Abstract
The salivary proteome consists of thousands of proteins, which include, among others, hormonal modulators of energy intake and output. Although the functions of this prominent category of hormones in whole body energy metabolism are well characterized, their functions in the oral cavity, whether as a salivary component, or when expressed in taste cells, are less studied and poorly understood. The respective receptors for the majority of salivary metabolic hormones have been also shown to be expressed in salivary glands, taste cells, or other cells in the oral mucosa. This review provides a comprehensive account of the gastrointestinal hormones, adipokines, and neuropeptides identified in saliva, salivary glands, or lingual epithelium, as well as their respective cognate receptors expressed in the oral cavity. Surprisingly, few functions are assigned to salivary metabolic hormones, and these functions are mostly associated with the modulation of taste perception. Because of the well-characterized correlation between impaired oral nutrient sensing and increased energy intake and body mass index, a conceptually provocative point of view is introduced, whereupon it is argued that targeted changes in the composition of saliva could affect whole body metabolism in response to the activation of cognate receptors expressed locally in the oral mucosa.
Introduction
It would be misleading to describe the Nobel Prize in physiology awarded to Ivan Pavlov in 1904 as the recognition of research of saliva’s composition and functions. Yet it is apparently due to Pavlov’s inadvertent influence that saliva attained a sovereign status of a bodily fluid worthy of scientific exploration. More than a century later, the progress in this area is unmistakable and the functions, composition, and utility of this once obscure fluid are now well studied and defined.
Salivary secretions derive from three pairs of major glands in the oral cavity - parotid, submandibular, and sublingual, as well as from hundreds of minor accessory glands in the tongue, palate and buccal mucosae. The flow of saliva is increased during a meal and it is utilized to form a moisturized bolus of foodstuff thus soaking the masticated food in salivary enzymes to increase its flavor perception, initiate the digestion, and promote swallowing. The digestive functions of saliva are supplemented by its protective functions to defend against bacterial and viral antigens with the help of the salivary antibody, secretory immunoglobulin A, as well as enzymes such as lactoferrin, sialoperoxidase, lysozyme, and histatins (Amerongen & Veerman, 2002). Moreover, salivary proteins form a protective coating layer on the teeth’s surface slowing down erosion and demineralization of the teeth’s enamel. Other salivary proteins (e.g. cytokines, fibroblast growth factor) mediate oral mucosal cell proliferation and differentiation; while interleukins and vascular endothelial growth factor contribute to oral mucosal inflammation and wound healing. With the salivary proteome including thousands of nonredundant proteins identified (Loo et al, 2010), it’s clear that their functions are diverse and many, as is reflected in the significant body of original work, as well as that summarized in several recent outstanding review articles. Some of these resources are listed below and the reader is encouraged to refer to them especially on the subjects of the functions of saliva (Aps & Martens, 2005; Groschl, 2009; Salles et al, 2011); the composition of the salivary proteome (Groschl, 2008; Loo et al, 2010; Yan et al, 2009); and diagnostics values of saliva (Malamud, 2011; Spielmann & Wong, 2011) (Schapher et al, 2011), (Pfaffe et al, 2011; Spielmann & Wong, 2011). The subject of the current review, on the other hand, is less explored and it is related to a small but prominent category of salivary peptides – hormonal modulators of energy intake and output, hereafter referred to as metabolic hormones. Moreover, the subject matter is approached from a therapeutic rather than a diagnostic perspective, a conceptually provocative point of view, whereupon it is argued that targeted changes in the composition of saliva could affect whole body metabolism in response to the activation of cognate receptors expressed locally in the oral mucosae rather than parenterally.
In general, the main rationale for advancing research related to saliva was attributed to its potential to be utilized as an alternative fluid for the diagnosis of various diseases, which, unlike blood, can be collected in non-invasive fashion. When considering pharmaceutical delivery, the advances in the oral application of drugs have been relatively slow and in no way related to intentionally attempting to change the composition of saliva. Orally applied drugs, by default, are swallowed and absorbed through the GI tract, unless the active compound was targeted for an oral trans-mucosal systemic delivery (e.g. glyceryl trinitrate), or topical drug applications for the treatment of diseases of the oropharynx. Using permeability enhancers to deliver hormones via oral mucosa (Fazen et al, 2011; Oh & Ritschel, 1990) served the same purpose of augmenting their concentration in the systemic circulation without any consideration of their possible interactions with orally expressed receptors. This review, on the other hand, will analyze the degree and extent of expression of metabolic hormones present both in plasma and saliva, as well as that of their cognate receptors in the oral mucosa, while addressing the possibility of their functional interaction and potential therapeutic applications.
Origins of salivary metabolic hormones
1. Salivary secretion overview
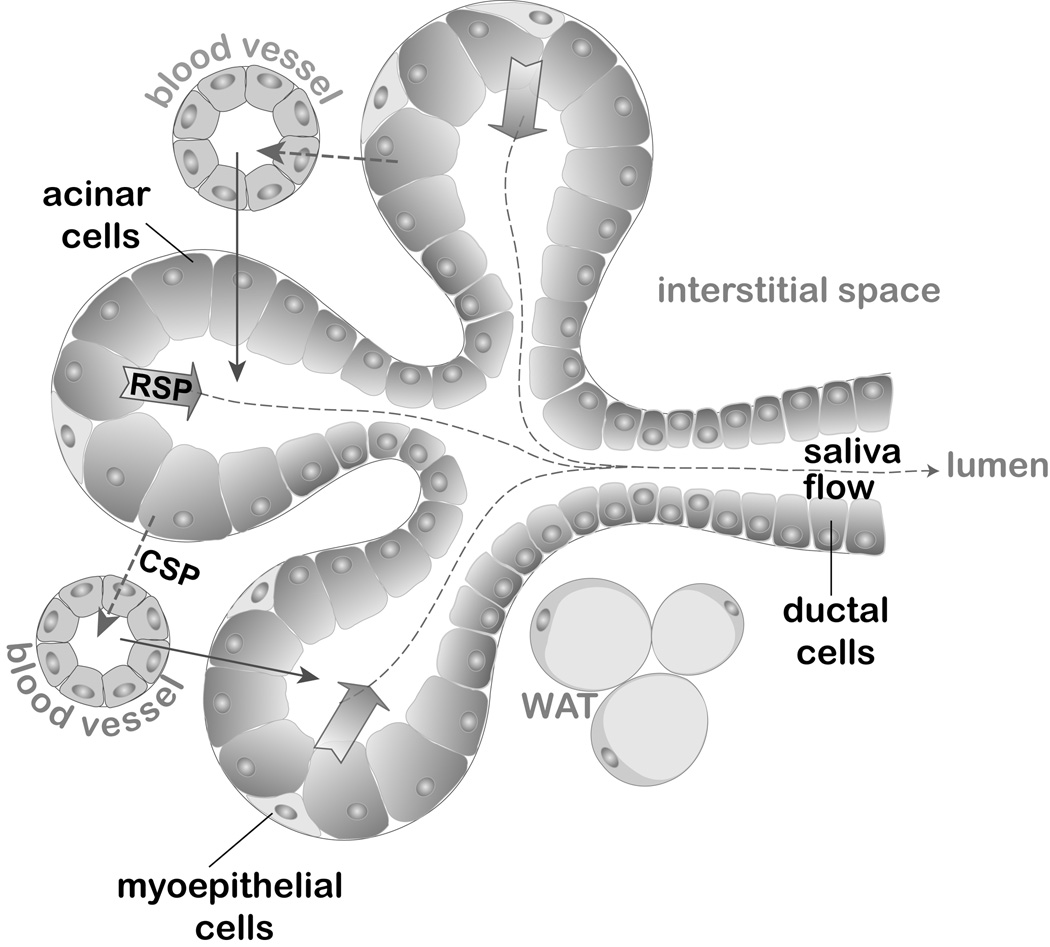
Salivary peptides and proteins derive from many sources including salivary glands (SGs), gingival crevicular fluid (GCF), and transudate from plasma (Fig. 1). There are three major types of secretory cells in salivary glands: acinar mucous, acinar serous, and ductal cells. Mucous cells, mainly residing in sublingual glands, produce a mucin-enriched secretion, while the secretion from serous cells in parotid gland acini is watery and less viscous. Acinar cells, as well as ductal cells, secrete their peptides predominantly into the luminal space using regulated secretory pathways (RSP) (Baum et al, 1999). The secretion is triggered by the neurotransmitters released by sympathetic (norepinephrine) or parasympathetic (acetylcholine, ACh) innervation of salivary glands. ACh, along with vasoactive intestinal peptide, also modulates the volume of fluid flow from glandular cells in response to food intake. In addition to saliva, acinar cells secrete some of the synthesized proteins and hormones into the circulating plasma. This endocrine, serosal mode of secretion utilizes constitutive secretory pathways (CSP) via the basolateral membrane (Isenman et al, 1999), reviewed in (Perez et al, 2010).
Fig. 1. Schematic representation of a submandibular salivary gland.
Acinar cells exhibit a predominant exocrine regulated secretory pathway (RSP) across the apical membrane and into the luminal space accumulating in saliva (depicted by block arrows). The endocrine constitutive secretory pathway (CSP) from acinar cells delivers peptides across the basolateral membrane into the interstitial space and into the bloodstream (depicted by dashed line arrows). The transport of peptides from plasma into the saliva includes both transcellular and paracellular mechanisms of passive diffusion, active transport and ultrafiltration (collectively depicted by uninterrupted line arrows). White adipose tissue (WAT) is shown within the interstitium as a possible source of salivary leptin and adiponectin.
As much as 27% of proteins present in the saliva derive from blood (Miller et al, 2010) entering either through gingival crevicular fluid by diffusion and filtration, or by active transport mechanisms. In fact, some consider saliva to be a “mirror to the body” (Schipper et al, 2007a) although, admittedly, a somewhat distorting mirror. The composition of the saliva is very dynamic depending on many physiological factors including flow rate, circadian rhythm, type and size of the salivary gland, duration and type of the stimulus, diet, age, gender, blood type, and physiological status (Schipper et al, 2007b). The latter variable depends on fasting vs. postprandial states, especially as it relates to fluctuating concentrations of salivary metabolic hormones that might correlate with the respective changes in plasma. It does not appear to be a byproduct of their presence in plasma that most of these hormones are also present in saliva, given that their cognate receptors are expressed in many cell types present in the oral mucosae. Moreover, these metabolic hormones appear to exhibit endocrine, or paracrine modes of action depending on their systemic or local origins. The prime examples of such diversity are hormones (e.g. leptin, insulin, GLP-1) whose main functions are to maintain whole body energy homeostasis and metabolism when circulating in blood, while, at the same time, modulating taste perception locally in the oral cavity. The latter function is associated with hormones and their cognate receptors expressed in the taste receptor cells (TRCs) in the taste buds. The following section describes the basic principles of the gustatory system structure and functions, as it is relevant to the subject of hormonal modulation of taste perception.
2. Gustatory system overview
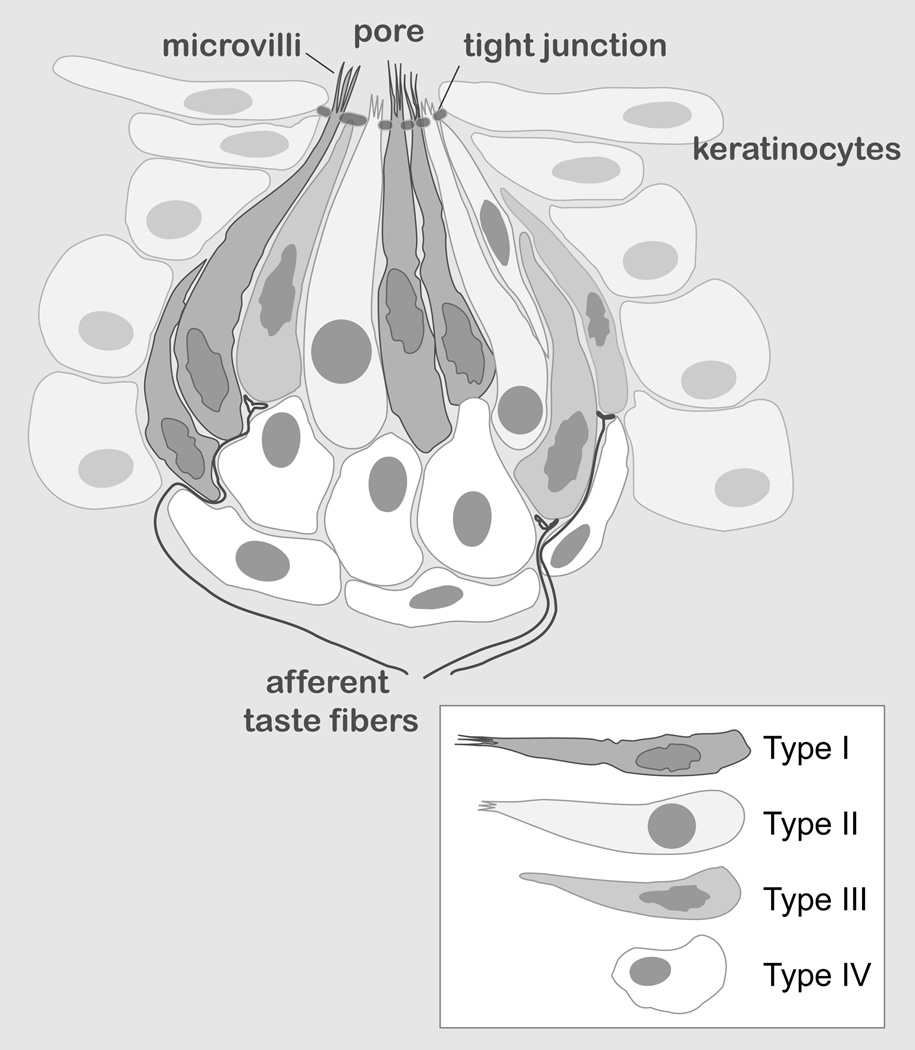
Ingestive behaviors are critical since they are required for survival, and an animal’s ability to “taste” chemical compounds is imperative for the evaluation of food quality. It’s been shown that saliva plays an essential role in taste perception and contributes to sensory stimulation (Pedersen et al, 2002). There are five basic taste modalities: sweet, bitter, sour, salty, umami (savory), and one putative sense modality - lipid sensing. Tastant detection begins in TRCs, elongated neuroepithelial cells which contain specific taste receptors. TRCs are organized in specialized structures, taste buds, which reside within three types of lingual gustatory papillae: fungiform (dorsal anterior surface), foliate (lateral posterior surface), and circumvallate (CV) (dorsal posterior border). Based on ultrastructural characteristics, TRCs are classified into three different types (Fig. 2): Type I cells, also known as glial-like cells, display a dark cytoplasm and indented irregular shaped nuclei. Type II cells, expressing chemosensory transduction proteins, have a light cytoplasm and large round nucleus. Type III cells, also known as presynaptic cells, have similar nuclei as Type I cells and a cytoplasm that is intermediate (i.e., lighter than in dark cells). Presynaptic cells form synapses with primary sensory afferent terminals, and the Type II cells can also communicate with nerve fibers via non-traditional synaptic signaling (Finger et al, 2005; Huang et al, 2007). Finally, cells located at the baso-lateral part of the taste bud are believed to be progenitor cells and sometimes referred to as Type IV cells.
Fig. 2. Schematic representation of a taste bud.
The diagram shows an onion-like taste bud structure embedded into the lingual epithelia. Taste receptor cell (TRCs) types are shown in the inset.
Once food is taken into the oral cavity, taste receptors relay a signal to the brainstem and higher centers in the central nervous system (CNS) through the gustatory nerve fibers. There is evidence pointing towards the fact that taste function and perception can be modulated by salivary compounds and hormones interacting with their respective receptors expressed in the lingual TRCs. The following sections provide a comprehensive account of the expression of salivary metabolic hormones and receptors, their functions in oral mucosal maintenance, as well as their putative roles in the modulation of gustatory signal transduction and energy metabolism. The hormones are arbitrarily assigned to one of several broad-range categories based on the origin of their expression or family identity. Because most of the hormones are expressed in several tissues/organs, the category assignments are subjective.
Gastrointestinal (GI) hormones
1. Insulin
The hormone insulin, produced by pancreatic β-cells of the islets of Langerhans, is the main hormone controlling carbohydrate and lipid metabolism. Insulin-like immunoreactivity has been extensively reported in rodent salivary glands, human parotid (Murakami et al, 1982) and submandibular salivary glands (Shubnikova et al, 1984), and in human saliva (Fekete et al, 1993; Marchetti et al, 1986; Marchetti et al, 1990). Fasting salivary insulin levels are lower in saliva than plasma, both increased following food intake (FI) but the rise in saliva was slower and less marked than in plasma (Messenger et al, 2003). In addition, salivary insulin remains unchanged following a sham-fed meal. These findings are consistent with insulin in saliva being an ultrafiltrate of that circulating in blood. The observation that salivary insulin has been shown to increase following an i.v. injection of insulin in humans (Vallejo et al, 1984), supports this hypothesis. On the other hand, both immunoreactive insulin and insulin mRNA have been found in the salivary glands of mice (Kerr et al, 1995) and rats (Taouis et al, 1995), and immunoreactive insulin secretion from mouse salivary glands is sensitive to changes in glucose concentrations (Shubnikova et al, 1984). It is therefore possible that the insulin found in saliva could be the product of local synthesis.
Is there a specific function that is attributed to salivary insulin? This question could be answered by characterizing the expression of its cognate receptors. Indeed, the expression of insulin receptor, insulin receptor substrate 1 and insulin receptor substrate 2 had been shown in murine TRCs (Baquero & Gilbertson, 2011). In behavioral tests, this group documented that insulin-treated acute hyperinsulinemic mice exhibited significant avoidance of NaCl at lower concentrations than the control group, suggesting that salivary insulin enhanced salty taste modality. Interestingly, these differences between groups were abolished when the drug amiloride was added to NaCl solutions, indicating that insulin was acting through the apical amiloride-sensitive epithelial sodium channel via PI3-kinase-mediated pathways and its phospholipid products (Baquero & Gilbertson, 2011).
2. Glucagon
The main function of the hormone glucagon, produced by pancreatic α-cells of the islets of Langerhans, is to increase blood glucose levels. Glucagon-like immunoreactivity has been previously reported in rat and human salivary glands (Bhathena et al, 1977; De Matteis et al, 2002; Lawrence et al, 1976; Lawrence et al, 1977; Perez-Castillo & Blazquez, 1980; Smith et al, 1979) and preproglucagon mRNA was shown to be expressed in adult rat submandibular glands (Egea et al, 2003). Although no specific function had been ascribed to glandular-expressed hormone, Elson et al demonstrated that glucagon enhances sweet taste responsiveness through local actions in the mouse gustatory epithelium (Elson et al, 2010). Previously, this group reported that both glucagon and the enzyme that processes the mature glucagon peptide from proglucagon, proprotein convertase 2 (PC2), are expressed in subsets of TRCs (Shin et al, 2008). The glucagon receptor and peptide 7B2 (an obligatory PC2 chaperone required for the production of mature glucagon and encoded by the Scg5 gene) were also shown to be expressed in mouse circumvallate papillae (Elson et al, 2010). These results indicate that key molecules required for the production and reception of glucagon signals are present in the TRCs of mouse gustatory epithelium. Most of these cells also express the T1R3 taste receptor implicated in sweet and/or umami taste. Genetic or pharmacological disruption of glucagon signaling in behavioral studies of mice indicated a critical role for glucagon in the modulation of taste responsiveness. Scg5-/- mice, which lack mature glucagon, had significantly reduced responsiveness to sucrose as compared to wild-type littermates in brief-access taste tests. Together, these data indicate a role for local glucagon signaling in the peripheral modulation of sweet taste responsiveness (Elson et al, 2010).
3. GLP-1
The gut hormone GLP-1 is produced in intestinal L cells. Because GLP-1 and glucagon are both derived from proglucagon, attempts had been made to detect GLP-1 in saliva. However, no GLP-1-like immunoreactivity could be detected in saliva samples from fasting human patients, or following a meal (Messenger et al, 2003). In the gustatory system, however, it was found that GLP-1 is produced in two distinct subsets of murine taste cells, while the GLP-1 receptor (GLP-1R) is expressed on adjacent intragemmal afferent nerve fibers (Shin et al, 2008). Moreover, GLP-1-positive taste cells express prohormone convertase 1/3, the enzyme necessary for the formation of GLP-1 by cleavage from proglucagon, and secrete a bioactive form of GLP-1. GLP-1R knockout (KO) mice showed reduced taste responses to sweeteners in behavioral assays, indicating that GLP-1 signaling normally acted to maintain or enhance sweet taste sensitivity. A modest increase in citric acid taste sensitivity in these KO mice suggested GLP-1 signaling may modulate sour taste, as well. In addition, GLP-1R KO mice exhibit an enhanced sensitivity to umami-tasting stimuli (Martin et al, 2009). Together, these findings suggest a novel paracrine mechanism for the regulation of taste function based on a locally produced effector acting on a receptor expressed in the juxtaposing dendrites.
The ability of GLP-1 to stimulate insulin secretion in a glucose-dependent manner makes it an attractive potential therapy for Type 2 diabetes mellitus. Reviewing the ongoing clinical trials for this indication (i.e., using protein replacement therapy with recombinant GLP-1, or its analogues such as Exendin-4), is beyond the scope of the current review. However, one nonconventional approach deserves attention: as a proof-of-principle for the SG-mediated treatment of diabetes, Voutetakis et al have recently shown that transduction of mouse SGs with a recombinant adenovirus encoding a GLP-1 peptide is able to delay the onset of Type 1 diabetes mellitus in mice (Voutetakis et al, 2010). The adenovirus used in these studies (Ad-GLP-1) contained a modified human GLP-1 cDNA sequence encoding the active GLP-1(7–37) peptide with an Ala to Gly substitution at position eight to confer resistance to dipeptidyl peptidase (DPP-IV), a catabolic enzyme that limits GLP-1’s half-life. This construct was demonstrated to produce a bioactive GLP-1 peptide that was resistant to DPP-IV degradation and was able to stimulate insulin secretion from pancreatic β-cells in vitro. Ad-GLP-1 was then delivered to the submandibular glands (SMGs) of intact mice by retroductal instillation to determine the route of GLP-1 peptide secretion and the capacity for bioactivity in vivo. GLP-1 expressed by the SMGs was detected in the serum of these mice and was able to rapidly reduce serum glucose levels in a glucose tolerance test when compared to animals treated with a control (Ad-Luc) adenovirus. Furthermore, in keeping with the glucose-dependent mechanism of GLP-1 action, the blood glucose levels of fasted animals treated with Ad-GLP-1 were indistinguishable from those treated with Ad-Luc (Voutetakis et al, 2010).
4. GIP
GIP, along with GLP-1, belongs to the incretin family of hormones and is produced by K cells in the gut. The main function of GIP is to inhibit GI motility and the secretion of gastric acid. The GI hormone GIP has also been detected in rodent salivary glands and saliva (Tseng et al, 1993; Tseng et al, 1994). Fasting GIP levels were significantly higher (9-fold) in saliva than plasma (Messenger et al, 2003). They decreased in saliva following both swallowed and sham-fed meals, but thereafter rose following the swallowed meal to peak levels. These fluctuations suggest that salivary GIP derives from local SG synthesis, whose secretion is influenced, directly or indirectly, by oral stimuli. The function of salivary GIP is unknown, but it was suggested that it may play a role in the regulation of gastric acid secretion in the fasting state (Messenger et al, 2003).
5. CCK
The hormone CCK is produced by L-cells in the small intestine and its secretion in the duodenum induces the release of enzymes from the pancreas and bile from the gallbladder. Studies from the Herness group (Herness et al, 2002; Shen et al, 2005) presented novel data demonstrating that CCK is expressed in a subset of TRCs, and that it may play a signaling role unknown previously within the taste bud. Immunocytochemistry revealed positively stained subsets of cells within taste buds throughout the oral cavity. Multiple physiological actions of CCK on TRCs were observed. An outward potassium current, recorded with the patch-clamp technique, was inhibited by exogenous application of sulfated CCK octapeptide in a reversible and concentration-dependent manner. Pharmacological analysis suggested that this inhibition is mediated by CCK-A receptors and involved protein kinase C phosphorylation. An inwardly rectifying potassium current, typically invariant to stimulation, was also inhibited by CCK. Additionally, exogenous CCK was effective in elevating intracellular calcium as measured by ratiometric techniques. Pharmacology similarly demonstrated that these calcium elevations were mediated by CCK-A receptors and were dependent on intracellular calcium stores. Collectively, these observations suggest a newly discovered role for peptide neuromodulation in the peripheral processing of taste information. In addition, another set of data indicated that TRCs expressing CCK-receptors also expressed receptors to bitter stimuli and/or muscarinic receptors suggesting that CCK might modulate bitter taste modality by acting as an autocrine agent to potentiate the excitatory actions of tastants on taste receptor cells (Herness & Zhao, 2009; Lu et al, 2003).
6. VIP
VIP is a gut hormone that modulates blood pressure, induces the relaxation of the GI tract and inhibits gastric acid secretion. VIP expression has been identified in the taste cells of the rat, hamster, and carp, as well as in humans (Herness, 1989; Kusakabe et al, 1998; Witt, 1995). In TRCs, VIP colocalizes with the taste transduction markers α-gustducin and T1R2 (Shen et al, 2005). More recently, both VIP receptors (VPAC1/2) were shown to be primarily localized to Type II TRCs, co-expressing with the VIP-encoding gene (Martin et al, 2010). The presence of VIP and its cognate receptors in the same cells suggests that the local VIP signaling within the taste bud may be autocrine in nature that it is likely affecting tongue-localized gustation. VIP KO mice demonstrated normal gross taste bud morphology; however, they exhibited significant increases in GLP-1R and decreases in leptin receptor (ObRb) expression in taste cells. In behavioral studies, VIP KOs demonstrated a greater preference for sweet compounds compared with wild-type mice (Martin et al, 2010). VIP KO mice also exhibited concentration-dependent changes in licking bitter stimuli and sour stimuli, but no change in salt perception, compared with wild-type mice.
7. Ghrelin
The orexigenic hormone ghrelin, a 28-amino acid acylated peptide, is produced predominantly in the stomachs of humans and rodents. Ghrelin plays a major role in the GI tract, stimulating gastric contractility and acid secretion. It is also responsible for the metabolic response to starvation by modulating insulin secretion, glucose metabolism, and amino acid uptake. Ghrelin is the ligand to the G-protein–coupled growth hormone secretagogue receptor (GHS-R) which is expressed in the brain and in various peripheral tissues in two isoforms, GHS-R 1a and 1b. Recently, Gröschl et al showed that ghrelin and both receptor isoforms are produced by the human salivary glands (Groschl et al, 2005a). While the concentrations of salivary ghrelin were lower than those in serum, there was a significant correlation between both body fluids suggesting transport from the blood vessels into the glandular cells. On the other hand, two other groups reported higher concentrations of ghrelin in human saliva compared with the plasma (Aydin et al, 2005; Ohta et al, 2011). Even more intriguing, the concentration of ghrelin found in GCF was approximately 500-fold higher than that detected in saliva (Ohta et al, 2011). Other studies documented an autonomous production of ghrelin by the salivary glands, in oral keratinocytes, and gingival fibroblasts (Benedix et al, 2011a; Benedix et al, 2011b; Groschl et al, 2005a; Li et al, 2011), as well as the expression of both cognate receptors in oral epithelial cells and fibroblasts (Ohta et al, 2011).
In gustatory tissues, ghrelin and the prepro-ghrelin cleaving enzyme prohormone convertase, 1/3, its cognate receptor (GHSR), and ghrelin-O-acyltransferase (the enzyme that activates ghrelin) were shown to be expressed in Type I, II, III and IV taste cells of mouse taste buds (Shin et al, 2010). In addition, ghrelin and GHSR colocalized in the same taste cells, suggesting that ghrelin works in an autocrine manner in taste cells. To determine a role for ghrelin in modifying taste perception, the authors performed taste behavioral tests using GHSR null mice. GHSR null mice exhibited significantly reduced taste responsivity to sour (citric acid) and salty (sodium chloride) tastants (Shin et al, 2010). These findings suggest that ghrelin plays a local modulatory role in determining taste bud signaling and function suggesting a novel mechanism for the modulation of salty and sour tastes modalities.
8. Obestatin
Obestatin is another hormone encoded by the ghrelin gene (Zhang et al, 2005). Contrary to the appetite-stimulating effects of ghrelin, treatment of rats with obestatin suppressed food intake, inhibited jejunal contraction, and decreased body weight gain. Obestatin binds to the G protein-coupled receptor GPR39. Immunohistochemical analysis demonstrated that obestatin was localized in the striated and excretory duct of human SGs (Ozbay et al, 2008). As expected, obestatin, like ghrelin, was also detected in saliva. Salivary ghrelin and obestatin levels are correlated with the blood levels in patients, and both were higher in epilepsy patients (Dag et al, 2010).
Adipokines
1. Leptin
The anorexigenic hormone leptin, released mostly from adipocytes, has been found to inhibit food intake (FI) and to increase energy expenditure via interacting with the signal-transducing long form of Ob-Rb. receptor. Similar to other metabolic hormones, leptin is also produced, stored, and secreted by the SGs and is expressed in oral mucosa (De Matteis et al, 2002; Groschl et al, 2001; Randeva et al, 2003), while Ob-Rb is expressed in the granular convoluted tubular cells within intralobular ducts of human SMG (De Matteis et al, 2002) and in keratinocytes (Groschl et al, 2005b). Leptin concentrations were significantly higher in plasma than saliva (Randeva et al, 2003), and there is a strong linear correlation between leptin concentrations from simultaneously collected saliva and plasma samples (Groschl et al, 2001; Randeva et al, 2003) suggesting an existence of active transport from blood vessels. Salivary leptin, like plasma leptin, showed circadian variations in both men and women, with a peak around 2400 h and a nadir at 1000 h (Randeva et al, 2003). Some studies indicate that leptin may also modulate sweet taste sensation in humans with a diurnal variation in sweet sensitivity. This leptin modulation of sweet taste information to the brain may influence individuals’ preference and ingestive behavior, thereby playing important roles in regulation of energy homeostasis (Sanematsu et al, 2009). Interestingly, a novel physiological role of salivary leptin as a growth factor for keratinocyte proliferation in the oral cavity has been also documented (Groschl et al, 2005b). Apparently, this function is similar to the proliferative function of the salivary ghrelin (Groschl et al, 2005a).
The expression of Ob-Rb had been shown in taste cells of CV and fungiform papillae in BALB mice (Kawai et al, 2000; Shigemura et al, 2004). Administration of leptin into lean mice suppressed responses of peripheral taste nerves (chorda tympani and glossopharyngeal) to sweet substances (sucrose and saccharin) without affecting responses to sour, salty, and bitter substances. Whole-cell patch-clamp recordings of activities of TRCs isolated from circumvallate papillae (innervated by the glossopharyngeal nerve) demonstrated that leptin activated outward K+ currents, which resulted in hyperpolarization of taste cells, thus suppressing taste cell responses and inhibiting afferent signals, which evoked sweet taste (Shigemura et al, 2004). The db/db mouse with impaired leptin receptors showed no such leptin-mediated suppression. The blunting of sweet taste leads to a decrease in consumption of sweet solutions in mice. These observations suggest that the taste organ is a peripheral target for leptin, and that leptin may be a sweet-sensing modulator (suppressor) that may take part in regulation of FI.
2. Adiponectin
Adiponectin (Acrp30) is a physiologically active polypeptide hormone secreted by adipose tissue that shows insulin-sensitizing, anti-inflammatory, and antiatherogenic properties. In humans, its levels are inversely related to the degree of adiposity. Adiponectin binds to two distant members of G protein-coupled receptor (GPCR) family of receptors, AdipoR1 and AdipoR2. The expression of adiponectin was investigated in minor salivary gland biopsy specimens obtained from patients with Sjogren’s syndrome (SS) and healthy adult subjects (Katsiougiannis et al, 2006). Immunohistochemical analysis for adiponectin revealed positive staining of adipocytes from primary SS lesions as well as ductal epithelial cells from both patients with primary SS and healthy subjects. In vitro, all cell lines tested were shown to express adiponectin, AdipoR1, and AdipoR2 mRNA. Adiponectin was also detected in human saliva, and its levels were significantly correlated with levels found in plasma (Mamali et al, 2012; Toda & Morimoto, 2008; Toda et al, 2007). Interestingly, salivary adiponectin appears to be present in multimeric forms, suggesting potential functional significance (Akuailou et al, 2012).
Neuropeptides
1. Galanin
The neuropeptide galanin is widely distributed in the CNS and peripheral nervous system and is expressed in many regions of the brain. Galanin is engaged in the regulation of FI, memory, neuroendocrine functions, gut secretion and motility. Galanin mediates its effects through the activation of three GPCR subtypes: GalR1, GalR2, and GalR3. In the gustatory epithelia of the rat, the expression of galanin and its cognate receptor had been examined using RT-PCR, immunohistochemistry, and in situ hybridization (Seta et al, 2006). Analysis showed the expression of galanin in Type II and Type III cells, and GalR2 in some not yet characterized cells in the taste buds of the CV papillae suggesting that galanin may play a role in taste perception.
2. OXT
The hormone OXT, known for its role in sexual reproduction, is produced by magnocellular neurons in the posterior pituitary gland. It’s also released in areas of the brain stem and hypothalamus whereupon it modulates feeding behavior. OXT acts through the activation of its receptor, the GPCR, OXTR, expressed primarily in myoepithelial cells of the mammary gland, uterine smooth muscle, and in CNS neurons. In mice, the expression of OXTR in taste buds throughout the oral cavity, but not in adjacent non-taste lingual epithelium has been shown by Sinclair et al (Sinclair et al, 2010). The OXTR is expressed in a subset of Glial-like (Type I) taste cells, and also in cells on the periphery of taste buds. At the same time, the OXT peptide is not produced in taste buds or in their associated nerves, and, is most likely, delivered to target cells through the circulation. The latter notion is consistent with the reports describing the presence of OXT in human saliva (Carter et al, 2007; White-Traut et al, 2009). OXT KO mice demonstrated a sweet preference phenotype, consuming solutions enriched in artificial sweeteners (Billings et al, 2006) or carbohydrates (Amico et al, 2005; Sclafani et al, 2007) while showing no preference for isocaloric lipid emulsions (Miedlar et al, 2007). Cumulatively, these data indicate that OXT may modulate sweet taste modality.
3. Nesfatin-1
Nesfatin-1, a proteolytic product of nucleobindin2, is a recently discovered peptide hormone and it is known to be involved in appetite-control functions including insulin sensitivity. It is produced in the nucleus of the solitary tract, and the hypothalamus where it colocalized with OXT and vasopressin, among other metabolic peptides. It acts through a yet uncharacterized GPCR inducing increases in intracellular Ca2+. Nesfatin-1 had been shown to be present in human saliva and also to be synthesized in the striated and interlobular parts of the human SMG (Aydin et al, 2011; Aydin et al, 2009). Salivary nesfatin-1 levels in tested subjects correlate closely with serum levels, with an average concentration 1.3 times higher than serum. The correlation between salivary and serum levels indicates transport from the blood vessels into glandular cells. The functions of salivary nesfatin-1 are not yet characterized.
PP-fold family
1. NPY
NPY, a 36 amino acid peptide, is a member of the PP-fold family of peptides. NPY is one of the most widely distributed peptides in the central and peripheral nervous system, and one of the most orexigenic effectors in the CNS. NPY, as well as the two other family members (PYY and PP) binds to a family of G protein-coupled Y receptors. At least five receptors have been cloned and classified as Y1, Y2, Y4, Y5 and y6 on the basis of their molecular and pharmacological properties. In the brain, the expression of all Y receptors has been demonstrated in high concentration in regions involved in energy intake and energy expenditure, such as hypothalamus. NPY expression had been also shown in a subset of TRCs where it specifically enhanced an inwardly rectifying potassium current via NPY-Y1 receptors (Herness & Zhao, 2009; Zhao et al, 2005). Preliminary examination suggested that the NPY-1 receptor-expressing TRCs appeared to colocalize with T1R3, a member of the sweet tastant receptor family. These expression patterns would suggest that NPY might inhibit activation of sweet-receptor expressing cells. No data is available yet whether NPY is present in the saliva.
2. PYY
PYY, a well-characterized molecular mediator of satiation, is released mostly by L-endocrine cells in the distal gut epithelia in response to the amount of calories ingested. The anorectic action of the truncated form PYY3-36 is apparently mediated through the inhibitory actions of its preferred Y2 receptor, which is highly expressed in orexigenic NPY neurons in the hypothalamic arcuate nucleus. Recently, Acosta et al demonstrated PYY3-36 is also present in murine as well as in human saliva (Acosta et al, 2011). In mice, salivary PYY3-36 derives from plasma and is also synthesized in taste cells from the CV papillae. Salivary PYY3-36 enters the oral cavity at least in part from the bloodstream. It is not known whether PYY3-36 is selectively transported from blood capillaries, or is non-specifically leaking into the gingival crevicular fluid. Similar to other metabolic peptides, salivary and plasma peptide concentrations in humans increase postprandially (Acosta et al, 2011). In addition, because PYY is also synthesized in TRCs of the CV papillae, it is conceivable that PYY3-36 is secreted from these cells into saliva. The expression of cognate receptors in lingual epithelia in mice has been extensively characterized by Hurtado et al (Hurtado et al, 2012b). The morphologically different layers of the keratinized stratified epithelium of the dorsal layer of the tongue express Y1R, Y2R, Y4R, and Y5R in a very distinctive yet overlapping pattern. In particular, the monolayer of basal progenitor cells expresses both Y1 and Y2 receptors; Y1R is present in the parabasal prickle cell layer and the granular layer, while differentiated keratinocytes display abundant Y5R. Y4 receptors are robustly expressed in the neuronal fibers innervating lamina propria and mechanoreceptors. In taste buds of the CV, significant fractions of TRCs express YRs localized primarily at the apical part of TRCs (Hurtado et al, 2012b). Indeed, in mice, it was shown that PYY signaling modulates behavioral responsiveness to the bitter-tasting stimuli, as well as to lipid emulsions (La Sala et al, 2012). Salivary PYY3-36 augmentation, via viral vector therapy, rescued behavioral responsiveness to a lipid emulsion but not to bitter stimuli suggesting distinct functions for PYY produced locally in taste cells vs. that circulating systemically and thus present in the saliva.
In a series of behavioral experiments, Acosta et al investigated the role of the salivary PYY3-36 in modulation of FI (Acosta et al, 2011). The acute augmentation of the hormone induced stronger satiation. In a long-term study involving diet-induced obese (DIO) mice, a sustained increase in PYY3-36 was achieved using viral vector-mediated gene delivery targeting salivary glands. The chronic increase in salivary PYY3-36 resulted in a significant long-term reduction in FI and body weight. Apparently, the anorexigenic action of salivary PYY was mediated through the preferred Y2R receptor as the selective Y2R antagonist BIIE0246 completely ablated the effect.
Paracrine and endocrine functions of metabolic hormones
Two discoveries in taste research in recent years have realigned our thinking about how taste perception is linked to mechanisms of appetite and satiety. The first was that many cells in the gut express the same molecular machinery required for nutrient detection as that found in taste cells. We now know these receptors in the gut detect ingested nutrients and mediate the secretion of gastric hormones. More recently, it was learned that these ‘gastrointestinal’ hormones are also expressed in taste cells in the peripheral gustatory system. As is evident from the overview given above, not only are GI hormones are expressed in TRCs, but many other metabolic peptides classified as adipokines and neuropeptides, are also detected in the oral cavity. It seems safe to imply that the presence of any given metabolic hormone in saliva is the rule rather than the exception. Moreover, their cognate receptors are, in most cases, found to be expressed in salivary glands, in the lingual epithelia, or in TRCs, sometimes in several tissues and cell types simultaneously. Anatomical proximity of agonists and receptors suggests that these hormones likely play a role in the functioning of the peripheral gustatory system, or playing other, as yet unidentified roles in local or even systemic metabolism.
All basic taste modalities are now known to be modulated by salivary metabolic hormones: sweet (glucagon, GLP-1, VIP, leptin, and OXT); bitter (VIP, PYY3-36); sour (GLP-1, VIP, and ghrelin); salty (insulin, ghrelin); umami (GLP-1); and lipids (PYY3-36). To better understand the mechanisms of such modulation, one has to discriminate endocrine and paracrine modes of effector actions. As noted earlier, salivary peptides and proteins derive from many sources including salivary glands, gingival crevicular fluid, and transudate from plasma. In addition, some of the peptides are also synthesized in the TRCs within the taste buds. Considering the structure of the taste bud and the composition of the salivary proteome, there is a distinct possibility that there exist two separate pools of peptides: 1) one pool synthesized and contained within the taste bud (paracrine); and 2) another derived from plasma and/or from salivary glands (endocrine). The first pool, most likely, consists of locally synthesized peptides and is contained within the anatomical structure of the taste bud. Only the apical parts of some TRCs, such as bitter- and sweet-detecting cells, are exposed to the saliva, and these parts contain microvilli protruding through the pore (Fig. 2). The intercellular barriers of the pore include tight junctions proteins such as occludin, junction adhesion molecules, and members of the claudin family (Michlig et al, 2007). Tight junctions serve as semipermeable barriers that make some taste receptors accessible only at the apical end in the taste pore and thus activated by tastants dissolved in the saliva, as well as any salivary derived endocrine effectors. On the other hand, paracrine neurotransmitters and neuromodulators expressed within TRCs inside the taste bud structure interact with their cognate ion channel receptors and GPCRs, as postulated by Herness and Zhao (Herness & Zhao, 2009). This notion of separate pools could be illustrated by the example of the satiation hormone PYY that exists in two active forms: PYY1-36 and PYY3-36, the latter being the truncated peptide resulted from the cleavage by the ubiquitous DPP-IV. Both forms activate Y1R and Y2R, while only PYY3-36 is capable of activating Y2R and inducing satiation. DPP-IV does not appear to be expressed in taste buds (Hartel et al, 1988; Shin et al, 2008), but is abundant in saliva (Ogawa et al, 2008; Sahara & Suzuki, 1984). Consequently, PYY produced in taste cells should remain as the relatively nonselective form, PYY1-36. However, salivary PYY largely consists of the highly Y2 receptor selective form, PYY3-36 (Acosta et al, 2011). Thus, PYY acting as a paracrine hormone in taste cells, relative to salivary PYY acting as an endocrine signal, may have substantially different functions. The former (TRC-derived PYY1-36) might be mediating bitter taste perception and/or modulating proliferation of Y1R-positive progenitors Type IV cells within the taste bud (La Sala et al, 2012), while the latter (plasma-derived salivary PYY3-36) could modulate lipid sensing (La Sala et al, 2012) and/or mediate proliferation/differentiation of Y2R/K5-positive basal epithelial cells (Acosta et al, 2011; Hurtado et al, 2012b). The latter function might supplement the proliferative potentials of salivary ghrelin and adiponectin (Groschl et al, 2005a; Groschl et al, 2005b). This exemplifies the pleotropic character of metabolic hormones in general, and the satiation peptide PYY in particular.
Oral nutrient sensing
As shown above, all taste qualities are modulated by one or more metabolic hormones. Because endocrine signaling in the oral cavity is likely to influence FI and satiety, it is important to understand how major hormones influence taste perception and FI. One purported mechanism for this phenomenon is that taste functioning may be modulated by circulating gastric peptides. It has been postulated that alterations in the levels of circulating gastric peptides mediate changes in taste perception observed after gastric bypass surgery in humans and in rodent models of bypass surgery (Chen et al, 2010; Hajnal et al, 2010; le Roux et al, 2011; Olbers et al, 2006; Shin et al, 2011; Tichansky et al, 2011; Umabiki et al, 2010). It has also been hypothesized that the peripheral taste system may be modulated in the context of an animal’s metabolic state. This notion may be especially relevant in case of gustatory perception for calorie-rich foods, such as carbohydrates and lipids. For example, an adipokine leptin not only regulates food intake via its impact on feed control centers in the CNS but also modulates the palatability of food by altering peripheral sweet taste sensitivity (Niki et al, 2010). It was also previously demonstrated that for VIP and sweet taste, there is a strong link between taste perception and energy homeostasis (Martin et al, 2010). The incretin hormone GLP-1 acts to maintain or enhance sweet taste sensitivity (Shin et al, 2008). Systemic and salivary PYY3-36 not only induces satiation (Acosta et al, 2011), but appears also to modulate the lipid taste modality (La Sala et al, 2012). Recent studies in humans have demonstrated an association between a decreased ability to detect the presence of free fatty acids in the oral cavity with increased energy intake and body mass index suggesting that impairment of oral fat sensing mechanisms may contribute to overeating and obesity (Stewart et al, 2010), reviewed in (Little & Feinle-Bisset, 2011). Incidentally, attenuated PYY release in obese subjects is associated with reduced satiety (le Roux et al, 2006), while obese adolescents show impaired secretion of PYY in response to a meal (Mittelman et al, 2010), suggesting that this blunted response may play a role in the pathophysiology of obesity (Stock et al, 2005). Because salivary PYY3-36 modulates lipid sensing (La Sala et al, 2012), impaired PYY-signaling in obese subjects may contribute to the diminished sensory-specific satiety towards the lipid component of foodstuffs, thus inducing compensatory binging. It would be then logical to hypothesize that by modulating salivary hormone composition, pharmacologically or even using gene therapy protocols, one could enhance the lipid and carbohydrate oral sensing, thus reducing overall FI.
This notion of intentional modulation of FI by the orally applied peptides targeting receptors in the lingual epithelia has been recently tested experimentally (Acosta et al, 2011). Answering the questions as to whether augmentation of salivary metabolic hormones functionally activates receptors in oral mucosa required rigorous and technically challenging experiments. For example, in some experiments involving wild type and PYY KO mice, Acosta et al were unable to detect any evidence that orally-applied PYY diffuses retrogradely into plasma in the opposite direction as its transport from plasma to saliva, thus leaving lingual Y receptors as the only candidates for the systemic effect the authors documented (Acosta et al, 2011). Moreover, considering the particularly protease-rich environment in the oral cavity (Thomadaki et al, 2011), it is highly unlikely that peptides applied orally without permeability enhancers would survive in the alimentary tract lumen, much less diffuse into plasma. Incretin hormones such as GLP-1 and its homolog, Exendin-4, when applied orally, showed similar anorexigenic effect, thus providing additional evidence for this model (Acosta et al, 2011). Conceptually, the anorexigenic effect of salivary hormones appears to be redundant considering that both PYY and GLP-1 induce satiation through hypothalamic receptors in a well-described fashion. However, it wouldn’t be out of the realm of possibility that they might also reduce FI through their influence on taste perception via modulatory effects on gustatory neuronal pathways in the peripheral (Hurtado et al, 2012a).
Concluding remarks
Given the recent increase in the prevalence of obesity and associated disorders, the absence of a reliable weight-reducing drug necessitates a search for new tactics. One of such approaches might involve targeted alterations in salivary hormone composition for the purpose of reducing appetite, or rather promoting faster satiation. In his Nobel Prize Lecture, Professor Ivan Pavlov stated: “One and the same food acts differently as a gland stimulator depending on whether the food was consumed by the dog eagerly or unwillingly. Generally, the following invariable phenomenon is observed: each kind of food ingested by the dog during this experiment acts as a strong stimulus only when it suits the dog's taste. We must admit that in the act of eating the craving for food, the appetite - and therefore a psychical phenomenon - serves as a powerful and constant stimulus. The physiological significance of this juice, which we termed appetite juice, proved to be outstandingly great” (Pavlov, 1967). Even now, more than a hundred years later, we’re still in the process of discovering the exact composition and functions of this “appetite juice” which, when fully characterized, might be utilized to curb obesity.
Acknowledgements
I thank my colleague Dr. Cedrick D. Dotson for critically reading and discussing the manuscript. The author was supported, in part, by 1R01DK62302-01 (NIH/NIDDK).
References
- Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, Baum BJ, Zolotukhin S. Salivary PYY: a putative bypass to satiety. PLoS One. 2011;6:e26137. doi: 10.1371/journal.pone.0026137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuailou EN, Vijayagopal P, Imrhan V, Prasad C. Measurement and validation of the nature of salivary adiponectin. Acta Diabetol. 2012 doi: 10.1007/s00592-012-0388-z. [DOI] [PubMed] [Google Scholar]
- Amerongen AV, Veerman EC. Saliva--the defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Aps JK, Martens LC. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int. 2005;150:119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aydin S, Dag E, Ozkan Y, Arslan O, Koc G, Bek S, Kirbas S, Kasikci T, Abasli D, Gokcil Z, Odabasi Z, Catak Z. Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients. Peptides. 2011;32:1276–1280. doi: 10.1016/j.peptides.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Aydin S, Dag E, Ozkan Y, Erman F, Dagli AF, Kilic N, Sahin I, Karatas F, Yoldas T, Barim AO, Kendir Y. Nesfatin-1 and ghrelin levels in serum and saliva of epileptic patients: hormonal changes can have a major effect on seizure disorders. Mol Cell Biochem. 2009;328:49–56. doi: 10.1007/s11010-009-0073-x. [DOI] [PubMed] [Google Scholar]
- Aydin S, Halifeoglu I, Ozercan IH, Erman F, Kilic N, Ilhan N, Ozkan Y, Akpolat N, Sert L, Caylak E. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides. 2005;26:647–652. doi: 10.1016/j.peptides.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Baquero AF, Gilbertson TA. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol Cell Physiol. 2011;300:C860–C871. doi: 10.1152/ajpcell.00318.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Berkman ME, Marmary Y, Goldsmith CM, Baccaglini L, Wang S, Wellner RB, Hoque AT, Atkinson JC, Yamagishi H, Kagami H, Parlow AF, Chao J. Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther. 1999;10:2789–2797. doi: 10.1089/10430349950016528. [DOI] [PubMed] [Google Scholar]
- Benedix F, Westphal S, Patschke R, Granowski D, Luley C, Lippert H, Wolff S. Weight loss and changes in salivary ghrelin and adiponectin: comparison between sleeve gastrectomy and Roux-en-Y gastric bypass and gastric banding. Obes Surg. 2011a;21:616–624. doi: 10.1007/s11695-011-0374-5. [DOI] [PubMed] [Google Scholar]
- Benedix F, Westphal S, Patschke R, Luley C, Lippert H, Wolff S. Comparison of serum and salivary ghrelin in healthy adults, morbidly obese, and patients with metastatic carcinoma. Obes Surg. 2011b;21:1265–1271. doi: 10.1007/s11695-010-0161-8. [DOI] [PubMed] [Google Scholar]
- Bhathena SJ, Smith SS, Voyles NR, Penhos JC, Recant L. Studies on submaxillary gland immunoreactive glucagon. Biochem Biophys Res Commun. 1977;74:1574–1581. doi: 10.1016/0006-291x(77)90622-2. [DOI] [PubMed] [Google Scholar]
- Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res. 2006;171:134–141. doi: 10.1016/j.bbr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Chen K, Yan J, Suo Y, Li J, Wang Q, Lv B. Nutritional status alters saccharin intake and sweet receptor mRNA expression in rat taste buds. Brain Res. 2010;1325:53–62. doi: 10.1016/j.brainres.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Dag E, Aydin S, Ozkan Y, Erman F, Dagli AF, Gurger M. Alteration in chromogranin A, obestatin and total ghrelin levels of saliva and serum in epilepsy cases. Peptides. 2010;31:932–937. doi: 10.1016/j.peptides.2010.02.009. [DOI] [PubMed] [Google Scholar]
- De Matteis R, Puxeddu R, Riva A, Cinti S. Intralobular ducts of human major salivary glands contain leptin and its receptor. J Anat. 2002;201:363–370. doi: 10.1046/j.0021-8782.2002.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea JC, Hirtz C, Deville de Periere D. Preproglucagon mRNA expression in adult rat submandibular glands. Diabetes Nutr Metab. 2003;16:130–133. [PubMed] [Google Scholar]
- Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010;24:3960–3969. doi: 10.1096/fj.10-158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazen CH, Valentin D, Fairchild TJ, Doyle RP. Oral delivery of the appetite suppressing peptide hPYY(3-36) through the vitamin B12 uptake pathway. J Med Chem. 2011;54:8707–8711. doi: 10.1021/jm2012547. [DOI] [PubMed] [Google Scholar]
- Fekete Z, Korec R, Feketeova E, Murty VL, Piotrowski J, Slomiany A, Slomiany BL. Salivary and plasma insulin levels in man. Biochem Mol Biol Int. 1993;30:623–629. [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Groschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54:1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- Groschl M. The physiological role of hormones in saliva. Bioessays. 2009;31:843–852. doi: 10.1002/bies.200900013. [DOI] [PubMed] [Google Scholar]
- Groschl M, Rauh M, Wagner R, Neuhuber W, Metzler M, Tamguney G, Zenk J, Schoof E, Dorr HG, Blum WF, Rascher W, Dotsch J. Identification of leptin in human saliva. J Clin Endocrinol Metab. 2001;86:5234–5239. doi: 10.1210/jcem.86.11.7998. [DOI] [PubMed] [Google Scholar]
- Groschl M, Topf HG, Bohlender J, Zenk J, Klussmann S, Dotsch J, Rascher W, Rauh M. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin Chem. 2005a;51:997–1006. doi: 10.1373/clinchem.2004.040667. [DOI] [PubMed] [Google Scholar]
- Groschl M, Topf HG, Kratzsch J, Dotsch J, Rascher W, Rauh M. Salivary leptin induces increased expression of growth factors in oral keratinocytes. J Mol Endocrinol. 2005b;34:353–366. doi: 10.1677/jme.1.01658. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel S, Gossrau R, Hanski C, Reutter W. Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry. Histochemistry. 1988;89:151–161. doi: 10.1007/BF00489918. [DOI] [PubMed] [Google Scholar]
- Herness MS. Vasoactive intestinal peptide-like immunoreactivity in rodent taste cells. Neuroscience. 1989;33:411–419. doi: 10.1016/0306-4522(89)90220-0. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado MD, Acosta A, Gorbatyuk O, Sergeyev GG, Dodson CD, Herzog H, Zolotukhin S. Anorectic signaling pathway mediated by salivary PYY. 2012a [Google Scholar]
- Hurtado MD, Acosta A, Riveros PP, Baum BJ, Ukhanov K, Brown AR, Dodson CD, Herzog H, Zolotukhin S. Expression of NPY family genes in lingual cells: novel anatomical domain. 2012b doi: 10.1371/journal.pone.0046358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman L, Liebow C, Rothman S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am J Physiol. 1999;276:E223–E232. doi: 10.1152/ajpendo.1999.276.2.E223. [DOI] [PubMed] [Google Scholar]
- Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54:2295–2299. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M, Lee A, Wang PL, Purushotham KR, Chegini N, Yamamoto H, Humphreys-Beher MG. Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice. Effects of type 1 diabetes mellitus. Biochem Pharmacol. 1995;49:1521–1531. doi: 10.1016/0006-2952(95)00017-t. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Matsuda H, Gono Y, Furukawa M, Hiruma H, Kawakami T, Tsukuda M, Takenaka T. Immunohistochemical localisation of regulatory neuropeptides in human circumvallate papillae. J Anat. 1998;192(Pt 4):557–564. doi: 10.1046/j.1469-7580.1998.19240557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Sala M, Hurtado MD, Brown AR, Bohόrquez DV, Liddle RA, Herzog H, Zolotukhin S, Dodson CD. Modulation of Taste Responsiveness by PYY Signaling. 2012 doi: 10.1096/fj.13-228064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AM, Kirstens L, Mitton L. Parotid gland insulin: an extrapancreatic source of insulin in rats. Diabetes. 1976;25:328. [Google Scholar]
- Lawrence AM, Tan S, Hojvat S, Kirsteins L. Salivary gland hyperglycemic factor: an extrapancreatic source of glucagon-like material. Science. 1977;195:70–72. doi: 10.1126/science.63992. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BB, Chen ZB, Li BC, Lin Q, Li XX, Li SL, Ding C, Wu LL, Yu GY. Expression of ghrelin in human salivary glands and its levels in saliva and serum in Chinese obese children and adolescents. Arch Oral Biol. 2011;56:389–394. doi: 10.1016/j.archoralbio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity--oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J Dent Res. 2010;89:1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhao FL, Herness S. Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett. 2003;351:157–160. doi: 10.1016/j.neulet.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamali I, Roupas ND, Armeni AK, Theodoropoulou A, Markou KB, Georgopoulos NA. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides. 2012;33:120–124. doi: 10.1016/j.peptides.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Benzi L, Masoni A, Cecchetti P, Giannarelli R, Di Cianni G, Ciccarone AM, Navalesi R. Salivary insulin concentrations in type 2 (non-insulin-dependent) diabetic patients and obese non-diabetic subjects: relationship to changes in plasma insulin levels after an oral glucose load. Diabetologia. 1986;29:695–698. doi: 10.1007/BF00870278. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Giannarelli R, Masoni A, Cecchetti P, Di Carlo A, Navalesi R. Salivary immunoreactive insulin concentrations are related to plasma free-insulin levels in insulin-treated diabetic patients. Diabete Metab. 1990;16:16–20. [PubMed] [Google Scholar]
- Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 2010;59:1143–1152. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger B, Clifford MN, Morgan LM. Glucose-dependent insulinotropic polypeptide and insulin-like immunoreactivity in saliva following sham-fed and swallowed meals. J Endocrinol. 2003;177:407–412. doi: 10.1677/joe.0.1770407. [DOI] [PubMed] [Google Scholar]
- Michlig S, Damak S, Le Coutre J. Claudin-based permeability barriers in taste buds. J Comp Neurol. 2007;502:1003–1011. doi: 10.1002/cne.21354. [DOI] [PubMed] [Google Scholar]
- Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063–R1068. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Redding SW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomark Med. 2010;4:171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 2010;18:918–925. doi: 10.1038/oby.2009.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Taniguchi H, Baba S. Presence of insulin-like immunoreactivity and its biosynthesis in rat and human parotid gland. Diabetologia. 1982;22:358–361. doi: 10.1007/BF00253582. [DOI] [PubMed] [Google Scholar]
- Niki M, Jyotaki M, Yoshida R, Ninomiya Y. Reciprocal modulation of sweet taste by leptin and endocannabinoids. Results Probl Cell Differ. 2010;52:101–114. doi: 10.1007/978-3-642-14426-4_9. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31:1059–1062. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- Oh CK, Ritschel WA. Biopharmaceutic aspects of buccal absorption of insulin. Methods Find Exp Clin Pharmacol. 1990;12:205–212. [PubMed] [Google Scholar]
- Ohta K, Laborde NJ, Kajiya M, Shin J, Zhu T, Thondukolam AK, Min C, Kamata N, Karimbux NY, Stashenko P, Kawai T. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J Dent Res. 2011;90:1286–1292. doi: 10.1177/0022034511420431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay Y, Aydin S, Dagli AF, Akbulut M, Dagli N, Kilic N, Rahman A, Sahin I, Polat V, Ozercan HI, Arslan N, Sensoy D. Obestatin is present in saliva: alterations in obestatin and ghrelin levels of saliva and serum in ischemic heart disease. BMB Rep. 2008;41:55–61. doi: 10.5483/bmbrep.2008.41.1.055. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Nobel Lectures, Physiology or Medicine 1901–1921, Including Presentation Speeches and Laureates' Biographies. Amsterdam: Elsevier Publishing Company; 1967. [Google Scholar]
- Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- Perez-Castillo A, Blazquez E. Synthesis and release of glucagon by human salivary glands. Diabetologia. 1980;19:123–129. doi: 10.1007/BF00421858. [DOI] [PubMed] [Google Scholar]
- Perez P, Rowzee AM, Zheng C, Adriaansen J, Baum BJ. Salivary epithelial cells: an unassuming target site for gene therapeutics. Int J Biochem Cell Biol. 2010;42:773–777. doi: 10.1016/j.biocel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Lewandowski KC, Sailesh S, O'Hare P, Hillhouse EW. Circadian rhythmicity of salivary leptin in healthy subjects. Mol Genet Metab. 2003;78:229–235. doi: 10.1016/s1096-7192(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Sahara N, Suzuki K. Ultrastructural localization of dipeptidyl peptidase IV in rat salivary glands by immunocytochemistry. Cell Tissue Res. 1984;235:427–432. doi: 10.1007/BF00217869. [DOI] [PubMed] [Google Scholar]
- Salles C, Chagnon MC, Feron G, Guichard E, Laboure H, Morzel M, Semon E, Tarrega A, Yven C. In-mouth mechanisms leading to flavor release and perception. Crit Rev Food Sci Nutr. 2011;51:67–90. doi: 10.1080/10408390903044693. [DOI] [PubMed] [Google Scholar]
- Sanematsu K, Horio N, Murata Y, Yoshida R, Ohkuri T, Shigemura N, Ninomiya Y. Modulation and transmission of sweet taste information for energy homeostasis. Ann N Y Acad Sci. 2009;1170:102–106. doi: 10.1111/j.1749-6632.2009.03893.x. [DOI] [PubMed] [Google Scholar]
- Schapher M, Wendler O, Groschl M. Salivary cytokines in cell proliferation and cancer. Clin Chim Acta. 2011;412:1740–1748. doi: 10.1016/j.cca.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Schipper R, Loof A, de Groot J, Harthoorn L, Dransfield E, van Heerde W. SELDI-TOF-MS of saliva: methodology and pre-treatment effects. J Chromatogr B Analyt Technol Biomed Life Sci. 2007a;847:45–53. doi: 10.1016/j.jchromb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Schipper RG, Silletti E, Vingerhoeds MH. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol. 2007b;52:1114–1135. doi: 10.1016/j.archoralbio.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta Y, Kataoka S, Toyono T, Toyoshima K. Expression of galanin and the galanin receptor in rat taste buds. Arch Histol Cytol. 2006;69:273–280. doi: 10.1679/aohc.69.273. [DOI] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- Shin AC, Townsend RL, Patterson LM, Berthoud HR. "Liking" and "wanting" of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1267–R1280. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Martin B, Kim W, White CM, Ji S, Sun Y, Smith RG, Sevigny J, Tschop MH, Maudsley S, Egan JM. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One. 2010;5:e12729. doi: 10.1371/journal.pone.0012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubnikova EA, Volkova EF, Printseva O. Submandibular glands as organs of synthesis and accumulation of insulin-like protein. Acta Histochem. 1984;74:157–171. doi: 10.1016/s0065-1281(84)80003-3. [DOI] [PubMed] [Google Scholar]
- Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, Chaudhari N. Oxytocin signaling in mouse taste buds. PLoS One. 2010;5:e11980. doi: 10.1371/journal.pone.0011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Mazur A, Voyles N, Bhathena S, Recant L. Is submaxillary gland immunoreactive glucagon important in carbohydrate homeostasis? Metabolism. 1979;28:343–347. doi: 10.1016/0026-0495(79)90105-7. [DOI] [PubMed] [Google Scholar]
- Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- Taouis M, Deville de Periere D, Hillaire-Buys D, Derouet M, Gross R, Simon J, Ribes G. Biological activity of immunoreactive insulin-like activity extracted from rat submandibular gland. Am J Physiol. 1995;269:E277–E282. doi: 10.1152/ajpendo.1995.269.2.E277. [DOI] [PubMed] [Google Scholar]
- Thomadaki K, Helmerhorst EJ, Tian N, Sun X, Siqueira WL, Walt DR, Oppenheim FG. Whole-saliva proteolysis and its impact on salivary diagnostics. J Dent Res. 2011;90:1325–1330. doi: 10.1177/0022034511420721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc. 2011;25:1176–1181. doi: 10.1007/s00464-010-1335-0. [DOI] [PubMed] [Google Scholar]
- Toda M, Morimoto K. Comparison of saliva sampling methods for measurement of salivary adiponectin levels. Scand J Clin Lab Invest. 2008;68:823–825. doi: 10.1080/00365510802147006. [DOI] [PubMed] [Google Scholar]
- Toda M, Tsukinoki R, Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007;44:20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- Tseng CC, Jarboe LA, Landau SB, Williams EK, Wolfe MM. Glucose-dependent insulinotropic peptide: structure of the precursor and tissue-specific expression in rat. Proc Natl Acad Sci U S A. 1993;90:1992–1996. doi: 10.1073/pnas.90.5.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CC, Jarboe LA, Wolfe MM. Regulation of glucose-dependent insulinotropic peptide gene expression by a glucose meal. Am J Physiol. 1994;266:G887–G891. doi: 10.1152/ajpgi.1994.266.5.G887. [DOI] [PubMed] [Google Scholar]
- Umabiki M, Tsuzaki K, Kotani K, Nagai N, Sano Y, Matsuoka Y, Kitaoka K, Okami Y, Sakane N, Higashi A. The improvement of sweet taste sensitivity with decrease in serum leptin levels during weight loss in obese females. Tohoku J Exp Med. 2010;220:267–271. doi: 10.1620/tjem.220.267. [DOI] [PubMed] [Google Scholar]
- Vallejo G, Mead PM, Gaynor DH, Devlin JT, Robbins DC. Characterization of immunoreactive insulin in human saliva: evidence against production in situ. Diabetologia. 1984;27:437–440. doi: 10.1007/BF00273907. [DOI] [PubMed] [Google Scholar]
- Voutetakis A, Cotrim AP, Rowzee A, Zheng C, Rathod T, Yanik T, Loh YP, Baum BJ, Cawley NX. Systemic delivery of bioactive glucagon-like peptide 1 after adenoviral-mediated gene transfer in the murine salivary gland. Endocrinology. 2010;151:4566–4572. doi: 10.1210/en.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009;51:367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt M. Distribution of vasoactive intestinal peptide-like immunoreactivity in the taste organs of teleost fish and frog. Histochem J. 1995;27:161–165. doi: 10.1007/BF00243912. [DOI] [PubMed] [Google Scholar]
- Yan W, Apweiler R, Balgley BM, Boontheung P, Bundy JL, Cargile BJ, Cole S, Fang X, Gonzalez-Begne M, Griffin TJ, Hagen F, Hu S, Wolinsky LE, Lee CS, Malamud D, Melvin JE, Menon R, Mueller M, Qiao R, Rhodus NL, Sevinsky JR, States D, Stephenson JL, Than S, Yates JR, Yu W, Xie H, Xie Y, Omenn GS, Loo JA, Wong DT. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl. 2009;3:116–134. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]