Introduction
Why do obese patients get sick? What underlies the pathogenesis of the many diseases associated with obesity? As recently as fifty years ago, the answer was mechanical stress on a variety of organ systems from increased body weight. But while this may explain osteoarthritis and venous stasis disease, it is not a satisfying explanation for diabetes, liver disease, allergic disease, cancer, and the many other diseases that are increased in incidence in obesity. What is it about excess adipose tissue that causes metabolic disease? The answer lies in one of the most important realizations in the field of obesity in the past twenty years: that obesity is associated with a state of chronic systemic inflammation and this inflammatory state, this hyper-activation of the immune system, underlies the pathogenesis of virtually every metabolic disease that afflicts the obese.
One of the most exciting recent papers came from investigators at the University of Pennsylvania, who treated patients with end-stage leukemia with their own immune T-cells, genetically modified in the test tube to target leukemic cells. This high-tech immunotherapy achieved durable and complete remission in the majority of a small group of subjects who were otherwise expected to live only months, and has generated much excitement and hope (1). Manipulating physiologic processes as fundamental as the immune system also entails risk. In 2006, Tegenero Inc., a German Biotech company, administered a monoclonal antibody designed to treat autoimmune disease to human volunteers in a Phase I safety trial. Despite prior safety testing in rodents and primates, within minutes of administration all subjects experienced a “cytokine storm”, a hyper-activation of the immune system, went into multi-system organ failure, and required months of treatment in the intensive care unit (2). This incident generated much scrutiny and speaks to the perils of immunotherapy. While these anecdotes may seem unrelated to obesity and metabolic disease, I would suggest that the same ravages of the immune system that the Tegenero subjects experienced also afflict obese patients, but play out over years rather than hours or days. Furthermore, the same hope that immunotherapy provides for patients with cancer also exists for those with metabolic disease.
Why is the study of inflammation important? After all, we already have a highly effective treatment for metabolic disease in the form of bariatric surgery. Why expend time and resources studying inflammation? One reason is that bariatric surgery is likely to remain an under-utilized resource - there are simply too many patients and not enough resources in the form of surgeons and health care dollars to provide surgery to all in need (3). As such, patient selection for surgery is of utmost importance and, as we will discuss, inflammation distinguishes patients with the most severe metabolic disease and may provide diagnostic tools to identify those most likely to benefit from surgical therapy. More importantly, however, the study of inflammation will lead to transformative immunotherapy that will be more cost-effective and less invasive than surgery with the potential to treat a wide range of metabolic diseases simultaneously with an enormous impact on public health.
Defining inflammation
Inflammation, while highly complex, may be simply defined as “an immune response to cellular injury”. But we must expand our understanding of injury for this definition to be useful. We typically think of immune responses as directed toward exogenous infectious stimuli such as bacteria, viruses, or parasites. But immune response also plays a central role in the scavenging, cleanup, and tissue remodeling that results from cell turnover, the daily “wear and tear” on all tissues. As a result, inflammation is triggered not only by exogenous infectious stimuli, but by endogenous stimuli as well, the very nutrients and metabolites that make up our cells that are released as cells die. Inflammation is therefore not an on-off switch, but rather a constant ubiquitous process. And not just a single process, but a complex set of processes carried out by the immune system that involve virtually all aspects of physiology, including energy balance.
What are the tools that the immune system uses to carry out the processes we collectively refer to as inflammation? The immune system is comprised of a primitive innate arm, as well as a more recently evolved adaptive arm. The term inflammation has typically been used to define those processes carried out by the innate immune system and indeed, until recently, obesity-related immune dysfunction has been considered to be primarily a disorder of innate immunity. As such we will discuss the role of macrophages, key cellular mediators of innate immune responses, in the pathogenesis of metabolic disease. But we will also discuss recent data which implicate adaptive immunity in obesity-related inflammation as well.
The complex processes that comprise inflammation are energy-intensive. The immune system utilizes up to 2–3% of total energy requirements in health and as much as 20% during active infection. It is therefore easily understood why evolution selected for a tight link between immunity and energy metabolism, and why in the case of nutrient deficit such as starvation, immune and inflammatory responses are markedly down-regulated. It is not as easily or intuitively understood, however, why in the case of obesity, that inflammation is increased. To understand this phenomenon, we must understand the concept of nutrient excess: that nutrients themselves, while necessary for health and life, when present in excess, are pro-inflammatory, detrimental to cellular function, and cause disease. It is this concept that lies at the heart of metabolic disease. But first, what were the initial observations that suggested a link between inflammation and obesity?
Obesity-related inflammation and serum inflammatory markers
Numerous reports dating back decades demonstrate suboptimal vaccine responses and increased risk of mortality from infectious disease in obese patients (4, 5), suggesting that something is wrong with the immune system in obesity. And, while the underlying mechanisms were not appreciated at the time, at the turn of the last century it was found that aspirin, a potent anti-inflammatory agent, ameliorated diabetes; this finding has led to current trials studying salicylate derivatives such as salsalate in diabetic humans (6, 7). These observations are among the earliest demonstrations of the intimate association between inflammation and metabolism, and the first example of immunotherapy for metabolic disease. But one of the most important early avenues of research was the study of serum markers, the circulating cytokines and inflammatory mediators that carry out the tasks of inflammation. Elevated levels of acute phase reactants in the serum of obese patients were reported as early as the 1960s (8), observations that evolved into an enormous body of literature that describes the association between obesity and literally hundreds of serum markers of inflammation (9–11). We will distill this body of literature to answer three questions. First, are serum markers of inflammation elevated in patients with obesity and metabolic disease? And the answer to this question is an unequivocal yes. The highest profile papers documenting this association study interleukin-6 and C-reactive protein (12–14). Elevated levels of these markers not only correlate with obesity and metabolic disease, but also predict incident diabetes prior to clinical or laboratory manifestations, suggesting their potential as diagnostic tools that would not only aid in management of non-surgical patients, but also identify patients who would benefit most from surgical therapy.
Do serum inflammatory markers revert to normal levels with weight loss and resolution of metabolic disease? Such a finding would suggest a causal relationship. Again the answer is yes, and an examination of the data is enlightening (15–19). First, the degree of weight loss correlates with the degree of reduction in serum inflammatory markers, with surgery, the most effective therapy, demonstrating the greatest reduction. Secondly, unlike resolution of insulin resistance after gastric bypass, which may be rapid and mediated by changes in incretin physiology, serum inflammatory markers decrease over time, and do not correlate with specific surgical procedures. Taken together these observations suggest that loss of adipose tissue mass is necessary for resolution of inflammation, a critical point to which we will return.
Finally, what are the obstacles to applying serum inflammatory markers to large patient populations as predictors of incident metabolic disease and tools for risk stratification? Unfortunately, these obstacles are not trivial. One obvious but nonetheless important obstacle is the presence of multiple confounding variables in large patient populations. Regression analysis of the association between serum C-reactive protein (CRP) levels and diabetes in a large group of patients demonstrates that the odds ratios progressively decrease as more confounding clinical variables are added to the regression model (20). Application of single serum markers to large populations will therefore be limited by inherent heterogeneity. One potential solution is to use multiple serum markers. This of course begs the question of which markers. Much attention has focused on CRP, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), but hundreds of others exist. Furthermore, their association with obesity and metabolic disease is complex. For example, while some markers correlate directly and linearly with obesity and metabolic disease, in other cases, this relationship may be indirect or non-linear. Other markers peak specific stages or types of metabolic disease. We are eventually faced with a large number of markers with variable and complex kinetics and degrees and directions of correlations with different aspects of disease. This complexity has so far limited application to the clinical setting, but of course also presents significant opportunity. Analysis of the serum inflammatory proteome, with simultaneous study of hundreds or thousands of markers, will eventually provide powerful personalized diagnostic tools. But to achieve this goal, we will need a much more sophisticated understanding of these many mediators. Furthermore, the study of serum alone will never provide a complete understanding of metabolic disease, because serum is simply a surrogate. To truly understand inflammation in obesity, we must go to the source of serum inflammatory markers and perhaps not surprisingly, when it comes to the pathogenesis of metabolic disease, adipose tissue is where the action is.
Adipose tissue inflammation- the heart of the matter
Inflammation in adipose tissue precedes the earliest signs of metabolic disease, correlates more closely with metabolic disease than serum markers and identifies patients with more severe disease (21–23). Over the past twenty years, we have learned that adipose tissue is much more than a storage depot for lipid, but rather elaborates hundreds of mediators that regulate virtually all aspects of physiology, including immunity and inflammation. The story of the modern understanding of the link between adipose tissue and inflammation begins in 1993 with the publication of a paper in the journal Science by Dr Bruce Spiegelman at Harvard University (24). Spiegelman studied expression of TNF-α, a classic inflammatory cytokine, in various tissues in mice. He found high levels of TNF-α in the spleen, an expected result since the spleen is rich in immune and inflammatory cells. He found virtually no TNF-α expression in other tissues, however, with one exception: adipose tissue from obese but not lean mice expressed high levels of TNF-α. Spiegelman went on to administer TNF-α neutralizing antibodies to obese diabetic mice and diabetes was markedly improved. These results were surprising - at the time, TNF-α was thought to function primarily as an inflammatory cytokine - the idea that such a molecule was expressed by adipose tissue and played a role in insulin resistance was a paradigm-shift. Indeed, this discovery led to further work by Spiegelman and others which demonstrated that, in addition to its inflammatory and immune functions, TNF-α regulated glucose and lipid homeostasis, energy expenditure, adipocyte proliferation, endocrine and reproductive functions, and a host of other metabolic processes in a wide range of cells and tissues.
Within a year of this discovery, Dr Jeffrey Friedman at the Rockefeller University published a paper in the journal Nature in which he described cloning the leptin gene and the central role of leptin in regulating body weight (25). This discovery ushered in the modern era of satiety research but, within a few years, it was found that, in addition to its role in regulating food intake, leptin had broad immunostimulatory and pro-inflammatory properties (26, 27). Again, this was a surprise - why did a molecule secreted by adipose tissue with a dominant role in regulating body weight also control immune function?
These discoveries led to an explosion of research in adipose tissue biology and the realization that adipose tissue is a rich source of many cytokines and adipokines, all of which manifest dual roles in regulating metabolism and immunity (Table 1). Cytokines and adipokines are phylogenetically closely related: leptin, for example, is a member of the same long-chain helical cytokine family that includes IL-6. Virtually every cytokine described has diverse effects on metabolic functions, in addition to their immunoregulatory roles, while virtually all adipokines regulate immune function as well as body weight and metabolism. Most of these proteins can be categorized as either pro-inflammatory and diabetogenic, with detrimental effects on metabolism (e.g. TNF-α, IL-6, leptin), or anti-inflammatory and insulinomimetic, with beneficial effects on metabolism (e.g. adiponectin, IL-10). In obesity, adipose tissue levels of the former are increased, while levels of the latter are decreased. This imbalance of inflammatory cytokines and adipokines is a dominant characteristic of adipose tissue inflammation.
Table 1. Adipose tissue cytokine/adipokine mediators.
A partial list of cytokines and adipokines expressed in adipose tissue with a simplified and incomplete description of effects on immunity and glucose homeostasis. Cytokines and adipokines are related, and share overlapping and diverse functions. Virtually all regulate inflammation and metabolism, including glucose, lipid and protein homeostasis, as well as endocrine, reproductive, and cognitive functions. Many mediators are expressed by both immune cells and adipocytes, and many are expressed in adipose and other tissues. Physiologic effects and disease associations listed are generalized- all mediators have diverse and context-dependent functions, and subtleties, conflicting data, and debate exist regarding the relationship of many mediators to obesity and metabolic disease.
| Mediator | Class | Source | Physiologic Effects | Disease association |
|---|---|---|---|---|
| TNF-α | Cytokine | Macrophages, other immune cells, adipocytes | Pro-inflammatory, diabetogenic | Increased in obesity, directly correlates with metabolic disease |
| Leptin | Adipokine | Adipocytes | Pro-inflammatory, diabetogenic | Increased in obesity, correlates with metabolic disease |
| IL-10 | Cytokine | Macrophages, other immune cells, adipocytes | Anti-inflammatory, insulinomimetic | Decreased in obesity, inversely correlates with metabolic disease |
| Adiponectin | Adipokine | Adipocytes | Anti-inflammatory, insulinomimetic | Decreased in obesity, inversely correlates with metabolic disease |
| IL-6 | Cytokine | Macrophages, other immune cells, adipocytes | Pro-inflammatory, diabetogenic | Increased in obesity, correlates with metabolic disease |
| IL-1 | Cytokine | Macrophages, other immune cells, | Pro-inflammatory, diabetogenic | Increased in obesity, correlates with metabolic disease |
| IFN-γ | Cytokine | T-cells, NK cells | Pro-inflammatory, diabetogenic | Unknown, possibly increased in obesity |
| Resistin | Adipokine | Macrophages, adipocytes | Pro-inflammatory, diabetogenic | Increased in obesity, correlates with metabolic disease |
| CCL2 | Adipokine, macrophage homing molecule | Adipocytes | Pro-inflammatory, diabetogenic | Unknown, likely increased in obesity |
What is the source of these mediators within adipose tissue and what generates cytokine imbalance? Adipocytes secrete adipokines such as leptin, as well as some cytokines, including TNF-α and IL-6. But over half of adipose tissue cells are non-adipocytes, the so-called stromovascular cell fraction (SVF), comprised of fibroblasts, endothelial cells, adipocyte precursors, and importantly immune leukocytes, which make up over half of the SVF and are a rich source of inflammatory cytokines. Within the SVF, macrophages, central cellular effectors of innate immune function, play a central role. Adipose tissue macrophages (ATM) comprise 10–20% of leukocytes within the SVF, and their numbers increase dramatically with increasing obesity, a finding that is reversed with weight loss (23, 28, 29). On microscopy, ATM wrap around adipocytes in “crown-like structures”, an intimate anatomic association that speaks to a complex functional relationship (Figure 1). TNF-α and leptin are paradigms for the many cytokines and adipokines that regulate macrophage-adipocyte interactions. Through expression of TNF-α and other pro-inflammatory and diabetogenic cytokines, ATM induce insulin resistance, altered lipid metabolism, and other detrimental effects in adipocytes (30, 31). Adipocytes in turn induce inflammatory responses in macrophages via expression of pro-inflammatory adipokines such as leptin (32–34). These interactions induce a vicious cycle that perpetuates inflammation and metabolic dysfunction. Macrophage-adipocyte interactions form the basis of the link between adipose tissue inflammation and metabolic dysfunction, and indeed are at the core of the link between metabolism and immunity (Figure 2). Underlying this relationship is a common phylogeny: macrophages and adipocytes derive from the same ancestral cell in our evolutionary past, a cell that carried out tasks of both immunity and metabolism, underscoring the intimate link between these physiologic systems (35, 36).
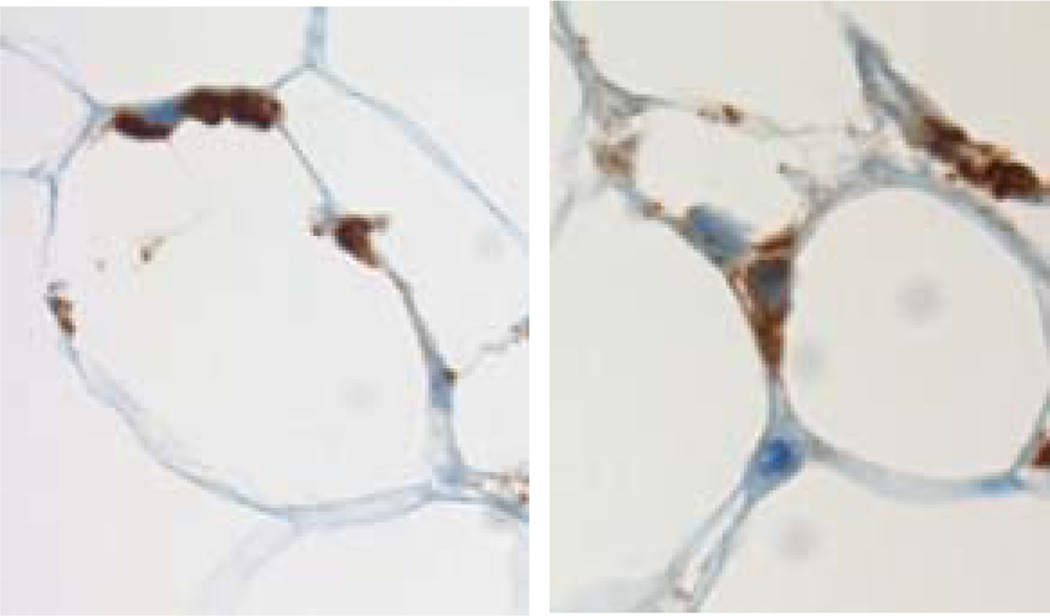
Figure 1. Macrophage-adipocyte anatomy.
Human adipose tissue macrophages, stained brown on immunohistochemistry with CD68 antibody, are found throughout adipose tissue, usually intimately associated with and often clustered around adipocytes.
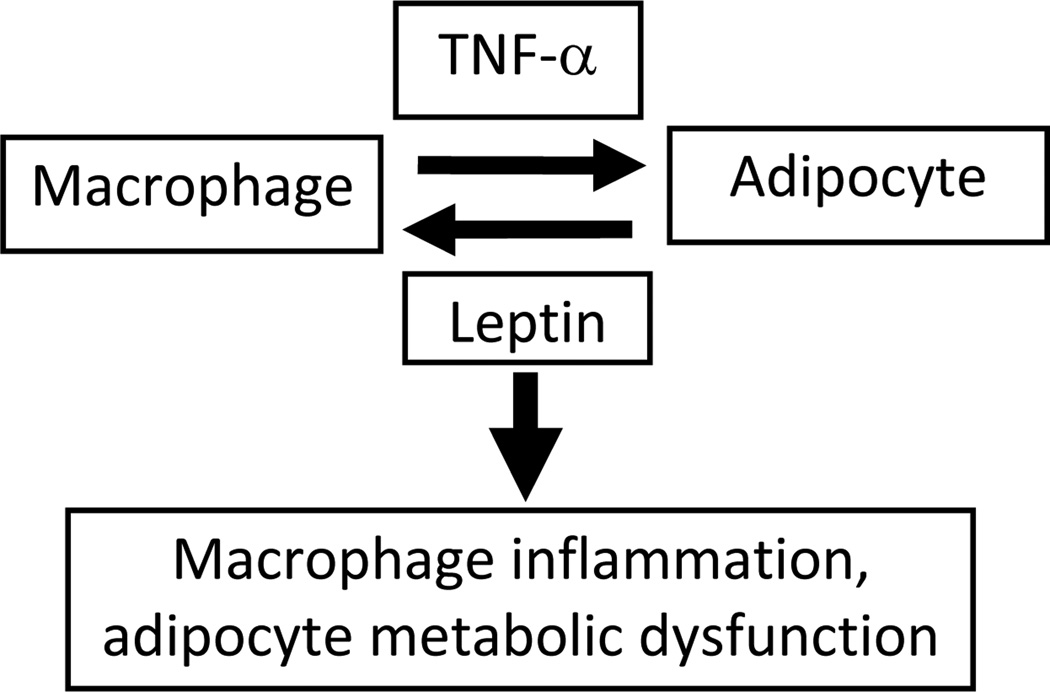
Figure 2. Macrophage-adipocyte interactions within adipose tissue.
Through expression of cytokines such as TNF-α, macrophages induce metabolic dysfunction in adipocytes, while through expression of pro-inflammatory adipokines such as leptin, adipocytes induce further inflammatory responses in macrophages. These complex interactions form the basis of a positive feedback loop which perpetuates inflammation and metabolic dysfunction in adipose tissue.
Early events in adipose tissue - root causes of inflammation and the basis of systemic metabolic disease
What initiates inflammation in adipose tissue and attracts macrophages? The answer lies in events that occur within adipose tissue in the early stages of obesity. One of the first responses of adipocytes to increased nutrient delivery in early obesity is hypertrophy: adipocytes grow as they store excess lipid. In fact, adipocytes are so well-designed to hypertrophy that they are among the few cell types that can reach diameters beyond 100 microns, the diffusion distance of oxygen. As adipocytes reach this 100 micron threshold, cellular oxygen diffusion is compromised, and adipocytes become hypoxic. This hypoxic state leads to adipocyte cell death, and macrophages are recruited to cleanup these dead and dying cells, a scavenging response that harkens back to our initial definition of inflammation. Much evidence exists for adipose tissue hypoxia in obesity, including direct measurement of tissue oxygen and demonstration of up-regulation of hypoxia-inducible genes (37, 38). Macrophages congregate in crown-like structures around hypoxic rather than healthy adipocytes. Adipocytes in obese humans are virtually all larger than 100 microns, while those from lean subjects are all below this size, and adipocyte diameter is a strong predictor of metabolic disease in humans (39, 40). Hypoxia is an early stimulus for inflammation in adipose tissue.
Hypoxia isn’t the only insult adipocytes experience in early obesity. The very reason adipocytes hypertrophy is that they are flooded with excess nutrients. Nutrient excess stresses all cells, including adipocytes, and cells express stress through a process called endoplasmic reticulum (ER) stress. The ER is a complex organelle present in all cells that is responsible for synthesizing all proteins, lipid, and RNA that make up the cell. To carry out these critical functions the ER depends on a constant, steady influx of nutrients. But the ER can be overwhelmed when nutrient delivery exceeds capacity. Adipocytes and their ER are especially well-designed to manage excess nutrients, but chronic nutrient excess in obesity overwhelms even the adipocyte ER, inducing ER stress. While a complex cellular process, a simplified but useful way to consider ER stress is that excess nutrients “leak out” of the overwhelmed adipocyte ER and induce damage to that cell and its neighbors. A dominant mechanism by which excess nutrients effect this damage is by directly triggering inflammation through activation of toll-like receptors (TLR), central immune pattern recognition receptors expressed by all innate immune cells, including macrophages. Free fatty acids are an important TLR ligand, binding TLR on macrophages and activating inflammatory responses (41). TLR are tools by which the immune system senses excess nutrients as a marker of increased cell damage and turnover and responds by scavenging damaged cells. TLR are thus a direct molecular link between inflammation and metabolism. Free fatty acids are not the only nutrients that activate inflammation; other nutrients and metabolites bind other pattern recognition receptors on macrophages to activate inflammation, including advanced glycation end-products, hexosamines, lipid metabolites including ceramide and diacylglycerol, and others.
This begs a question central to this discussion: why are nutrients toxic to cells? We think of nutrients as beneficial to health - why would evolution select for molecules in our diet that are toxic? And the answer is that the very properties that make nutrients efficient energy carriers also make them highly bioenergetic and capable of participating in energy-intensive and potentially damaging chemical reactions within cells. For this reason, cells carefully sequester and meter exposure to nutrients, and the ER is a critical tool that all cells use to accomplish this goal. Adipocytes are exquisitely well-designed to manage excess nutrients and sequester them, thus protecting other cells from nutrient excess. But as obesity progresses, adipocytes and their ER are overwhelmed. Initially ER stress and inflammation are confined to adipose tissue. But as nutrient excess persists, adipose tissue failure ensues, and nutrients, metabolites, and inflammatory mediators overflow from adipose tissue into the systemic circulation. Overflow from visceral adipose tissue affects the liver via direct communication through the portal circulation, and establishes the liver as a dominant secondary site of inflammation and metabolic dysfunction. Overflow progresses to affect all peripheral tissues, and processes initially confined to adipose tissue, including inflammation, ER stress, nutrient toxicity, and metabolic dysfunction, unfold in virtually all tissues. Cells in the liver, muscle, and vascular endothelium accumulate lipid much like adipocytes, but unlike adipocytes, are poorly equipped to tolerate lipotoxicity. Inflammation develops in peripheral tissues which accumulate an inflammatory macrophage infiltrate much like adipose tissue. As a result, metabolic dysfunction unfolds in peripheral tissue cells just as it initially does in adipocytes. These observations speak to an important property of adipose tissue. Far from being detrimental to health, adipose tissue serves a critical role in protecting other tissues from the toxic inflammatory effects of excess nutrients. This protective role is demonstrated in patients with lipodystrophy, who lack adipose tissue and develop severe metabolic disease, demonstrating that its absence is just as detrimental as its excess (42). Adipose tissue serves as a buffer for valuable but potentially toxic nutrients, and failure of this critical buffering capacity and resultant “overflow” or “metastasis” of inflammation and metabolic dysfunction from adipose tissue to other tissues underlies the pathogenesis of systemic metabolic disease (Figure 3).
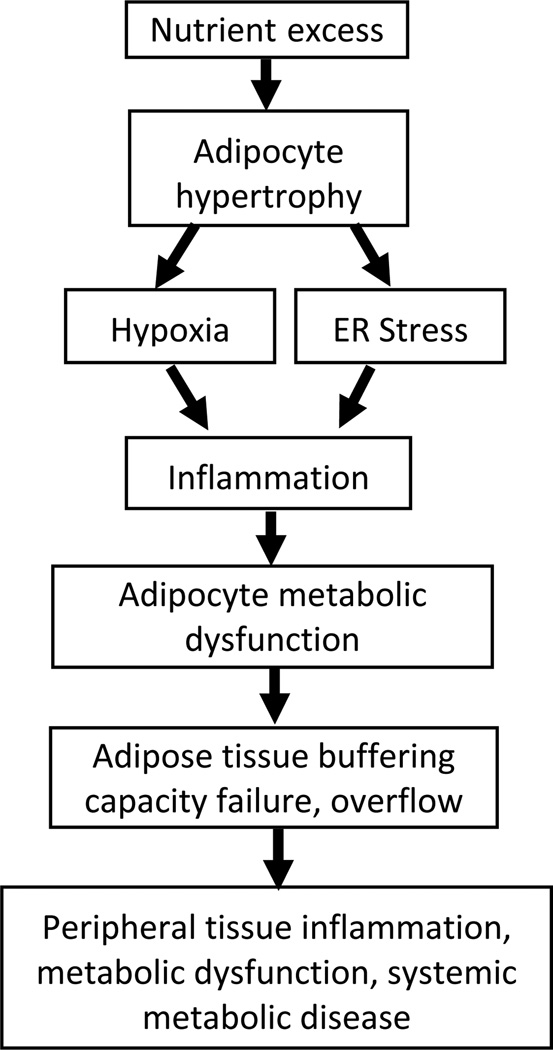
Figure 3. The pathogenesis of metabolic disease.
In a simplified schematic model, nutrient excess induces adipocyte hypertrophy, leading to hypoxia and ER stress, which in turn triggers adipocyte cell death and damage, and recruitment of an inflammatory response that includes adipose tissue macrophages. Inflammation and ER stress potentiate one another and create a vicious cycle. Inflammatory and cellular stress mediators induce adipocyte metabolic dysfunction. Initially this process is confined to adipose tissue, but as damage persists, adipose tissue buffering capacity is impaired, leading to systemic overflow. Nutrient excess, inflammation, and ER stress go on to affect peripheral tissues, and thus induce systemic metabolic disease.
Immunotherapy for metabolic disease
An understanding of adipose tissue dysfunction should lead to therapy for metabolic disease. An important line of research towards this goal is directed towards modifying the early events in adipose tissue that trigger inflammation. A recent study that reinforces the importance of adipose tissue as a buffer for nutrients was based on the observation that adipose tissue inflammation is accompanied by fibrosis, which is thought to limit adipocyte hypertrophy and lead to early overflow and systemic disease. After noting that collagen-VI was an important mediator of adipose tissue fibrosis, investigators studied collagen-VI knockout mice. These animals developed less severe metabolic disease later in the course of obesity, along with decreased adipose tissue fibrosis and increased adipocyte hypertrophy (43). These results underscore the importance of adipocyte buffering capacity in the pathogenesis of metabolic disease and suggest the potential for therapy directed towards increasing buffering capacity. Other research has been directed towards attenuating the early insults of hypoxia and ER stress on adipocytes. ER stress mediators are increased in humans with metabolic disease, and ER stress inhibitors ameliorate metabolic disease in mice (44, 45). Our laboratory has identified specific mediators of hypoxia-induced ER stress in human adipose tissue, including the stress activated kinase p38, inhibition of which abrogates inflammation in human adipose tissue (39). The goal of these and similar efforts is to engineer a “lean”, metabolically beneficial adipocyte, a cell with increased nutrient buffering capacity and increased ability to tolerate the insults of hypoxia and ER stress.
Macrophages and their inflammatory cytokine products are important therapeutic targets. Cytokine imbalance is a key feature of metabolic disease, and cytokine-specific monoclonal antibody therapy has a successful track record for autoimmune diseases such as rheumatoid arthritis and vasculitis, inflammatory bowel disease, cancer, and other inflammatory disorders. Despite Spiegelman’s initial observations in mice, trials of short courses of anti-TNF-α antibodies did not prove effective for human diabetes; recent data studying long-term treatment with these agents for rheumatoid arthritis in diabetic patients, however, demonstrate improvement in diabetes and have renewed interest in anti-TNF-α antibody therapy (46). Recent phase I trials in diabetic humans of antibodies directed towards IL-1 have also shown promising results (47).
Knockdown of molecules that mediate macrophage homing to adipose tissue prevents insulin resistance in obese mice (48, 49), testament to the central role of adipose tissue macrophages in the pathogenesis of diabetes. Human trials are in progress studying a macrophage homing molecule antagonist with early promising results (50). Targeting specific pathogenic macrophage subpopulations is another important line of research. Macrophages are quite heterogeneous, and the M1-M2 classification system is used to describe broad categories of pro-inflammatory, acute response cells, and anti-inflammatory, scavenging and remodeling cells. This classification scheme, while conceptually useful, is of course overly simplistic, as macrophages span a gamut of functional heterogeneity and are comprised of many distinct subpopulations. A pathogenic diabetogenic adipose tissue macrophage subpopulation specific to obesity and defined by expression of the cell adhesion molecule CD11c has been identified in mice and humans, and in vivo ablation of this CD11c+ macrophage subpopulation ameliorates diabetes in obese mice (51–53), suggesting the potential for therapy targeting pathogenic macrophage subpopulations. Other efforts are directed towards modifying macrophage phenotype and function, creating so-called “designer macrophages” (54).
Finally, adipose tissue inflammation is not all about macrophages: recent data implicate the adaptive immune system, and include aberrations in adipose tissue T-cells, B-cell, and NK cells (55). Observations by our laboratory that NK cells are increased in frequency in adipose tissue from obese humans led to transcriptional profiling that revealed, in addition to increased macrophage-related cytokines, increased expression of a non-macrophage cytokine, IFN-γ, a product of T-cells and NK cells, in visceral adipose tissue from obese humans. We went on to identify a small population of adipose tissue NK cells that constitutively express IFN-γ and found that IFN-γ induced a strong inflammatory cytokine response in human ATM (56). These observations led us to hypothesize that adipose tissue NK cells promote ATM inflammation via IFN-γ. Subsequent study of obese IFN-γ knockout mice revealed that these animals had decreased ATM and adipose tissue NK cells, decreased systemic inflammation and adipocyte size, and improved insulin sensitivity (57). Future experiments will develop methods to directly target IFN-γ or NK cells in wild-type diabetic animals. Other research targeting B-cells and regulatory T-cells has shown promise in animal studies (55, 58). The macrophage-adipocyte axis that forms the basis of adipose tissue inflammation and metabolic dysfunction is regulated by a complex cellular communication network.
Conclusion
Research in inflammation and obesity is in nascent stages. Most research directed towards therapy has focused on diabetes, and indeed, type II diabetes is a paradigmatic inflammatory disease responsive to anti-inflammatory treatment. Nonetheless, given the central role of inflammation in the pathogenesis of vascular disease, liver disease, allergic disease, and cancer, anti-inflammatory therapy should prove effective for these diseases as well. Of interest, the inflammatory disorders of obesity in many ways parallel those of the aging process, and metabolic disease can be considered in some ways similar to accelerated aging. Not coincidentally caloric restriction appears to reduce systemic inflammation, prevent metabolic disease, and prolong life; aging, energy balance, metabolism, and inflammation are intimately associated. An understanding of the mechanisms underlying this complex relationship will lead to novel immunotherapy effective for a wide spectrum of pathology with the potential to attenuate the aging process itself.
Acknowledgments
Dr O’Rourke is supported by grants K08DK074397 and R03DK095050 from NIH-NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr O’Rourke has no financial conflicts of interest to report.
References
- 1.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hünig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol. 2012;12(5):317–318. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- 3.Purnell JQ, Flum DR. Bariatric surgery and diabetes: who should be offered the option of remission? JAMA. 2009;301(15):1593–1595. doi: 10.1001/jama.2009.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254(22):3187–3189. [PubMed] [Google Scholar]
- 5.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT. California Pandemic (H1N1) Working Group. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52(3):301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 6.Williamson RT. On the Treatment of Glycosuria and Diabetes Mellitus with Sodium Salicylate. Br Med J. 1901;1(2100):760–762. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2011 Sep 22; doi: 10.1007/s00592-011-0329-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Ogston D, Mcandrew GM. Fibrinolysis in obesity. Lancet. 1964;2(7371):1205–1207. doi: 10.1016/s0140-6736(64)91042-6. [DOI] [PubMed] [Google Scholar]
- 9.Bulló M, García-Lorda P, Megias I, Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11(4):525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RB. Cytokine and Cytokine-Like Inflammation Markers, Endothelial Dysfunction, and Imbalanced Coagulation in Development of Diabetes and Its Complications. J Clin Endocrinol Metab. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Gallimore JR, Pepys MB, Campbell LV. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109(24):3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 12.Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, Stijnen T, Hofman A, Schram MT, Witteman JC. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 15.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 16.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59(9):1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 17.Selvin E, Paynter NP, Erlinger TP. The Effect of Weight Loss on C-Reactive Protein: A Systematic Review. Arch Int Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Tziomalos K, Dimitroula HV, Katsiki N, Savopoulos C, Hatzitolios AI. Effects of LifestyleMeasures, Antiobesity Agents, and Bariatric Surgery on SerologicalMarkers of Inflammation in Obese Patients. Med Inflamm. 2010;2010:364957. doi: 10.1155/2010/364957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31(9):2063–2069. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Festa A, D'Agostino R, Jr, Tracy RP, Haffner SM. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51(4):1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 21.Akagiri S, Naito Y, Ichikawa H, Mizushima K, Takagi T, Handa O, Kokura S, Yoshikawa T. A Mouse Model of Metabolic Syndrome; Increase in Visceral Adipose Tissue Precedes the Development of Fatty Liver and Insulin Resistance in High-Fat Diet-Fed Male KK/Ta Mice. J Clin Biochem Nutr. 2008;42(2):150–157. doi: 10.3164/jcbn.2008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, El Bekay R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J. 2010;430(1):141–149. doi: 10.1042/BJ20100285. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 26.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates pro-inflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- 27.Lord G, Matarese G, Howard J, Baker R, Blooms S, Lechler R. Leptin Modulates T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 28.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 29.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 30.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341(2):507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Spinas GA, Niessen M. ER Stress in Adipocytes Inhibits Insulin Signaling, Represses Lipolysis, and Alters the Secretion of Adipokines Without Inhibiting Glucose Transport. Horm Metab Res. 2010;42:643–651. doi: 10.1055/s-0030-1255034. [DOI] [PubMed] [Google Scholar]
- 32.Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J Biol Chem. 2010;285(14):10273–10280. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 35.Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278(11):9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 36.Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6(3):e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Varmalov O, Cheang EC, Corless CL, Roberts CT, Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54(6):1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003;149(2):129–135. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- 41.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 42.Huang-Doran I, Sleigh A, Rochford JJ, O'Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207(3):245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 43.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ursini F, Naty S, Grembiale RD. Infliximab and insulin resistance. Autoimmun Rev. 2010;9(8):536–539. doi: 10.1016/j.autrev.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1 receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 48.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 49.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan TJ, Dairaghi DJ, Krasinski A, Miao Z, Wang Y, Zhao BN, Baumgart T, Berahovich R, Ertl LS, Pennell A, Seitz L, Miao S, Ungashe S, Wei Z, Johnson D, Boring L, Tsou CL, Charo IF, Bekker P, Schall TJ, Jaen JC. Characterization of CCX140-B, an orally bioavailable antagonist of the CCR2 chemokine receptor, for the treatment of type 2 diabetes and associated complications. J Pharmacol Exp Ther. 2012 Feb 29; doi: 10.1124/jpet.111.190918. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-Positive Cells Normalizes Insulin Sensitivity in Obese Insulin Resistant Animals. Cell Metabolism. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lacy-Hulbert A, Moore KJ. Designer macrophages: oxidative metabolism fuels inflammation repair. Cell Metab. 2006;4(1):7–8. doi: 10.1016/j.cmet.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin I, Jobe BA, Slifka MK, Roberts CT, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN–γ in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33(9):978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Rourke RW, White AE, Metcalf MD, Winters B, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin resistance in obese IFN-γ knockout mice. Metabolism. 2012 Mar 2; doi: 10.1016/j.metabol.2012.01.018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]