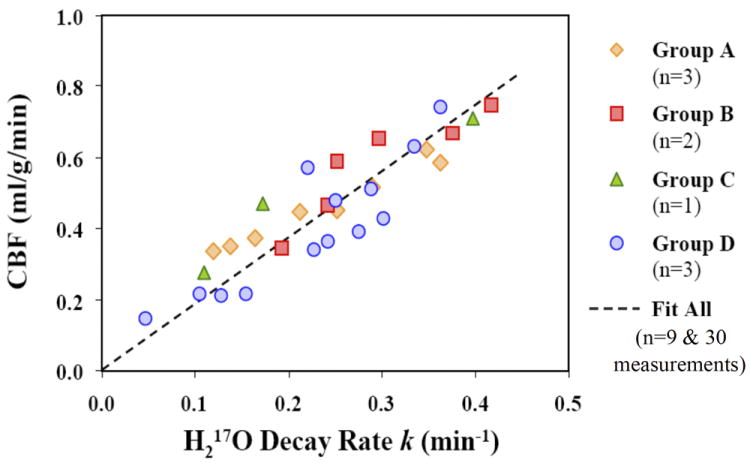
Summary of the correlation between CBF values and the metabolic H217O decay rate constants (k) following brief 17O2 inhalations in rat brains underwent different preparations and/or with varied physiological conditions (nine rats and 30 measurements). A linear correlation between CBF and k is observed: CBF = 1.86 k (R=0.88).
The linear regression of the experimental data shown in Fig. 5 led to Eq. [3]
| [3] |
with a correlation coefficient R=0.88 (9 animals and 30 measurements). This result suggests that the measured k should provide a good approximation for calculating the absolute CBF value with the unit of ml/g/min.
DISCUSSION AND CONCLUSION
The ability to image both CMRO2 and CBF is vital for biomedical research aiming to understand the cerebral metabolism, hemodynamics, functions and neuroenergetics of normal and diseased brains. There are various MR methods capable of assessing CBF in a living brain; however, very few are able to directly image CMRO2 in vivo. Recent success in development of the high/ultrahigh field 17O MR technique has shown that the 17O-MR based CMRO2 imaging approach can be readily applied to image CMRO2 and its change in small animal brains (11-13), and potentially in the human brain (16,28). This provides new opportunities for advanced brain research. However, to obtain both CMRO2 and CBF in the same animal or human, a combination of different MR or non-MR neuroimaging techniques are commonly utilized.
In this study, we confirmed our previous observation, i.e. the CBF value calculated from the decay rate of the metabolic H217O is much slower than that obtained from the H217O tracer technique. Different from an externally introduced H217O tracer, the metabolic H217O produced inside the mitochondrion has to cross two layers of mitochondrial membranes before entering the cytosol and extracellular spaces and, finally, the venous blood. Thus, its washout rate is expected to reflect the brain perfusion with a slower clearance process due to possible mitochondrial permeability limitations (12,13).
The result of the present study demonstrates that the metabolically generated H217O in a rat brain can indeed serve as a perfusion tracer, and that the absolute CBF can be determined from its decay rate according to Eq. [3]. The advantage of this method is that both CMRO2 and CBF can be simultaneously and non-invasively determined in a single measurement with only a few minutes of 17O2 gas inhalation.
A tight correlation between CBF and CMRO2 across a range of brain temperature (e.g. 32°C-37°C) has been previously examined and confirmed in rat brain (11-13). However, the correlation between CBF and CMRO2 observed earlier together with the relation of CBF vs. k observed herein do not necessarily mean that a correlation between k and CMRO2 can be established for different physiological conditions. For instance, hypercapnia leads to large increases in both CBF and decay rate k (see Fig. 1C) but a negligible change in CMRO2 (34). Therefore, we do not expect a linear correlation between CMRO2 and k (or CBF) across all the animal conditions studied herein.
The CBF value reflects the ratio between cerebral perfusion pressure (CPP) and cerebrovascular resistance (CVR), and it can be regulated by many factors. In this study, a number of physiological manipulations were applied to alter the CBF levels for understanding the relation between the decay rate constant k and CBF across a wide range of animal conditions. The investigated CBF alterations via various underlying perfusion regulation mechanisms included those induced by acute ischemia (Group A), by vasodilation due to the chemical effect of CO2 (Group B), by the effects of different anesthetic drugs (Group C) or by the metabolic regulation associated with brain temperature change (Group D). The results suggest a tight correlation between the decay rate k and the CBF across all these conditions.
The relationship between CBF and k may be extended to pathological conditions beyond acute brain ischemia if the brain mitochondrial membrane permeability is unchanged. This could potentially open new opportunities for many disease studies. One promising application is imaging the abnormal brain oxygen extraction fraction (OEF) in stroke patients, because the OEF value can be calculated from the ratio of CMRO2 to CBF (35,36).
One possible complication involved in CBF quantification is the loss of 17O-labeled water through the hydrolytic processes which incorporates 17O atoms into phosphate molecules (16,19,21,37). One dominant hydrolytic process in the brain is the ATP hydrolysis that serves to generate chemical energy and support neuronal activity (38). This process can convert some H217O molecules to 17O-labeled inorganic phosphate (Pi) compounds, potentially can lead to complications in quantifying CBF from the H217O signal decay rate during the post-inhalation period. Nevertheless, the H217O signal loss caused by the hydrolytic processes in the brain is considered to be insignificant. This is due to the fact that the inhaled 17O2 molecules are consumed inside the mitochondria for generating 17O-labeled water while, in contrast, the ATP hydrolysis reaction occurs in the cytosol and it can use both 17O-labled and non-labeled water molecules. Since the mitochondria volume is much smaller than the cytosol volume in a brain cell and the extra-cellular, non-labeled water can rapidly exchange with the intracellular water, thus, a rapid 17O label dilution occurs when the metabolically generated H217O molecules crossing the mitochondrial membranes and entering the cytosol space. Therefore, the 17O enrichment fraction of the water in the cytosol space can be approximated by the extremely low natural abundance enrichment. This isotope dilution effect significantly reduces the loss of 17O-labeled water due to the hydrolytic processes occurring in the cytosol; thus, its confounding effect is negligible in quantifying the CBF value.
The chemical shift of 17O-labeled Pi has been identified before (i.e., at 95.5 ppm) (39) and it was only detected from a global rat brain at 11.7T with a poor SNR after 6 hours of acquisition and 1.5 million signal averages. However, this 17O resonance peak was undetectable as demonstrated in Fig. 1A with the temporal resolution and signal averaging applied in the present study. This observation confirms that the process of converting H217O to 17O-labeled Pi in the rat brain is negligible unless a majority (or significant portion) of the tissue water in the animal is labeled with 17O.
To our knowledge, this work is the first demonstration of a practical MR technique that is capable of simultaneous and non-invasive imaging of both CMRO2 and CBF in a living brain. Compared to the in vivo 2H MRS study for assessing the brain oxygen and glucose consumption after an infusion of deuteriated glucose (23), the in vivo 17O-MR approach described here is simpler and more robust for imaging CBF. The metabolically produced deuterium water (HDO) may also serve as a perfusion tracer (similar to the H217O water). Its NMR signal, however, is more difficult to quantify due to the chemical shift overlapping with other adjacent deuteron resonances. In addition, the reported data in that study (23) indicates that determining the absolute CBF value from the brain HDO time course may be challenging since the decay of the HDO signal does not follow an exponential function, thus, a more sophisticated modeling is required for CBF quantification.
In conclusion, the findings from the present study reveal that the high/ultrahigh field 17O MR approach can provide a completely non-invasive and robust tool for simultaneously imaging CMRO2 and CBF and their respective changes in the animal brain. Furthermore, this 17O MR based CMRO2-CBF imaging method could be readily extended to obtain OEF, another important physiological variable reflecting the balance between brain oxygen metabolism and oxygen supply. The ability for simultaneous CMRO2, CBF and OEF imaging could provide invaluable utilities for studying the cerebral oxygen metabolism and tissue perfusion associated with brain function, physiology and diseases. Finally, the same 17O imaging approach should also be applicable for other organs, such as the heart.
Acknowledgments
Grant sponsor: National Institute of Health, Grant numbers: NS057560, NS041262, NS041262S1, NS070839, NS070839S1, P41 RR08079, P41 EB015894 and P30 NS057091; and the W.M. Keck Foundation.
References
- 1.Coles JP. Imaging of cerebral blood flow and metabolism. Curr Opin Anaesthesiol. 2006;19:473–480. doi: 10.1097/01.aco.0000245270.90377.00. [DOI] [PubMed] [Google Scholar]
- 2.Kety SS. The measurement of cerebral blood flow by means of inert diffusible tracers. Keio J Med. 1994;43:9–14. doi: 10.2302/kjm.43.9. [DOI] [PubMed] [Google Scholar]
- 3.Kim SG, Ackerman JJ. Quantification of regional blood flow by monitoring of exogenous tracer via nuclear magnetic resonance spectroscopy. Magn Reson Med. 1990;14:266–282. doi: 10.1002/mrm.1910140212. [DOI] [PubMed] [Google Scholar]
- 4.Larson KB, Perman WH, Perlmutter JS, Gado MH, Ollinger JM, Zierler K. Tracer-kinetic analysis for measuring regional cerebral blood flow by dynamic nuclear magnetic resonance imaging. J Theor Biol. 1994;170:1–14. doi: 10.1006/jtbi.1994.1164. [DOI] [PubMed] [Google Scholar]
- 5.Neil JJ. The validation of freely diffusible tracer methods with NMR detection for measurement of blood flow. Magn Reson Med. 1991;19:299–304. doi: 10.1002/mrm.1910190218. [DOI] [PubMed] [Google Scholar]
- 6.Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 7.Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- 8.Mateescu GD, Yvars GM, LaManna JC, Lust WD. Oxygen-17 MRS: In vivo evalution of water uptake and residence time in the mouse brain after injection of O-17 labelled water. Proc Inter Soc Magn Reson Med. 1990;9:1236. [Google Scholar]
- 9.Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- 10.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- 14.Arai T, Mori K, Nakao S, Watanabe K, Kito K, Aoki M, Mori H, Morikawa S, Inubushi T. In vivo oxygen-17 nuclear magnetic resonance for the estimation of cerebral blood flow and oxygen consumption. Biochem Biophys Res Commun. 1991;179:954–961. doi: 10.1016/0006-291x(91)91911-u. [DOI] [PubMed] [Google Scholar]
- 15.Arai T, Nakao S, Mori K, Ishimori K, Morishima I, Miyazawa T, Fritz-Zieroth B. Cerebral oxygen utilization analyzed by the use of oxygen-17 and its nuclear magnetic resonance. Biochem Biophys Res Commun. 1990;169:153–158. doi: 10.1016/0006-291x(90)91447-z. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51:723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 17.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging: Part 1. Theory and data analysis methods. Neurol Res. 1992;14:303–311. doi: 10.1080/01616412.1992.11740074. [DOI] [PubMed] [Google Scholar]
- 18.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging. Part 2. Determination of CMRO2 for the rat by 17O NMR, and CMRO2, rCBF and the partition coefficient for the cat by 17O MRI. Neurol Res. 1993;15:7–22. doi: 10.1080/01616412.1993.11740100. [DOI] [PubMed] [Google Scholar]
- 19.Mateescu GD. Functional oxygen-17 magnetic resonance imaging and localized spectroscopy. Adv Exp Med Biol. 2003;510:213–218. doi: 10.1007/978-1-4615-0205-0_35. [DOI] [PubMed] [Google Scholar]
- 20.Mateescu GD, Cabrera ME. In vivo 17O magnetic resonance spectroscopy. Determination of temperature effects on metabolic rates (Q10 factor) Adv Exp Med Biol. 1997;411:585–590. [PubMed] [Google Scholar]
- 21.Mateescu GD, Fercu D. Interleave 17O/ 31P MRS: Novel Approach for In Vivo Determination of Defects in Oxidative Phosphorylation. Proc Inter Soc Magn Reson Med. 1993;12:110. [Google Scholar]
- 22.Mateescu GD, LaManna JC, Lust WD, Mars LM, Tseng J. Oxygen-17 magnetic resonance: in vivo detection of nascent mitochondrial water in animals breathing 17O2 enriched air. Proc Soc Magn Reson Med. 1991;10:1031. [Google Scholar]
- 23.Mateescu GD, Ye A, Flask CA, Erokwu B, Duerk JL. In vivo assessment of oxygen consumption via Deuterium Magnetic Resonance. Adv Exp Med Biol. 2011;701:193–199. doi: 10.1007/978-1-4419-7756-4_26. [DOI] [PubMed] [Google Scholar]
- 24.Mateescu GD, Yvars GM, Dular T. Oxygen-17 Magnetic Resonance Imaging. Proc Inter Soc Magn Reson Med. 1987;6:929. [Google Scholar]
- 25.Mateescu GD, Yvars GM, Pazara DI, Alldridge NA, LaManna JC, Lust WD, Mattingly M, Kuhn W. Combined oxygen-17/proton magnetic resonance microscopy in plants, animals and materials: present status and potential. In: Bailie TA, Jones JR, editors. Synthesis and applications of isotopically labelled compounds. Amsterdam: Elsevier; 1989. pp. 499–508. [Google Scholar]
- 26.Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- 27.Zhu XH, Merkle H, Kwag JH, Ugurbil K, Chen W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn Reson Med. 2001;45:543–549. doi: 10.1002/mrm.1073. [DOI] [PubMed] [Google Scholar]
- 28.Zhu XH, Zhang X, Zhang N, Zhang Y, Strupp J, Ugurbil K, Chen W. High-field 17O Study of 3D CMRO2 Imaging in human visual cortex. Proc Intl Soc Mag Reson Med. 2006;14:409. [Google Scholar]
- 29.Fiat D, Ligeti L, Lyon RC, Ruttner Z, Pekar J, Moonen CT, McLaughlin AC. In vivo 17O NMR study of rat brain during 17O2 inhalation. Magn Reson Med. 1992;24:370–374. doi: 10.1002/mrm.1910240218. [DOI] [PubMed] [Google Scholar]
- 30.Pekar J, Sinnwell T, Ligeti L, Chesnick AS, Frank JA, McLaughlin AC. Simultaneous measurement of cerebral oxygen consumption and blood flow using 17O and 19F magnetic resonance imaging. J Cereb Blood Flow Metab. 1995;15:312–320. doi: 10.1038/jcbfm.1995.36. [DOI] [PubMed] [Google Scholar]
- 31.Garwood M, Schleich T, Bendall MR, Pegg DT. Improved Fourier series windows for localization in in vivo NMR spectroscopy. J Magn Reson. 1985;65:510. [Google Scholar]
- 32.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 33.Herscovitch A, Raichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 34.Siesjo BK. Brain energy metabolism. New York: Wiley; 1978. pp. 101–110. [Google Scholar]
- 35.Heiss WD. The ischemic penumbra: correlates in imaging and implications for treatment of ischemic stroke. The Johann Jacob Wepfer award 2011. Cerebrovasc Dis. 2011;32:307–320. doi: 10.1159/000330462. [DOI] [PubMed] [Google Scholar]
- 36.Delapaz R, Gupte P. Potential application of 17O MRI to human ischemic stroke. Adv Exp Med Biol. 2011;701:215–222. doi: 10.1007/978-1-4419-7756-4_29. [DOI] [PubMed] [Google Scholar]
- 37.LaNoue KF, Jeffries FM, Radda GK. Kinetic control of mitochondrial ATP synthesis. Biochemistry. 1986;25:7667–7675. doi: 10.1021/bi00371a058. [DOI] [PubMed] [Google Scholar]
- 38.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Graaf RA, Brown PB, Rothman DL, Behar KL. Natural abundance 17O NMR spectroscopy of rat brain in vivo. J Magn Reson. 2008;193:63–67. doi: 10.1016/j.jmr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


