Abstract
Immunity to sand fly saliva in rodents induces a TH1 delayed-type hypersensitivity (DTH) response conferring protection against leishmaniasis. The relevance of DTH to sand fly bites in humans living in a leishmaniasis-endemic area remains unknown. Here, we describe the duration and nature of DTH to sand fly saliva in humans from an endemic area of Mali. DTH was assessed at 24, 48, 72, and 96 hours post bite in volunteers exposed to colony-bred sand flies. Dermal biopsies were obtained 48 hours post bite; cytokines were quantified from peripheral blood mononuclear cells (PBMCs) stimulated with sand fly saliva in vitro. A DTH response to bites was observed in 75% of individuals aged 1–15 years, decreasing gradually to 48% by age 45, and dropping to 21% thereafter. Dermal biopsies were dominated by T lymphocytes and macrophages. Abundant expression of IFN-γ and absence of TH2 cytokines establishes the TH1 nature of this DTH response. PBMCs from 98% of individuals responded to sand fly saliva. Of these, 23% were polarized to a TH1 and 25% to a TH2 response. We demonstrate the durability and TH1 nature of DTH to sand fly bites in humans living in a cutaneous leishmaniasis-endemic area. A systemic TH2 response may explain why some individuals remain susceptible to disease.
Introduction
Leishmaniasis is a neglected vector-borne disease transmitted by the bite of phlebotomine sand flies. It is prevalent in 88 countries and comprises several diseases with predominantly cutaneous, mucocutaneous, or visceral manifestations (WHO, 2010). As natural transmission involves the deposition of Leishmania parasites into the bite wound, a better understanding of the immunologic environment at the bite site is needed. The inhabitants of leishmaniasis-endemic areas are bitten on a daily basis during the sand fly season. Considering that the proportion of Leishmania-infected sand flies in such areas is low (Janini et al., 1995; Anderson et al., 2011), it is generally accepted that most individuals will frequently receive bites from uninfected sand flies before encountering Leishmania parasites. Upon repeated exposures, insect bites typically induce one of the several hypersensitivity reactions. For sand flies, the bite site in experimentally exposed humans or animals has been mostly associated with development of a delayed-type hypersensitivity (DTH) response (Theodor, 1935; Belkaid et al., 2000; Vinhas et al., 2007; Oliveira et al., 2009b).
In animal models, a DTH response induced by previous exposure to sand fly saliva conferred substantial protection against cutaneous and visceral leishmaniases (Belkaid et al., 1998; Kamhawi et al., 2000; Oliveira et al., 2006, 2008; de Moura et al., 2007; Gomes et al., 2008; Collin et al., 2009; Drahota et al., 2009). The protection observed in exposed rodents has been closely associated with the development of a potent anti-sand fly saliva TH1-mediated DTH response at the bite site, adversely affecting the establishment of parasites (Valenzuela et al., 2001; Oliveira et al., 2006, 2008; Gomes et al., 2008). In general, a proportion of inhabitants of cutaneous leishmaniasis (CL)-endemic areas convert to a positive leishmanin skin test indicative of exposure to Leishmania without ever developing clinically apparent cutaneous lesions (Sassi et al., 1999; Follador et al., 2002). Although these individuals were bitten by infected sand flies and were likely infected by the parasite, the absence of disease in these individuals suggests that they mounted a rapid and protective immune response. This outcome could be mediated by a pre-established TH1 immune response to sand fly saliva that ultimately leads to Leishmania-specific immunity as was demonstrated in animal models (Kamhawi et al., 2000; Valenzuela et al., 2001; Oliveira et al., 2008; Rohousova et al., 2011).
DTH responses to insect bites are widely believed to be transient. This common perception has been established and reinforced by studies on mosquito bites (Mellanby, 1946) that initially induce a transient DTH response followed by an immediate-type hypersensitivity (ITH) reaction after repeated exposure to bites and later desensitization (Mellanby, 1946; Peng et al., 1996; Peng et al., 2004a, 2004b; Billingsley et al., 2006; Kulthanan et al., 2010). In the case of mosquitoes, desensitization of experimentally exposed humans occurs within 26 weeks to 5 years (Peng and Simons, 2004; Peng et al., 2004b). Owing to limited information regarding the DTH response to sand fly saliva in humans (Theodor, 1935; Belkaid et al., 2000; Vinhas et al., 2007), we investigated its prevalence, duration, and nature in a cohort of individuals from a CL-endemic area in Mali, West Africa, where Phlebotomus duboscqi is the incriminated sand fly vector of Leishmania major (Anderson et al., 2011).
Results
DTH and ITH at the bite site of P. duboscqi sand flies in individuals from a CL-endemic area of mali
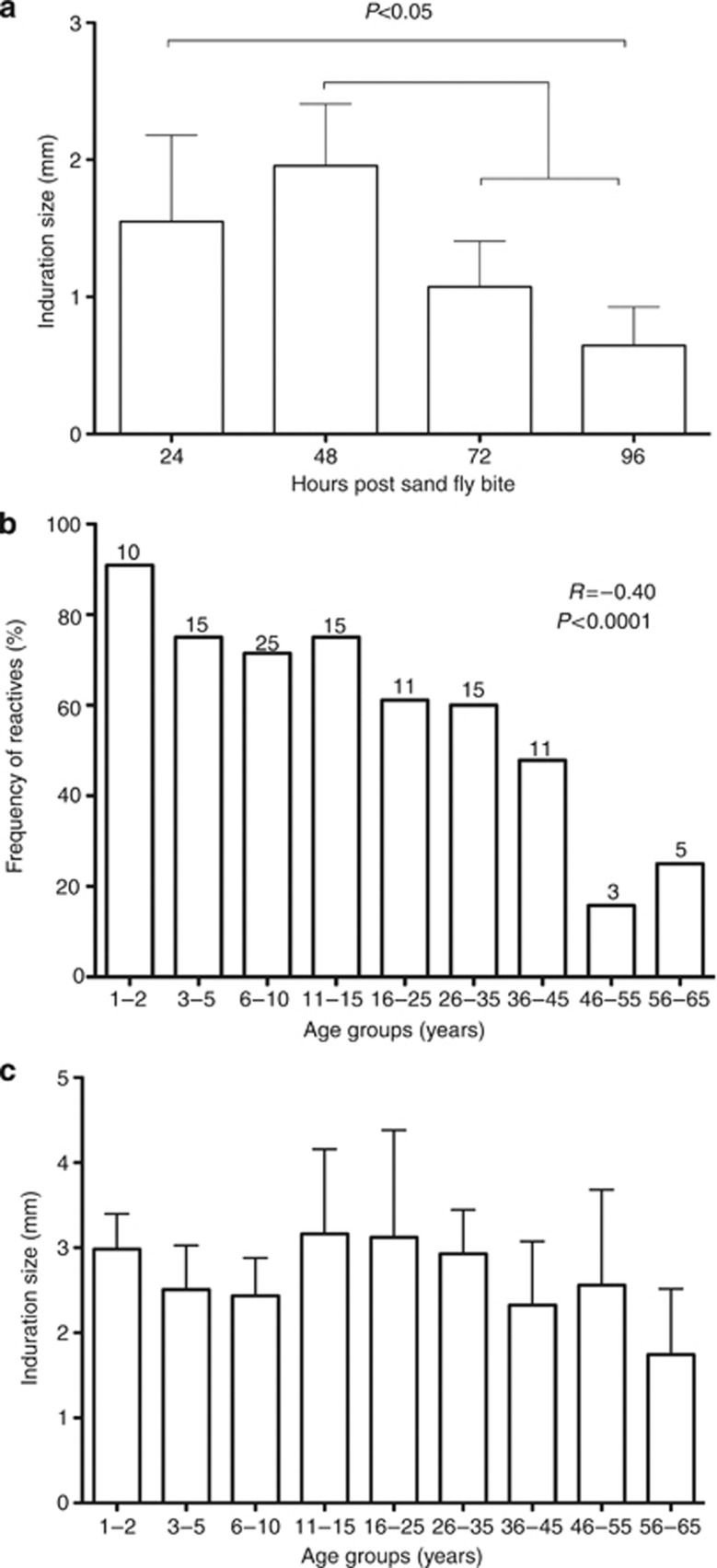
Twenty minutes after exposure, two of the three colony-bred sand flies had fed to completion in the majority of volunteers. Overall, 64% (122/191) individuals had a positive DTH response. In the majority (58%) of volunteers the induration size peaked at 48 hours and subsided by 96 hours, typical of a DTH response (Figure 1a). A gradual decrease in the frequency of DTH-positive reactions was observed with age (Figure 1b); however, this decrease was only substantially diminished in older age groups, where 48% of individuals 35–45 years old maintained a DTH reaction (Figure 1b). Importantly, 95% of 1–2-year olds were DTH-positive, indicating that children rapidly acquire a DTH response. The magnitude of the DTH response (induration size) was not influenced by age (Figure 1c). Gender had no effect on the frequency of DTH positives (data not shown).
Figure 1.
Delayed-type hypersensitivity (DTH) response to uninfected sand fly bites in volunteers from a cutaneous leishmaniasis-endemic area. Following exposure to three colony-bred sand flies, the diameter of the largest indurated nodule was measured in 191 volunteers (20±15 from each age group) at 24, 48, 72, and 96 hours post bite. (a) Kinetics of the DTH response indicated by the mean induration size from reactive individuals at different time points. Statistically significant differences were tested by analysis of variance (ANOVA) test, followed by Tukey's multiple comparison test (P<0.05). (b) Frequency of DTH+ individuals stratified by age at the 48 hours time point. Numbers atop each bar represent the number of reactive individuals per age group. A Spearman test indicated a negative correlation with a coefficient r=0.40. (c) Induration measurements of DTH+ individuals taken at the 48 hour time point. Bars in graphs represent the upper 95% confidence limit of the mean.
An ITH response was observed within minutes of sand fly exposure in 21% of volunteers. These responses were observed mainly in children (73%, 32%, and 8% in age groups 1–2, 3–15, and 16–65 years, respectively). Of these volunteers, 97.5% later developed a DTH response.
Characterization of the DTH response to sand fly bites
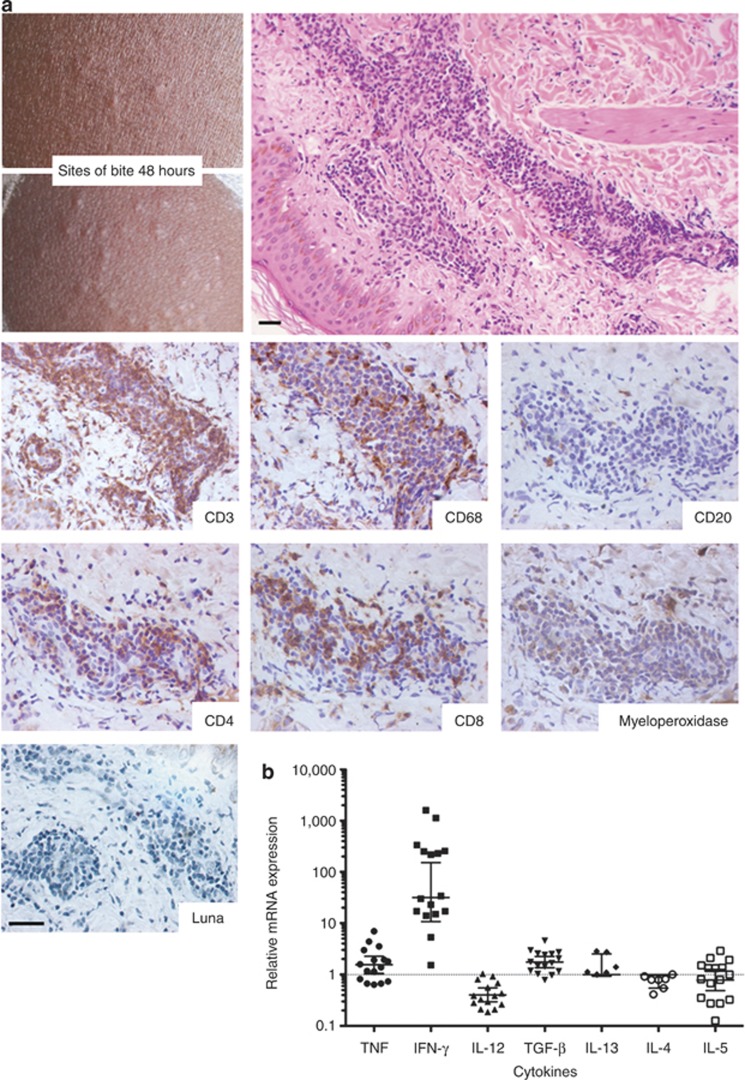
The DTH reaction manifested as indurated nodules at the bite sites (Figure 2a). These sites were dominated by T lymphocytes (CD3+CD20–) and to a lesser extent by macrophages (CD68+) (Figure 2a). Neutrophils—defined by myeloperoxidase (shown) and elastase (not shown) staining—and eosinophils (Luna stain) were mostly absent (Figure 2a). Despite strong staining for CD3 in all six biopsies, CD4+ and/or CD8+ lymphocytes were identified in only three of them. Compared with normal skin, DTH biopsies showed a 59-fold (95% confidence interval, 0.78, 4,497.0) mean increase in IFN-γ expression (Figure 2b). This finding contrasts with low TNF (tumor necrosis factor), TGF-β (tumor growth factor-beta), IL-13 and IL-5 expression and the absence of IL-12 and IL-4 expression (Figure 2b). Together, these results demonstrate the TH1 nature of the observed DTH response.
Figure 2.
Characterization of the delayed-type hypersensitivity (DTH) response to sand fly bites in human volunteers from a cutaneous leishmaniasis-endemic area. Biopsies of the DTH response were obtained 48 hours after sand fly bites. (a) Cellular infiltration representative of a DTH+ site shown by hematoxylin–eosin staining (200 × ) and by immunohistochemical staining of T lymphocytes (CD3, CD4, and CD8), B lymphocytes (CD20), macrophages (CD68), neutrophils (myeloperoxidase), and eosinophils (Luna stain) (640 × ). Bar=20 μm. (b) Cytokine mRNA expression in biopsies from 16 DTH+ sites measured by real-time PCR. Expression of the housekeeping gene GAPDH was used to normalize samples. Values represent fold-change over control biopsies (two biopsies from unchallenged skin). A two-sample t-test on the log-transformed response was used to test for statistical differences. Means and 95% confidence intervals are shown in graphs. TGF, tumor growth factor; TNF, tumor necrosis factor.
The systemic immune response to P. duboscqi salivary proteins
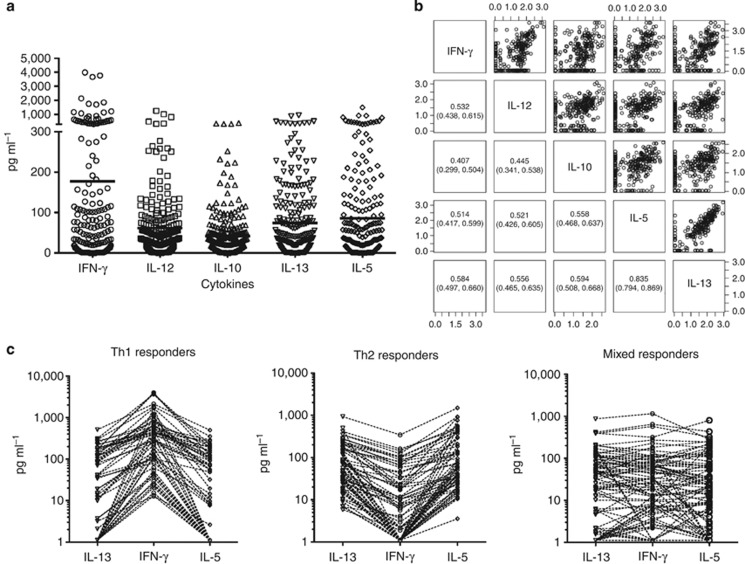
Peripheral blood mononuclear cells (PBMCs) from 98% of 255 volunteers 11–65 years old responded specifically to stimulation with P. duboscqi salivary gland homogenate (SGH), producing IL-5, IL-10, IL-12p40, IL-13, and IFN-γ in cell supernatants recovered 96 hours post stimulation (Figure 3a). We were unable to detect IL-4 or TNF in these supernatants. Of note, responsive PBMC samples showed a positive pairwise correlation among all five cytokines detected (Figure 3b). The correlation between IL-13 and IL-5 levels (r=0.835), both TH2 cytokines, was particularly strong (Figure 3b).
Figure 3.
The systemic cellular immune response to sand fly saliva in volunteers in a cutaneous leishmaniasis-endemic area of Mali. Peripheral blood mononuclear cells (PBMCs) were obtained from 255 individuals aged 11–65 years from the villages of Kemena and Sougoula. IFN-γ, IL-12, IL-10, IL-13, and IL-5 levels were measured in the supernatants of PBMCs from exposed volunteers 96 hours after stimulation with Phlebotomus duboscqi salivary gland homogenate. (a) Overall cytokine production by tested individuals. (b) Pairwise correlation of expressed cytokines (log-transformed cytokine levels). Numbers in insets indicate the Spearman correlation coefficient and the 95% confidence intervals for each correlation. (c) Cytokine responses of individuals in (a) stratified by TH1-predominant, TH2-predominant, or TH1/TH2 mixed responses to sand fly salivary proteins.
Three subpopulations were distinguished by their systemic immune response to SGH: 23% were polarized toward a TH1 response producing high IFN-γ and low IL-13 and IL-5 levels; 25% were polarized toward a TH2 response inducing high IL-13 and IL-5 and low IFN-γ levels; and 52% displayed a mixed TH1/TH2 response (Figure 3c).
There was no significant correlation between age (a) or gender (b) and the production of IFN-γ, IL-12p40, IL-10, IL-5, or IL-13 cytokines from PBMCs of Malian volunteers stimulated with sand fly salivary proteins (Supplementary Figure S2 online).
Discussion
Here, we report the kinetics, duration, and cellular composition of the DTH response to sand fly bites in individuals from a CL-endemic area, to our knowledge previously unreported, and the evidence that this DTH response is predominantly TH1-mediated. To date, the DTH response to sand fly bites has been documented only in human volunteers who do not reside in leishmaniasis-endemic areas (Theodor, 1935; Belkaid et al., 2000; Vinhas et al., 2007). Here, we conducted our study in a large cohort of 191 randomly selected volunteers living in a CL-endemic area of Mali. We found that the frequency of positive DTH responses to bites of colony-reared sand flies is highly prevalent (75%) in children up to the age of 15 years. Unlike the DTH response to mosquito bites, we observed that the DTH response to sand fly bites is long-lasting and diminishes appreciably only after 46 years of age. This gradual loss of DTH positivity implies that in our study site, a stable area of Leishmania transmission (Oliveira et al., 2009a), inhabitants will likely encounter an infected bite before losing their reactivity to sand fly saliva. We hypothesize that those that loose or never develop a DTH to sand fly saliva may not benefit from its modulation of their immune response to Leishmania shown to be protective in animal models (Gomes and Oliveira, 2012). Most of the ITH responses in our study were transient and occurred in children (8 of 11) who later on developed a DTH response. The difference in the hypersensitivity responses of humans to mosquitoes and sand flies is not surprising. Salivary components from sand flies and mosquitoes are considerably different sharing only a small number of molecules with significantly divergent sequences (Anderson et al., 2006; Calvo et al., 2010). In fact, several sand fly salivary proteins are unique to these insects (Anderson et al., 2006), potentially accounting for the distinct hypersensitivity responses observed here.
Our results show that exposure to sand fly bites induces a TH1-biased DTH response in inhabitants of a CL-endemic area over a considerable period of their lifetime. These immune responses are reminiscent of those that protect against leishmaniasis in animal models (Belkaid et al., 1998; Kamhawi et al., 2000; Oliveira et al., 2006, 2008; Collin et al., 2009). It is pertinent to mention that the DTH responses in this study were induced by saliva from colony-bred sand flies. Assuming that a TH1-mediated DTH response in humans correlates with protection against leishmaniasis, our observations differ from those in studies of animal models where colonized sand flies lose the ability to induce the protective immune response generated by wild-caught flies (Laurenti et al., 2009; Ahmed et al., 2010). In addition, Rohousova et al. (2011) reported the loss of saliva-mediated protection from CL in BALB/c mice exposed long-term to a large number of sand fly bites. In our study site, where P. duboscqi sand flies are present throughout the year (Anderson et al., 2011) and inhabitants are exposed to bites on a daily basis over their lifetime, half of the individuals maintained their DTH responses until their mid-forties. These findings highlight the important differences between studies conducted in animal models and human populations.
PBMC from most individuals tested in this cohort displayed a systemic immune response to sand fly saliva involving the production of TH1 and TH2 cytokines. This is not surprising as sand fly saliva consists of multiple proteins that may induce different immune profiles. Nevertheless, despite an overall correlation between TH1 and TH2 cytokines in these subjects that was independent of gender and age, one of the most interesting findings is the presence of individuals whose systemic immune response to sand fly saliva was polarized. Although the majority (52%) of individuals produced a mixed response, 23 and 25% displayed a TH1- or TH2-polarized response, respectively. These proportions are significant and lead us to hypothesize that individuals with a TH1-polarized response are relatively resistant to CL or manifest less severe CL lesions. On the other hand, individuals with a TH2-polarized response would reflect a proportion of the population that would not be protected from—and perhaps are made more susceptible to—leishmaniasis by previous exposure to sand fly saliva. The factors underlying the induction of TH1, TH2, or mixed type anti-saliva immune responses remain unknown and may be due to genetic factors inherent to these individuals, co-infections, or environmental factors.
In a CL-endemic area of Tunisia, where P. papatasi is the main vector of L. major, Abdeladhim et al. (2011) reported that stimulation of PBMCs with P. papatasi saliva induced a predominantly TH2 response (IL-4 and IL-10) produced mostly by CD8+ lymphocytes. This TH2 predominance may be attributed to adenosine and AMP present in P. papatasi saliva (Ribeiro et al., 1999) and absent from P. duboscqi saliva (Kato et al., 2007). In vitro, adenosine is known to drive immune cells toward a TH2 response by inducing IL-10 production in human monocytes (Hasko et al., 1996, 2000; Katz et al., 2000). Of note, rodents exposed to P. papatasi saliva mount a protective TH1 DTH response even in the presence of salivary adenosine indicating that the activity of adenosine in the skin may not be as potent as the one observed in vitro.
Experimental exposure of non-endemic volunteers to the bites of Lutzomyia longipalpis induced both ITH and DTH reactions (Vinhas et al., 2007). PBMCs from exposed volunteers produced IFN-γ and IL-10 following SGH stimulation (Vinhas et al., 2007). This study demonstrated that lymphocytes from exposed volunteers reduced the number of amastigotes in macrophages upon the addition of SGH, demonstrating that a saliva-induced TH1 environment has an adverse effect on Leishmania parasites (Vinhas et al., 2007). It is important to note that PBMC responses to saliva, whether TH1 or TH2, reflect systemic responses that may be distinct from those occurring locally at the bite site and require further validation by associating them with DTH responses in the same individual.
Most individuals in our study were leishmanin skin test-positive in 2008 with no recorded history of CL (Oliveira et al., 2009a). Unfortunately, due to the high prevalence of scars, and fungal and other cutaneous infections, we could not correlate the TH1, TH2, and mixed type anti-saliva immune responses to the incidence of CL. Studies in other human populations will thus be necessary to correlate the incidence of CL and severity of lesions in leishmanin skin test-negative individuals to TH1, TH2, or mixed type anti-saliva immune responses.
In summary, this study highlights the evolution of the DTH response to sand fly bites in a naturally exposed human population and establishes the TH1 nature of this response at the bite site. In light of the transiency of the DTH response to mosquitoes and the expectation of immune tolerance to antigens after repetitive and prolonged exposure, it is remarkable that the DTH response to sand fly saliva is long-lasting. It remains to be determined whether this sustained TH1-mediated DTH response confers significant protection against CL in humans as it does in animal models.
Materials and Methods
Study sites and populations
This study was conducted in a CL-endemic area of Central Mali. Individuals residing permanently in two villages (Kemena and Sougoula) (Oliveira et al., 2009a) were enrolled in a longitudinal study (Clinical protocol NCT00344084) approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (NIAID) and the Ethics Committee of the Faculty of Medicine, Pharmacy, and Odontostomatology at the University of Bamako, Mali and was externally monitored for protocol agreement, data integrity, and protection of human subjects. All clinical investigations have been approved by the author's institution and conducted according to Declaration of Helsinki principles, and written informed consent was obtained from all patients.
In a period of 5 years, 400 permanent residents of Kemena and Sougoula were recruited to participate in the study. Participating adults and the parent or guardian of participating minors provided a written informed consent. In the first 3 years of this study, PBMCs were obtained from 255 individuals aged 11–65 years to assess systemic immunity to sand fly saliva. During the final 2 years of this study, 191 individuals arranged in age groups (years): 1–2 (n=11), 3–5 (n=20), 6–10 (n=35), 11–15 (n=20), 16–25 (n=18), 26–35 (n=25), 36–45 (n=23), 46–55 (n=19), and 56–65 (n=20) participated in the study of DTH responses to uninfected sand fly bites. The two cohorts were randomly selected from a population of 1,549 individuals using a computer-generated list based on age and subject identification numbers.
Sand fly rearing and SGH preparation
P. duboscqi sand flies were collected in Mali (Kato et al., 2006; Anderson et al., 2011) and reared for approximately 42 generations at the Laboratory of Malaria and Vector Research (LMVR), NIAID. Late-stage P. duboscqi pupae were shipped from the LMVR to the University of Bamako and reared at the insectary for 5 days before use. To perform in vitro experiments, salivary glands were dissected from 5–7-day old colonized P. duboscqi females in phosphate-buffered saline. SGH was prepared by ultrasonication followed by centrifugation at 10,000 g for 3 minutes at 4 °C. Supernatants were collected and dried using a vacuum concentrator (Thermo, Asheville, NC). SGH was shipped to the University of Bamako and rehydrated with ultra-pure water (KD-Medical, Columbia, MD) immediately before use.
Exposure of human volunteers to uninfected sand flies
A secure, custom-designed Plexiglas capsule (Precision Plastics, Beltsville, MD) with a meshed surface was used in exposure experiments. A capsule containing three P. duboscqi females was strapped to the forearm of each individual for 20 minutes while covered with a dark fabric. The bite sites were traced using a permanent marker. A vernier caliper (Mitutoyo, Edgemont, PA) recorded measurements of the skin reaction 24, 48, 72, and 96 hours post bites. Photographs were taken before exposure and at each time point thereafter. All sand flies were accounted for and killed after each exposure.
Blood collection and cytokine measurements
To evaluate cytokine levels, PBMCs isolated by density-gradient centrifugation (Ficoll-Paque PLUS; GE Healthcare, Pittsburgh, PA) from blood collected in heparinized Vacutainer tubes (BD Diagnostics, Hunt Valley, MD). Two hundred microliters (5 × 106 cells ml–1) were seeded in 96-well micro-titer plates in RPMI medium 1640 supplemented with 10% heat-inactivated fetal bovine serum, penicillin (10 U ml–1), streptomycin (10 μg ml–1), and L-glutamine (0.3 mg ml–1) (Life Technologies, Grand Island, NY). Cells were stimulated with 2.5 salivary gland pairs of P. duboscqi or 2.5 μg ml–1 Concanavalin A (Sigma, St Louis, MO). Supernatants were collected 96 hours later and frozen at –70 °C until use. Levels of IL-4, IL-5, IL-10, IL-12p40, IL-13, IFN-γ, and TNF were quantified using a multiplex bead-based platform (Life Technologies).
Quantification of cytokine expression in skin biopsies using real-time PCR
A single 2-mm punch biopsy was obtained from the DTH-affected area of 16 individuals aged 18–54 years who manifested a DTH response 48 hours after sand fly bites. A control skin biopsy was taken from the opposite arm of two DTH-positive individuals biopsied following sand fly bites. Biopsies were stored in RNAlater (Ambion, Grand Island, NY). RNA was extracted using the RNEASY fibrous kit (Qiagen, Valencia, CA) and treated with DNase I. Total RNA (100 ng) was used for cDNA synthesis using the qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD). Genomic DNA contamination was measured by PCR of total RNA. Relative quantification of IFN-γ, IL-12, TNF, TGF-β, IL-13, IL-4, and IL-5 expression was performed in a LightCycler 480 (Roche Applied Science, Indianapolis, IN) using the Universal ProbeLibrary system (Roche). Primers and probes were designed using ProbeFinder software Version 2.45 (Roche) (Supplementary Figure S1 online). Relative quantification analysis of target genes versus GAPDH was performed using the LightCycler 480 software; cDNA from control skin were used as normalizing calibrators.
Histologic analyses of skin biopsies
We obtained a 2-mm punch biopsy from the DTH-affected area of six individuals aged 21–40 years who manifested a DTH response 48 hours after sand fly bites. Biopsies were stored in 10% buffered formalin solution and shipped to Histoserv for histological staining. Primary antibodies against CD3 (Dako#A0452, Carpinteria, CA) at a dilution of 1:100, CD4 (Dako#M7310) at 1:80, CD8 (Dako#M7103) at 1:75, CD20 (Dako#M0755) at 1:300, CD68 (Dako#M0814) at 1:100 and myeloperoxidase (Dako#A0398) at 1:400 were used. For secondary antibodies biotinylated anti-mouse IgG against CD4, CD8, CD20, CD68, and biotinylated anti-rabbit IgG against CD3 at 1:500 dilution were used followed by streptavidin-horseradish peroxidase. Slides were then counterstained with hematoxylin and eosin.
Statistical analysis
DTH measurements were taken as the largest induration diameter. Individuals with at least one DTH response at bite site were considered reactive. Individual transparency sheets were used to track the induration overtime, verifying that the same induration was measured at different time points. Responders to stimulation with P. duboscqi SGH were placed into three categories: TH1 (1.5 × IFN-γ>IL-13 and 1.5 × IFN-γ>IL-5 levels), TH2 (1.5 × IL-13>IFN-γ and 1.5 × IL-5>IFN-γ levels) or mixed responders. To test for differences between biopsies from the 16 DTH responders and normal skin, we used a two-sample t-test on the log-transformed response. The confidence intervals are wide because both control biopsies were combined and treated as a single observation. Spearman correlation was used to determine whether age is related to reactivity (0,1), with the P-value calculated by permutational central limit theorem so that it properly handles ties. Induration size by hours post bite was tested by analysis of variance followed by Tukey's multiple comparison test. Calculations were done in GraphPad Prism (version 5.0d) (La Jolla, CA) or R (version 2.14.2) (Vienna, Austria).
Acknowledgments
We sincerely thank the residents of Sougoula and Kemena, Mali, for participating in this study. We thank Elvin Morales, Anika T. Haque, and Nathan Smith for their help with sand fly colonization. We thank Drs Jose M.C. Ribeiro, Ryan Jochim, and Hamide Aslan for critical review of the manuscript. We thank Robert Gwadz and Thomas Wellems for continuous support of our research program, and NIAID intramural editor Brenda Rae Marshall for assistance. This study was funded by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Disclaimer
Because R.G., F.O., C.M., D.C.G., S.K., and J.G.V. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMed Central for display and use by the public, and PubMed Central may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Glossary
- CL
cutaneous leishmaniasis
- DTH
delayed-type hypersensitivity
- IFN-γ
interferon gamma
- ITH
immediate-type hypersensitivity
- PBMC
peripheral blood mononuclear cells
- SGH
salivary gland homogenate
- TGF-β
tumor growth factor-beta
- TNF
tumor necrosis factor
- Th1
T cell helper type 1
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Abdeladhim M, Ben Ahmed M, Marzouki S, et al. Human cellular immune response to the saliva of Phlebotomus papatasi is mediated by IL-10-producing CD8+ T cells and Th1-polarized CD4+ lymphocytes. PLoS Negl Trop Dis. 2011;5:e1345. doi: 10.1371/journal.pntd.0001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SB, Kaabi B, Chelbi I, et al. Lack of protection of pre-immunization with saliva of long-term colonized Phlebotomus papatasi against experimental challenge with Leishmania major and saliva of wild-caught P. papatasi. Am J Trop Med Hyg. 2010;83:512–514. doi: 10.4269/ajtmh.2010.09-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Oliveira F, Kamhawi S, et al. Comparative salivary gland transcriptomics of sandfly vectors of visceral leishmaniasis. BMC Genomics. 2006;7:52. doi: 10.1186/1471-2164-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Samake S, Jaramillo-Gutierrez G, et al. Seasonality and prevalence of Leishmania major infection in Phlebotomus duboscqi Neveu-Lemaire from two neighboring villages in central Mali. PLoS Negl Trop Dis. 2011;5:e1139. doi: 10.1371/journal.pntd.0001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Valenzuela JG, Kamhawi S, et al. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: An adaptive response induced by the fly. Proc Natl Acad Sci USA. 2000;97:6704–6709. doi: 10.1073/pnas.97.12.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley PF, Baird J, Mitchell JA, et al. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 2006;28:143–153. doi: 10.1111/j.1365-3024.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Calvo E, Sanchez-Vargas I, Kotsyfakis M, et al. The salivary gland transcriptome of the eastern tree hole mosquito, Ochlerotatus triseriatus. J Med Entomol. 2010;47:376–386. doi: 10.1603/me09226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin N, Gomes R, Teixeira C, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura TR, Oliveira F, Novais FO, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007;1:e84. doi: 10.1371/journal.pntd.0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahota J, Lipoldova M, Volf P, et al. Specificity of anti-saliva immune response in mice repeatedly bitten by Phlebotomus sergenti. Parasite Immunol. 2009;31:766–770. doi: 10.1111/j.1365-3024.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- Follador I, Araujo C, Bacellar O, et al. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–E58. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on leishmania immunity. Front Immunol. 2012;3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R, Teixeira C, Teixeira MJ, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Németh ZH, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- Janini R, Saliba E, Khoury S, et al. Incrimination of Phlebotomus papatasi as vector of Leishmania major in the southern Jordan Valley. Med Vet Entomol. 1995;9:420–422. doi: 10.1111/j.1365-2915.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Belkaid Y, Modi G, et al. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- Kato H, Anderson JM, Kamhawi S, et al. High degree of conservancy among secreted salivary gland proteins from two geographically distant Phlebotomus duboscqi sandflies populations (Mali and Kenya) BMC Genomics. 2006;7:226. doi: 10.1186/1471-2164-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Jochim RC, Lawyer PG, et al. Identification and characterization of a salivary adenosine deaminase from the sand fly Phlebotomus duboscqi, the vector of Leishmania major in sub-Saharan Africa. J Exp Biol. 2007;210 (Part 5:733–740. doi: 10.1242/jeb.001289. [DOI] [PubMed] [Google Scholar]
- Katz O, Waitumbi JN, Zer R, et al. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am J Trop Med Hyg. 2000;62:145–150. doi: 10.4269/ajtmh.2000.62.145. [DOI] [PubMed] [Google Scholar]
- Kulthanan K, Wongkamchai S, Triwongwaranat D. Mosquito allergy: clinical features, natural course. J Dermatol. 2010;37:1025–1031. doi: 10.1111/j.1346-8138.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- Laurenti MD, da Matta VL, Pernichelli T, et al. Effects of salivary gland homogenate from wild-caught and laboratory-reared Lutzomyia longipalpis on the evolution and immunomodulation of Leishmania (Leishmania) amazonensis infection. Scand J Immunol. 2009;70:389–395. doi: 10.1111/j.1365-3083.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- Mellanby K. Man's reaction to mosquito bites. Nature. 1946;158:554. doi: 10.1038/158554c0. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Doumbia S, Anderson JM, et al. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on Leishmanin skin test surveys. PLoS Negl Trop Dis. 2009a;3:e565. doi: 10.1371/journal.pntd.0000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F, Jochim RC, Valenzuela JG, et al. Sand flies, Leishmania, and transcriptome-borne solutions. Parasitol Int. 2009b;58:1–5. doi: 10.1016/j.parint.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F, Kamhawi S, Seitz AE, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Lawyer PG, Kamhawi S, et al. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS Negl Trop Dis. 2008;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Beckett AN, Engler RJ, et al. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol. 2004a;114:1189–1194. doi: 10.1016/j.jaci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Peng Z, Ho MK, Li C, et al. Evidence for natural desensitization to mosquito salivary allergens: mosquito saliva specific IgE and IgG levels in children. Ann Allergy Asthma Immunol. 2004b;93:553–556. doi: 10.1016/s1081-1206(10)61262-8. [DOI] [PubMed] [Google Scholar]
- Peng Z, Simons FE. Mosquito allergy: immune mechanisms and recombinant salivary allergens. Int Arch Allergy Immunol. 2004;133:198–209. doi: 10.1159/000076787. [DOI] [PubMed] [Google Scholar]
- Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG antibodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238–244. doi: 10.1016/S1081-1206(10)63262-0. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Katz O, Pannell LK, et al. Salivary glands of the sand fly Phlebotomus papatasi contain pharmacologically active amounts of adenosine and 5′-AMP. J Exp Biol. 1999;202 (Part 11:1551–1559. doi: 10.1242/jeb.202.11.1551. [DOI] [PubMed] [Google Scholar]
- Rohousova I, Hostomska J, Vlková M, et al. The protective effect against Leishmania infection conferred by sand fly bites is limited to short-term exposure. Int J Parasitol. 2011;41:481–485. doi: 10.1016/j.ijpara.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Sassi A, Louzir H, Ben Salah A, et al. Leishmanin skin test lymphoproliferative responses and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin Exp Immunol. 1999;116:127–132. doi: 10.1046/j.1365-2249.1999.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodor O. A study of the reaction of Phlebotomus bites with some remarks on harara. Transcactions Royal Soc Trop Med Hyg. 1935;29:273–284. [Google Scholar]
- Valenzuela JG, Belkaid Y, Garfield MK, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinhas V, Andrade BB, Paes F, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007;37:3111–3121. doi: 10.1002/eji.200737431. [DOI] [PubMed] [Google Scholar]
- WHO 2010Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of LeishmaniasesTRS No. 949
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.