Abstract
We investigated whether the perifornical-lateral hypothalamic area (PF-LHA), where the orexin neurons reside, is a central chemoreceptor site by microdialysis of artificial cerebrospinal fluid (aCSF) equilibrated with 25% CO2 into PF-LHA in conscious rats. This treatment is known to produce a focal tissue acidification like that associated with a 6 to 7 mm Hg increase in arterial P CO2. Such focal acidification in the PF-LHA significantly increased ventilation up to 15% compared with microdialysis of normal aCSF equilibrated with 5% CO2 only in wakefulness but not in sleep in both the dark (P=0.004) and light (P<0.001) phases of the diurnal cycle. This response was predominantly due to a significant increase in respiratory frequency (11%, P< 0.001). There were no significant effects on ventilation in the group with probes misplaced outside the PF-LHA. These results suggest that PF-LHA functions as a central chemoreceptor site in the central nervous system in a vigilant state dependent manner with predominant effects in wakefulness.
Keywords: perifornical-lateral hypothalamus, central chemoreception, orexin
1. Introduction
Central chemoreception, which regulates breathing by detecting changes in CO2/pH in the interstitial tissue of the brain, has been studied mostly in the brain stem at sites including but not limited to the retrotrapezoid nucleus (RTN) (Guyenet, 2008; Li and Nattie, 2002), the medullary raphe (Nattie and Li, 2001; Taylor et al., 2005; Wang et al., 1998), the locus ceruleus (Biancardi et al., 2008; Noronha-de-Souza et al., 2006), nucleus tractus solitaries (NTS) (Dean et al., 1989; Nattie and Li, 2002) and the caudal ventrolateral medulla (cVLM) (da Silva et al., 2010). The role of higher regions of the central nervous system such as the lateral hypothalamus, which participate in multiple physiological functions, in chemoreception is less well understood.
There are studies that suggest a role for the lateral hypothalamus in the regulation of breathing. Destruction of the lateral hypothalamus in lightly anesthetized cats by high frequency currents caused an immediate decrease in rate and/or depth of respiration (Redgate and Gellhorn, 1958). Extracellular recording showed that the neurons located in dorsal and lateral hypothalamic areas increased firing rate in responding to hypercapnic stimulation in lightly-anesthetized rabbits (Cross and Silver, 1963). In the past decade, many studies have shown that orexin/hypocretin neurons, which are exclusively localized in the lateral hypothalamus, are sensitive to CO2/pH changes both in vitro and in vivo. A switch from 5% to 10% CO2 in the artificial cerebrospinal fluid (aCSF) (pH change from 7.02 to 7.25) can induce an increased firing rate of orexin neurons (Williams et al., 2007). A measure of c-Fos activation shows that systemic CO2 activates orexin neurons in the perifornical region in mice (Sunanaga et al., 2009). In transgenic mice lacking the precursor gene of orexin (OX-KO), prepro-orexin, there is a 50% decrease in the ventilatory CO2 response in wakefulness but not during sleep (Deng et al., 2007). This hypothesis was further investigated in pharmacological studies with inhibition of both orexin receptors (OX1R and OX2R) using the oral administration of a dual orexin receptor antagonist, Almorexant. The CO2 response was attenuated by 26% only in wakefulness and only during the dark, active, period of the diurnal cycle (Li and Nattie, 2010).
We hypothesize that the neurons in the perifornical lateral hypothalamic area (PF-LHA) including orexin neurons are chemosensitive to CO2/H+ and they participate in the central chemoreception in a vigilance-state dependent manner. To test this hypothesis we focally acidified PF-LHA area with high CO2 (25%) by using a reverse microdialysis technique and measured the ventilation in wakefulness and NREM sleep during both dark and light phase of the diurnal cycles.
2. Methods
2.1. Ethical approval
The animal experiments and surgical protocols were within the guidelines of the National Institutes of Health for animal use and care and the American Physiological Society’s Guiding Principles in the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at the Dartmouth College Animal Resource Center.
2.2. General and Diurnal cycle
A total of 28 adult male Sprague–Dawley rats (250–350 g) were used for the experiments and were individually housed in the Animal Resource Center in a light- and temperature-controlled environment with a 12h light and12h dark cycle. The light cycle was set as 12:00 am lights on and 12:00 pm lights off. Food and water were provided ad libitum. Animals were randomly divided into two groups to be tested during either light or dark cycle. The experiments were carried out from 8:00 am ~11:00 am for the light cycle study and from 1:00 pm ~4:00 pm for the dark cycle test.
2.3. Surgery
All the rats were surgically implanted with a microdialysis guide cannula, electroencephalography (EEG) & electromyography (EMG) electrodes, and a telemetric temperature probe. The detailed surgical procedures have been described in our earlier publications (Li et al., 2006; Nattie and Li, 2001). Briefly, sterile surgeries were performed under general anesthesia produced by intramuscular injection of ketamine (100 mg/kg) and xylazine (15 mg/kg). Rats were fixed in a Kopf stereotaxic frame and a microdialysis guide cannula (CMA11, Microdialysis AB, Stockholm, Sweden) was implanted into the PF-LHA. The coordinates for probe placement were 3.3 mm caudal and 1.8 mm lateral from the lambda and 8.5 mm below the surface of the skull (Paxinos and Watson, 1998). Three EEG electrodes were screwed onto the skull and two EMG electrodes were inserted into the dorsal neck muscles, and all electrode wires were connected to a six-prong plastic pedestal attached to the skull. The guide cannula and the plastic pedestal were secured with cranioplastic cement on the top of the rat’s head. A sterile telemetry temperature probe (TA-F20, Data Sciences, St Paul, MN, USA) was implanted into the peritoneal cavity. After the incisions were sutured and cleaned, the animal was allowed to recover for at least 7 days.
2.4. CO2 Microdialysis Solution
The aCSF was equilibrated with 5 or 25% CO2. The composition of the aCSF was (in mM) 152 sodium, 3.0 potassium, 2.1 magnesium, 2.2 calcium, 131 chloride and 26 bicarbonate. The calcium was added after the aCSF was acidified by equilibration with CO2. The measured pH of this solution is ~6.8 for equilibration with 25% CO2 and ~7.45 with 5% CO2. The syringe pump for the dialysis was set at a speed of 40 μl /min for all the experiments.
2.5. Ventilation, Oxygen Consumption and Temperature Measurement
The methods used to measure the ventilation (V̇E), body temperature (Tb) and oxygen consumption (V̇o2) in conscious rats were those commonly used in our lab (Li et al., 2006; Nattie and Li, 2001). In brief, a whole body plethysmograph was used to measure ventilation and the volume of the plethysmograph chamber was 7.6L. The analog output from the pressure transducer was sampled at 150Hz and converted into a digital signal by a computer using the DATAPAC 2K2 system (RUN Technologies, USA). The rate of gas inflow was maintained at ≥1.4 l min−1 with the plethysmograph at atmospheric pressure. CO2 and O2 fractions were sampled from the outflow line at ~100 ml min−1 by the CO2 and O2 gas analyser (Applied Electrochemistry). Before each experiment, we calibrated the plethysmograph by obtaining pressure response data from five 0.3 ml air injections made with a 1 ml syringe. Breath-by-breath analysis was performed using the pressure deflections and the respiratory cycle time for each breath.
Tidal volume (VT) and respiratory frequency (fR) were calculated per breath to estimate ventilation per breath. VT was calculated by using the equation published by Bartlett and Tenney in 1970 (Bartlett and Tenney, 1970). V̇E was calculated as the product of VT*fR. All ventilatory parameters are expressed as Mean ± S.E.M. in quiet wakefulness (wakefulness) or NREM sleep. Oxygen consumption was calculated by application of the Fick principle, using the difference in O2 content between inflow air and outflow air and the flow rate as described previously (Li et al., 2006; Nattie and Li, 2001). Core body temperature was measured continuously using the signal from the telemetric temperature probe inside the abdomen. The temperature of the experimental chamber was measured using a thermometer within the plethysmograph.
2.6. Determination of vigilance state
The EEG and EMG signals from the electrodes implanted on the skull and in the neck muscles were sampled at 150Hz, filtered at 0.3–70 and 0.1–100 Hz, respectively, and were acquired continuously using the DATAPAC system. The vigilance states were determined by analysis of EEG and EMG records as previously described (Li et al., 2006; Nattie and Li, 2001). In brief, a fast Fourier transform was performed on the EEG and EMG signal at 4.0-s-long epochs. We categorized the vigilance states as wakefulness, NREM and REM sleep. Both wakefulness and NREM sleep states were observed consistently through the experiments but periods of REM sleep were short and sometimes were absent during the experiment period. Thus ventilation events that occurred during REM or when sleep state was indeterminant were excluded from our analysis.
2.7. Experimental protocol
At least 7 days after surgery, the rat was weighed and then a microdialysis probe was gently inserted into the guide cannula and EEG and EMG electrode cable was connected. The animals were placed into the plethysmograph chamber and allowed 1 h to acclimate. All experiments were performed at room temperature (24–25 °C) with the rat breathing room air. During acclimation, we began reverse-microdialysis with 5% CO2 - equilibrated aCSF without recording any data. After acclimatization, baseline measurements were recorded for 30 min with dialysis of 5% CO2. The microdialysis solution was then switched to 25% CO2-equilibrated aCSF and measurements were continued for another 30min followed by second period of dialysis with 5% CO2 with data recorded for another 30 min. All animals were submitted to the same protocol and received only one microdialysis probe insertion and 1-day of experimentation. The animals first were randomly divided into two groups based on the diurnal cycle that the experiments were conducted, as dark (N=14) and light (N=14) groups, and then within the dark and light group the animals were further divided into two sub-groups based on the placement of the microdialysis probe, as within-PF-LHA (N=8 & 9 respectively) and outside-PF-LHA groups (N=6 & 5 respectively) (Fig1B & C).
Figure 1.
Anatomical locations of the tip of the microdialysis probe. (A) a photomicrograph of a stained coronal section of the lateral hypothalamus of one representative rat with the tip of microdialysis probe near the fornix indicated in the white rectangle. Scale bar represents 0.5 mm. The schematized anatomical cross sections show in (B) the probe placement within the PF-LHA in the dark (left, N=8) and light (right, N=9) phase groups; and (C) the probe placement outside PF-LHA in the dark (left, N=6) and light (right, N=5) phase groups. The dark filled ovals represent the locations of the microdialysis probe tips. The numbers on the left refer to millimeters caudal to Bregma. Abbreviations: mt, mammillothalamic tract; f, fornix; 3v, third ventricle. The drawings are modified from the atlas of Paxinos & Watson (Paxinos and Watson, 1998).
2.8. Anatomy analysis
Upon conclusion of the experiment, the rats were euthanized with an overdose of sodium pentobarbitone injected intraperitoneally (>75mg/kg). The whole brain was removed, frozen, and sectioned at 50 μm thickness with a Reichert–Jung cryostat. The sections were then stained with cresyl violet to identify the locations of the microdialysis probe tips.
2.9. Statistics
The data are presented as mean ± standard error of the mean (S.E.M.). We analyzed data measured during wakefulness and NREM sleep separately with a one-way repeated measures (RM) ANOVA to compare VT, fR, V̇E, V̇O2, and Tb changes within each of three groups; 1) control (5% CO2 dialysis), 2) acidification (25% CO2 dialysis), and 3) control post focal acidosis (5% CO2 dialysis). Bonferroni post hoc test was applied when appropriate.
3. Results
3.1. Microdialysis probe placement
A representative example of the anatomic location of a microdialysis probe placement in the PF-LHA is shown in Fig. 1A (4x magnification). A series of schematic cross-sections of the hypothalamus in a caudal to rostral organization demonstrating the placement of the microdialysis probe tips (black filled symbols) are shown in Fig 1B and 1C. Based on the diurnal cycle and anatomic locations of the probe placement the rats were divided into four groups, 1) the probe tips were within the PF-LHA and rats studied in the dark phase (Fig 1B left, N=8); 2) the probe tips were within the PF-LHA and rats studied in the light phase (Fig 1B right, N=9); 3) the probe tips were misplaced outside PF-LHA and rats studied in the dark phase (Fig 1C left, N=6); the probe tips were outside PF-LHA and rats studied in the light phase (Fig 1B right, N=5). The anatomical location of the microdialysis probes of 17 rats in group 1 and 2 are within the PF-LHA, which are comparable with the location of the orexin neurons in the LHA demonstrated by orexin-immunoreactive perikarya staining in the adult rat brain (Nambu et al., 1999).
Minor tissue damage and gliosis were observed surrounding the location of the probe tip, but never extended more than a 0.4-mm radius from the tip center. This damage did not appear to alter ventilatory responses induced by focal acidification in control rats.
3.2 Typical experiments
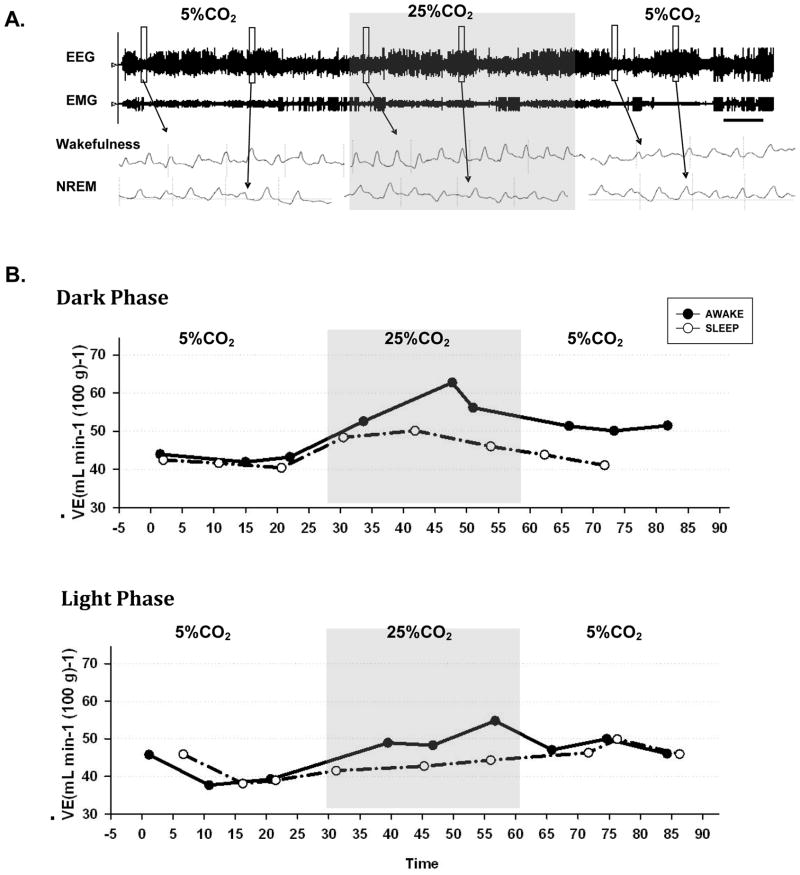
The representative experiments with time course analysis of before, during and after high 25% CO2 microdialysis are illustrated in Fig. 2, which shows that the increase of V̇E during focal acidification depended on the animal’s arousal state. Fig. 2A shows EEG and EMG recording throughout the experimental protocol for this individual animal. As expected, the animal cycled between wakefulness and sleep periods before, during and after focal acidification. Fig. 2B shows V̇E changes during the time course throughout the experimental periods in both dark (Fig. 2B, upper panel) and light (Fig. 2B, lower panel) phases in two individual animals. Comparing to the control 5% CO2 dialysis, 25% CO2 dialysis into the PF-LHA significantly increased V̇E only in wakefulness but not in NREM sleep. The similar response was observed during both dark and light cycles.
Figure 2.
Illustrative example of one single experiment in one animal with the microdialysis probe located in the PF-LHA. (A) EEG and EMG recordings with breathing tracing before, during and after focal acidification. Scale bar represents 6 min. The open rectangles refer to 6 s. (B) time course of changes in V̇E before, during and after 25% CO2 microdialysis in the perfornical hypothalamic area in dark (upper panel) and light (lower panel) phase in two individual rats. Dark circles refer to V̇E during quiet wakefulness and open circles NREM sleep. The grey rectangles represent the period of microdialysis of 25% CO2.
3.3. Ventilatory effects: focal acidification of PF-LHA
Fig. 3 summarizes the effects of focal acidification of PF-LHA on V̇E, fR and VT in wakefulness and NREM sleep during both dark and light phases.
Figure 3.
V̇E, fR and VT (Mean ± S.E.M.) during CO2 microdialysis in the groups with the tips located within the PF-LHA in both dark (N = 8, left panel) and light (N=9, right panel) phases. V̇E and fR increased significantly during high CO2/H+ (25% CO2) dialysis compare to normal 5% CO2 in wakefulness (! ) but not in NREM sleep (! ) during dark and light phase of the diurnal cycle. * P < 0.01, ** P< 0.001, one-way RM ANOVA; Bonferroni post hoc test.
In wakefulness during the dark cycle, V̇E was 70 ± 3 (ml min−1 100g−1) during first 5% CO2 dialysis period and increased to 80 ±2 (ml min−1 100g−1) during higher 25% CO2 dialysis, which was a 15% increase (P = 0.004). V̇E then returned to 71 ± 3 (ml min−1 100g−1) after switching back to 5% CO2 dialysis. The significantly increased V̇E by the focal acidification of PF-LHA was primarily contributed by respiratory frequency. During dark cycle, fR increased from 71 ± 2 (min−1) during the first period of 5% CO2 microdialysis to 79 ± 3 (min−1) during 25% CO2 microdialysis, an 11% increase (P<0.001). During the second period of 5% CO2 dialysis, fR fell back to 70 ± 3 (min−1). VT was not significantly affected by any period of CO2 microdialysis. In wakefulness during the dark cycle, VT was 1.00±0.04, 1.04±0.05, and 1.03±0.05 (ml 100g−1) during first 5% CO2, 25% CO2 and second 5% CO2 dialysis period, respectively. There is no significant difference on V̇E, fR and VT between the first and second periods of 5% CO2 dialysis (P≥0.05). The similar ventilatory effects were observed in wakefulness during the light phase (Fig. 2B and 3).
Distinct from the responses observed during wakefulness, focal acidification did not induce any significant changes on V̇E, fR or VT during NREM sleep in either dark or light phase.
3.4. Ventilatory effects: focal acidification outside of PF-LHA
Fig. 4 shows the changes of V̇E, fR and VT in the groups that microdialysis probe tips were mis-located outside the PF-LHA during 5% and 25% CO2 dialysis in wakefulness and sleep during either dark or light phase. Focal acidification outside the PF-LHA with 25% CO2 compared to 5% CO2 did not significantly change V̇E, fR, VT, body temperature and metabolic rate in wakefulness or NREM sleep during either light or dark phases.
Figure 4.
V̇E, fR and VT (Mean ± S.E.M.) during CO2 microdialysis in the animals with the tips located outside the PF-LHA in both dark (N = 6, left panel) and light (N=4, right panel) phases. V̇E, fR and VT were not significantly changed during high CO2/H+ (25% CO2) dialysis compare to normal 5% CO2 in both wakefulness (! ) and NREM sleep (! ) during dark and light phase of the diurnal cycle (P > 0.05, one-way RM ANOVA).
3.5. Body temperature and metabolic rate
There were no significant effects on body temperature and oxygen consumption by microdialysis of either 5% or 25% CO2 in wakefulness and sleep during dark and light phases (data not shown).
4. Discussion
The key finding of this study is that focal acidification of PF-LHA by CO2 significantly increased ventilation predominantly by increasing respiratory frequency only in wakefulness but not in NREM sleep during both light and dark phases of the diurnal cycle. Our data suggest that the PF-LHA may play a role in the central chemoreception depending on the animal’s arousal state.
4.1. Focal acidification by microdialysis of CO2/H+
Using the reverse microdialysis with a high CO2/H+ aCSF to produce a focal acidosis in a specific brain site for studying the central chemoreception in wakefulness and sleep in conscious animals has been well established (Biancardi et al., 2008; da Silva et al., 2010; Li and Nattie, 2002; Martino et al., 2006; Nattie and Li, 2001, 2002). The approach does not distinguish the specificity of the stimulation at cellular level, e.g., CO2 vs H+, but it does address the functional difference in the central chemoreception at various brain regions in different arousal state and diurnal cycle.
We have demonstrated in our previous study that aCSF solution equilibrated with 25% CO2 only provides a mild to moderate focal acidification in the region (Li and Nattie, 2002). Focal acidification of the RTN area by 25% CO2, the same stimulating intensity used in this study, induced an average focal pH change of 0.069 pH units measured within 200 μm of the microdialysis probe, which was equivalent to that induced by a 6.6 mm Hg increase in arterial PCO2 (Li and Nattie, 2002). Here we showed that this modest acidification in the area of PF-LHA significantly stimulated breathing mainly by increasing respiratory frequency in wakefulness while the same acidification in the area outside PF-LHA showed no effects on breathing in either arousal state (Fig. 1C & 4).
It is also important to note that in this study the enhanced respiratory response to the focal acidification by dialysis of CO2 was only noted when the probe was placed within the area of PF-LHA; no such respiratory response to the same focal acidification was observed when the probe was placed outside PF-LHA (see figures 1, 3 and 4). These data indicate that: 1) not all neurons or areas of the brain are chemosensitive to CO2/H+ and respond to such focal acidosis generated by microdialysis of high CO2; 2) microdialysis of CO2 can produce a specific stimulation in specific areas that can excite breathing.
4.2. Lateral hypothalamus and central chemoreception
The LHA has been found to regulate body homeostasis, blood pressure, heart rate, food intake, and energy balance and stress response. Orexin (or hypocretin) neurons are specifically localized within the hypothalamus (LHA) including the PF-LHA, dorsomedial hypothalamic nucleus (DMH), and posterior hypothalamic area (Nambu et al., 1999). The orexin receptors (OX1R and OX2R) and orexin axonal projections are widely distributed in the central nervous system including many sites that are important in cardiorespiratory regulation (Nambu et al., 1999; Trivedi et al., 1998). Recent studies have found that the orexin neurons may participate in central chemoreception (Deng et al., 2007; Song et al., 2012; Sunanaga et al., 2009; Williams et al., 2007). The c-Fos expression in orexin neurons were significantly increased in the perifornical region and the dorsomedial hypothalamus after systemic hypercapnic stimulation in mouse (Sunanaga et al., 2009). In mouse brain slices, the spontaneous firing rate of identified orexin neurons is profoundly affected by physiological changes of H+ and CO2. The firing rate of the orexin neurons increases as pH decreases with ~100% rate increase per 0.1 pH unit (Williams et al., 2007). Song et, al (2012) showed that the acid-sensing ion channel 1 (ASIC1) is co-localized with orexin-A in the LHA, and focal injection of acidified aCSF into the LH significantly increased phrenic nerve activity in anesthetized rats (Song et al., 2012). It is possible that the neurons in the PF-LHA area sense CO2/H+ and participate in the central chemoreception through the ASIC1 located on the orexin neurons in the region.
We acknowledge that other types of neurons are also located in the PF-LHA area, e.g. melanin concentrating hormone (MCH) neurons, and that they may also contribute to the response observed in this study.
4.3. Multiple central chemoreceptor sites and sleep-wake states
Previous studies by our group and others have shown that there are multiple central chemoreceptor sites in the brain, for example, the locus ceruleus, medullary raphe, caudal ventrolateral medulla, the RTN and caudal NTS (Coates et al., 1993; da Silva et al., 2010; Haxhiu et al., 1996; Hodges and Richerson, 2010; Li and Nattie, 2002; Nattie and Li, 2002). Many of these central chemoreceptor sites function in an arousal state dependent manner, e.g. focal microdialysis of 25% CO2: 1) in the RTN, increased ventilation (mostly VT) by 24% only in wakefulness (Li and Nattie, 2002); 2) in the caudal medullary raphé, increased ventilation (mostly fR) by 20% only in NREM sleep (Nattie and Li, 2001); 3) in the cVLM increased ventilation (both VT and fR) by 17% only in wakefulness (da Silva et al., 2010); 4) in the caudal NTS significantly increased ventilation by 16% in sleep and 28% in wakefulness (Nattie and Li, 2002). Here we show that focal dialysis of 25% CO2 into the PF-LHA increased ventilation (mostly FR) by 15% only in wakefulness. The data supports the hypothesis that among multiple central chemoreceptor sites in the brain some function in an arousal state dependent manner. The multiple central chemoreceptor sites may work together to control the breathing and maintain the homeostasis and physiological functions in different arousal states.
Orexin neurons discharge maximally during active wakefulness and the firing rate gradually slows down during quiet wakefulness in the absence of movement and ceases during sleep (Lee et al., 2005). The level of orexin in the CSF has a diurnal-rhythm, with a peak level at the end of the dark, predominantly wake-active period, and low levels toward the end of the light, predominantly sleep period) (Desarnaud et al., 2004). Studies have shown that orexin participates in the control of breathing in an arousal state dependent manner. For examples, OX-KO mice, lacking orexins, have a significantly attenuated CO2 response during wakefulness but not during sleep (Nakamura et al., 2007). Antagonism of both orexin receptors by Almorexant attenuates the CO2 response by 26% only in wakefulness during the dark period of the diurnal cycle (Li and Nattie, 2010). In this study we have demonstrated that the focal acidification of the PF-LHA only stimulates breathing when the animals are awake. All of these data suggest that the LHA and orexin system participate in central chemoreception in a vigilance-state-dependent manner.
In summary, focal acidification of the PF-LHA by CO2/H+ significantly increased breathing (predominantly respiratory frequency) only in wakefulness but not in sleep. Our study suggests that the higher regions of the central nervous system, such as the lateral hypothalamus, which participate in multiple physiological functions, may also play a role in the central chemoreception.
Highlights.
We focal acidified Perifornical-Lateral Hypothalamic Area (PF-LHA) by CO2/H+ in conscious rat.
Focal acidification of PF-LHA significantly increased ventilation mainly due to breathing frequency.
The enhanced ventilatory response was only observed in wakefulness not in sleep.
Focal acidification of outside the PF-LHA had no effect on respiration.
Acknowledgments
This research was supported by HL 28066 from the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respiration physiology. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Archiv: European journal of physiology. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Cross BA, Silver IA. Unit activity in the hypothalamus and the sympathetic response to hypoxia and hypercapnia. Experimental neurology. 1963;7:375–393. doi: 10.1016/0014-4886(63)90019-0. [DOI] [PubMed] [Google Scholar]
- da Silva GS, Li A, Nattie E. High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke’s area) increases ventilation in wakefulness. Respiratory physiology & neurobiology. 2010;171:46–53. doi: 10.1016/j.resp.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respiration physiology. 1996;105:35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respiratory physiology & neurobiology. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. The Journal of physiology. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. The Journal of physiology. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Forster HV. CO2/H+ chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain research. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Noronha-de-Souza CR, Bicego KC, Michel G, Glass ML, Branco LG, Gargaglioni LH. Locus coeruleus is a central chemoreceptive site in toads. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R997–1006. doi: 10.1152/ajpregu.00090.2006. [DOI] [PubMed] [Google Scholar]
- Paxinos, Watson The Rat Brain in Stereotaxic Coordinates. 1998 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Redgate ES, Gellhorn E. Respiratory activity and the hypothalamus. The American journal of physiology. 1958;193:189–194. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- Song N, Zhang G, Geng W, Liu Z, Jin W, Li L, Cao Y, Zhu D, Yu J, Shen L. Acid sensing ion channel 1 in lateral hypothalamus contributes to breathing control. PloS one. 2012;7:e39982. doi: 10.1371/journal.pone.0039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respiratory physiology & neurobiology. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. The Journal of physiology. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. The Journal of physiology. 1998;511 ( Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]