Abstract
Introduction
Fibrinogen is a major structural protein in blood clots, and is also a well-known acute phase reactant. The γ chain gene of fibrinogen has two alternative splice variants, γA and γ' chains. γ' fibrinogen constitutes about 7% of total fibrinogen. Total fibrinogen levels and γ' fibrinogen levels have been associated with cardiovascular disease, but the mechanisms regulating the production of the two isoforms are unknown. Several inflammatory cytokines are known to influence the production of total fibrinogen, but the role of cytokines in the production of γ' fibrinogen has not been examined. However, epidemiologic studies have shown an association between γ' fibrinogen levels and inflammatory markers in humans.
Materials and methods
The expression of γ' fibrinogen and total fibrinogen by HepG2 liver cells was quantitated after treatment with interleukin-1β, transforming growth factor-β, tumor necrosis factor-α, and interleukin-6.
Results
Interleukin-1β, transforming growth factor-β, and tumor necrosis factor-α, known down-regulators of total fibrinogen synthesis, also downregulate γ' fibrinogen synthesis in HepG2 cells. However, interleukin-6 differentially up-regulates the production of total and γ' fibrinogen, leading to a 3.6-fold increase in γA mRNA, but an 8.3-fold increase in γ' mRNA.
Conclusions
These findings indicate that γ' fibrinogen is disproportionately up-regulated by inflammatory responses induced by interleukin-6.
Keywords: Cytokines, expression, fibrinogen, inflammation, liver
γ' fibrinogen is a newly-emerging risk factor for cardiovascular disease, including coronary artery disease [1], myocardial infarction [2–4], and stroke [5]. Recent studies have also shown a significant association between γ' fibrinogen and CRP [4, 6], a marker of inflammation that is associated with cardiovascular disease [7]. In particular, patients from the PAVE study who had both periodontal inflammation and a history of coronary artery blockage of 50% or greater had an increased ratio of γ' fibrinogen/total fibrinogen. Whereas γ' fibrinogen typically constitutes about 7% of total fibrinogen in normal individuals [1, 8], γ' fibrinogen constituted 18.6% of total fibrinogen in the PAVE cohort. In addition, γ' fibrinogen levels were significantly associated with CRP levels (P<0.001) [6]. These results suggest that γ' fibrinogen may display differential regulation from total fibrinogen in the presence of combined cardiovascular and periodontal disease.
The γ' isoform arises from an alternative splicing event within intron 9 where intron 9 is cleaved before it can be spliced out, resulting in the loss of exon 10 and the retention of a portion of intron 9 in the mRNA. Translation of this mRNA isoform reads through the end of exon 9 into intron 9 (also known as exon 9a), leading to a 20 amino acid carboxyl-terminal extension, VRPEHPAETEYDSLYPEDDL, that is not found in the γA chain [9, 10]. The γ' chain harbors unique properties, and when incorporated into a mature fibrinogen molecule can lead to clots with altered architecture that are more resistant to breakdown [11].
The regulation of total human fibrinogen expression by cytokines has been investigated in past studies using the HepG2 hepatocellular carcinoma cell line [12]. This model system faithfully recapitulates fibrinogen expression, including γ' fibrinogen [13] and has been used successfully to study fibrinogen production and regulation in vitro [14]. We therefore investigated whether inflammatory cytokines can act directly on HepG2 cells to dysregulate the expression of γ' fibrinogen.
Materials and Methods
Cell culture
HepG2 cells (ATCC, Manassas, VA), between passage three and seven, were grown in 6-well plates to 70% confluence in HyClone MEM/EBSS (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% heat-inactivated fetal bovine serum/50 units/ml penicillin/50 µg/ml streptomycin. Cells were serum-starved for 24 hours and then treated for 24 hours with the follow recombinant cytokines; human IL-6, IL-1β, TGF-β, or TNF-α (Leinco Technologies, Inc, St. Louis, MO, USA). Conditioned media were harvested from the wells and total fibrinogen and γ' fibrinogen were quantitated by ELISA. γ' and γA mRNAs were isolated from the cells and quantitated by RT-PCR.
Total fibrinogen ELISA
To measure total fibrinogen produced by HepG2 cells, 96 well plates were coated with 1.5 µg/ml AXL203 rabbit anti-human fibrinogen polyclonal antibody (Accurate Chemical, Westbury, NY, USA) and incubated overnight at 4°C. Wells were washed three times with PBS/0.1% Triton and blocked in PBS containing 1% BSA/0.1% Triton X-100 at 37°C for 1 hour. Wells were washed with PBS/0.1% Triton, and 50 µl of conditioned medium diluted 1:10 in PBS/1% BSA/5 mM EDTA/0.1% Triton X-100 was added to each well and incubated at 37°C for one hour. Wells were washed again with PBS/0.1% Triton, and a 1:2500 dilution of sheep anti-human fibrinogen-HRP conjugate (Innovative Research, Novi, MI, USA) was added to the wells and incubated for 1 hour at 37°C. Wells were washed with PBS/0.1% Triton and incubated with TMB Super Sensitive 1 Component HRP Microwell Substrate (BioFX Laboratories, Owings Mills, MD, USA) for 30 minutes at room temperature. 450 nm liquid stop solution for TMB microwell (BioFX Laboratories) was added to each reaction and absorbance was measured at 450 nm.
γ' fibrinogen ELISA
γ' fibrinogen was measured using an assay that was originally developed to measure γ' fibrinogen in human plasma samples [1]. This assay was modified slightly for measuring γ' fibrinogen levels in cell culture supernatants. Briefly, 96 well plates were coated overnight at 4°C with the monoclonal antibody 2.G2.H9 which is directed against the unique carboxyl terminus of the γ' chain. Wells were washed three times with PBS/0.1% Triton and blocked in PBS containing 1% BSA/0.1% Triton at 37°C for 1 hour. Wells were washed with PBS/0.1% Triton, and 50 µl of conditioned medium diluted 1:1 in PBS/1% BSA/5 mM EDTA/0.1% Triton X-100 was added to each well and incubated at 37°C for one hour. Wells were washed again with PBS/0.1% Triton X-100, and a 1:2500 dilution of sheep anti-human fibrinogen-HRP conjugate was added to the wells and incubated for 1 hour at 37°C. Wells were washed with PBS/0.1% Triton and incubated with TMB substrate for 30 minutes at room temperature. Stop solution was added to each reaction and absorbance was measured at 450 nm.
RT-PCR
Total RNA was isolated from HepG2 cells using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and 1 µg of polyA mRNA was reverse-transcribed using SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). TaqMan primer/probe pairs (Applied Biosystems Inc., Carlsbad, CA, USA) used in RT-PCR reactions were as follows; GAPDH Inventoried Endogenous Control Assay ABI4333764T, FGG(γ) Inventoried Assay Hs00241038_m1, FGG(γ')-forward 5'-CAATTGGAGAAGGACAGCAACA-3', FGG(γ')-reverse 5'-TCTGAAACTTTGTGGGTCAATAGAAG-3', FGG(γ')-Probe(FAM) 5'-CACCCTGCGGAAACAGAATATGACTCACTT-3'. PCR reactions were performed using TaqMan Universal Master Mix II with UNG (Applied Biosystems, Inc.) and assayed on an ABI 7300 Real-Time PCR System. Relative mRNA levels were calculated using the 2−ΔΔCt method with GAPDH as the endogenous control.
Western Blots
HepG2 cells were prepared and treated as described above. Following 24 hours of IL-6 treatment, cells were washed with PBS and lysed in RIPA buffer. Total cellular protein concentration was determined using a BCA assay and 20 µg of total protein from the cell lysates were separated using 10% SDS-PAGE. Protein was transferred to a nitrocellulose membrane and the membrane was probed using rabbit anti-human primary antibodies (phospho-p38 Thr180/Tyr182, phospho MEK1/2 Ser217/221, phospho-Jun Ser 63; Cell Signaling Technology). The membrane was stripped and reprobed for total p38, MEK and Jun using rabbit anti-human antibodies (Cell Signaling Technology). Goat anti-rabbit HRP conjugated secondary antibodies were used and membranes were imaged using an ECL Chemiluminescence Kit (Pierce).
Results
IL-1β, TGF-β, and TNF-α inhibit γ' fibrinogen production
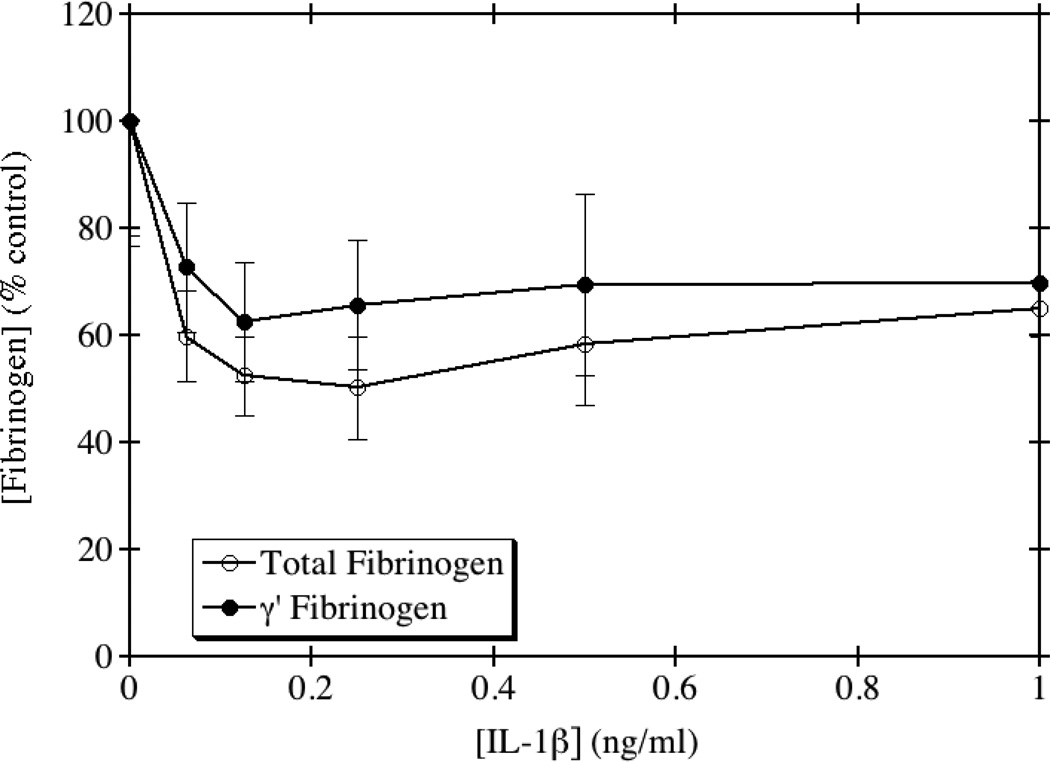
We tested the direct effects of several inflammatory cytokines on total and γ' fibrinogen expression in HepG2 cells. IL-1β is a member of the proinflammatory IL-1 superfamily produced by macrophages, monocytes and dendritic cells in response to infection. IL-1β has several effects on the coagulation system, including increasing the release of von Willebrand factor [15] and plasminogen activator [16], and inhibiting the anticoagulant protein C pathway [17]. Interestingly, IL-1β is also known to bind to fibrinogen and fibrin, and this binding leads to increased activity of IL-1β [18]. Previous studies have shown that IL-1β has an inhibitory effect on fibrinogen production in HepG2 cells [14], however the effects of IL-1β on γ' fibrinogen have not previously been examined. As shown in Fig. 1, the addition of recombinant IL-1β to HepG2 cells caused a decrease in the production of both total and γ' fibrinogen. There was no significant difference between the dose response curves by one-way ANOVA (p=0.35). Since both total and γ' fibrinogen decreased to a similar extent, this suggests that the inhibition may be occurring at the promoter level. The ratio of γ'/total fibrinogen remained relatively unchanged upon treatment with IL-1β.
Fig. 1. IL-1β inhibits the production of total and γ' fibrinogen.
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of IL-1β for 24 hours. Total and γ' fibrinogen levels were assayed by ELISA and the results normalized to the amount of total protein from whole cell lysates. The average of triplicate experiments is shown ± SE.
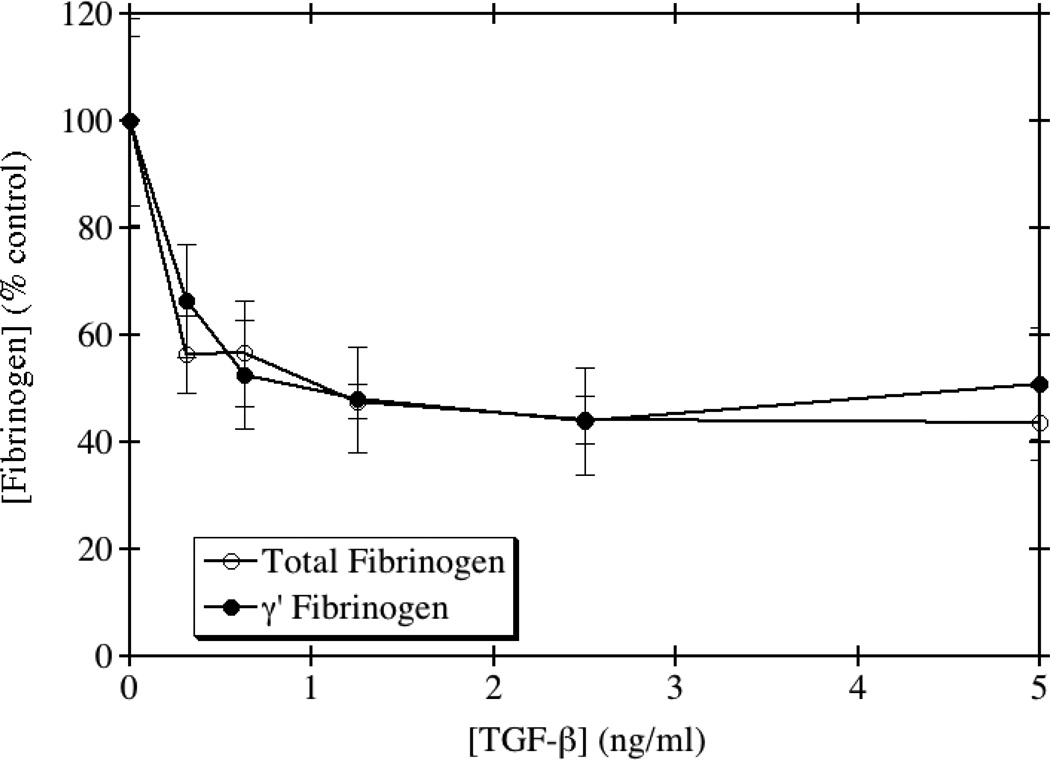
TGF-β is a cytokine known to have both pro- and anti-inflammatory functions, and is made in several different cell types. Its specific function depends on the extracellular environment and cues received from surrounding cells. TGF-β signaling has been implicated in numerous processes, including atherogenesis [19]. Therefore, we examined the effects of TGF-β on the production of total and γ' fibrinogen in HepG2 cells. Several previous studies have also shown that TGF-β inhibits the production of total fibrinogen in HepG2 and Hep3B hepatocellular carcinoma cell lines [20, 21]. As seen in Fig. 2, our data is consistent with these prior studies in that addition of TGF-β to HepG2 cells led to a dose-dependent decrease in total fibrinogen production. Additionally, a dose-dependent decrease in the production of γ' fibrinogen was also observed. However, there was no significant difference between the dose response curves by one-way ANOVA (p=0.86). Moreover, the ratio of γ'/total fibrinogen remained relatively unchanged upon treatment, except at the highest concentration tested, suggesting that the action of TGF-β on fibrinogen production, like IL-1β, also may occur at the promoter level.
Fig. 2. TGF-β inhibits the production of total and γ' fibrinogen.
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of TGF-β for 24 hours. Total and γ' fibrinogen levels were assayed by ELISA and the results normalized to the amount of total protein from whole cell lysates. The average of triplicate experiments is shown ± SE.
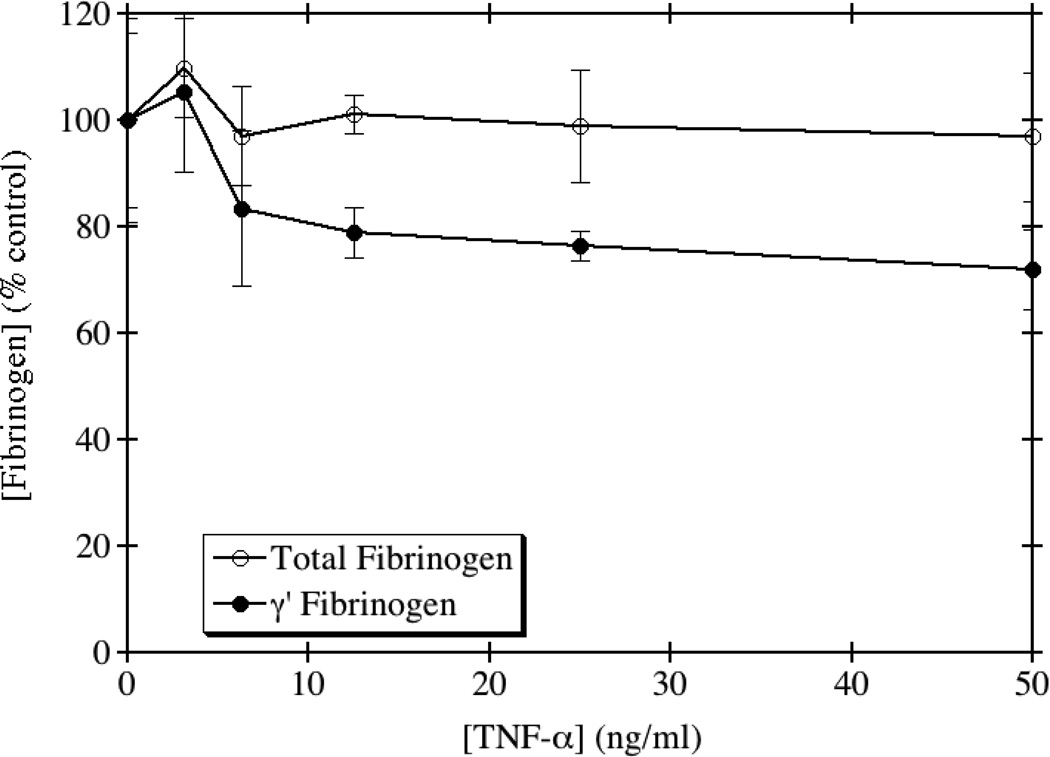
TNF-α is a known modulator of the acute phase response and is involved in systemic inflammation. The role of TNF-α in the modulation of the type II acute phase proteins, especially fibrinogen, has been controversial in the literature. An in vivo study examining the role of TNF-α in Candida albicans infection [22] demonstrated that administration of recombinant murine TNF-α to mice leads to a significant increase in the concentration of circulating fibrinogen. However, a study performed in HepG2 cells showed an inhibition of fibrinogen synthesis by TNF-α [14]. We therefore investigated the effects of TNF-α on the production of both total and γ' fibrinogen in HepG2 cells. As shown in Fig. 3, addition of recombinant TNF-α did not alter the levels of total fibrinogen, in contrast to both of the above-referenced findings. However, TNF-α had a slight inhibitory effect on the production of γ' fibrinogen, reducing γ' fibrinogen by up to 30% at the highest doses tested. There was a significant difference between the dose response curves by one-way ANOVA (p=0.031), although this was mainly apparent at higher doses and was not especially robust.
Fig. 3. TNF-α decreases the production of γ' fibrinogen, but not total fibrinogen.
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of TNF-α for 24 hours. Total and γ' fibrinogen levels were assayed by ELISA and the results normalized to the amount of total protein from whole cell lysates. The average of triplicate experiments is shown ± SE.
IL-6 differentially regulates total and γ' fibrinogen production
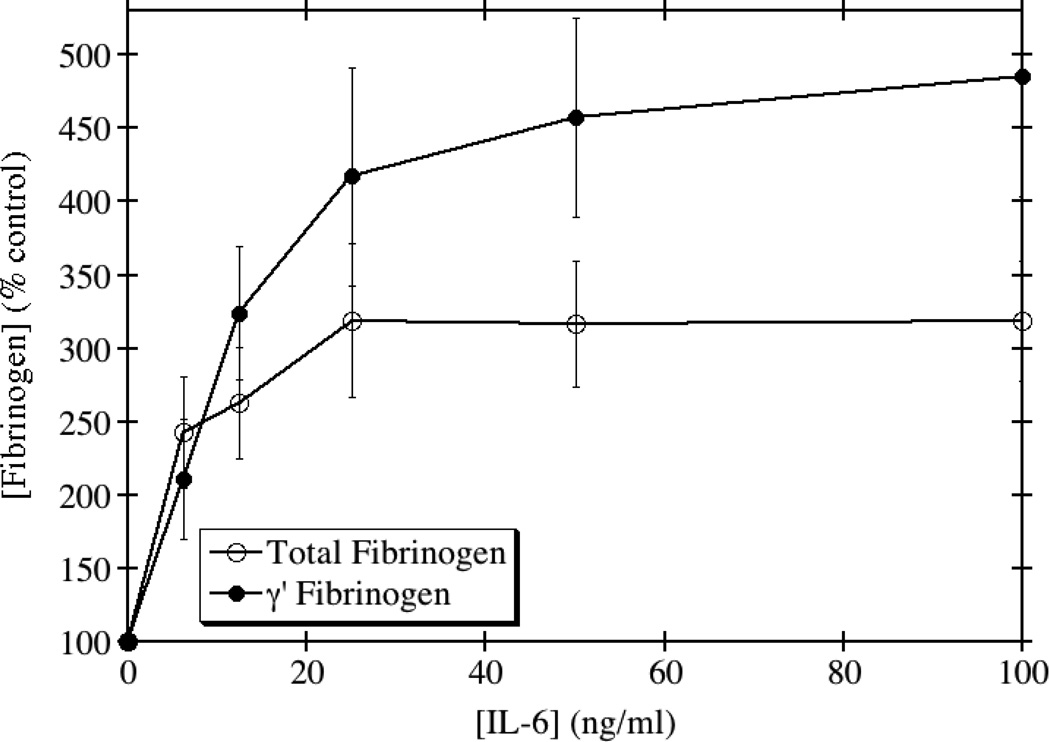
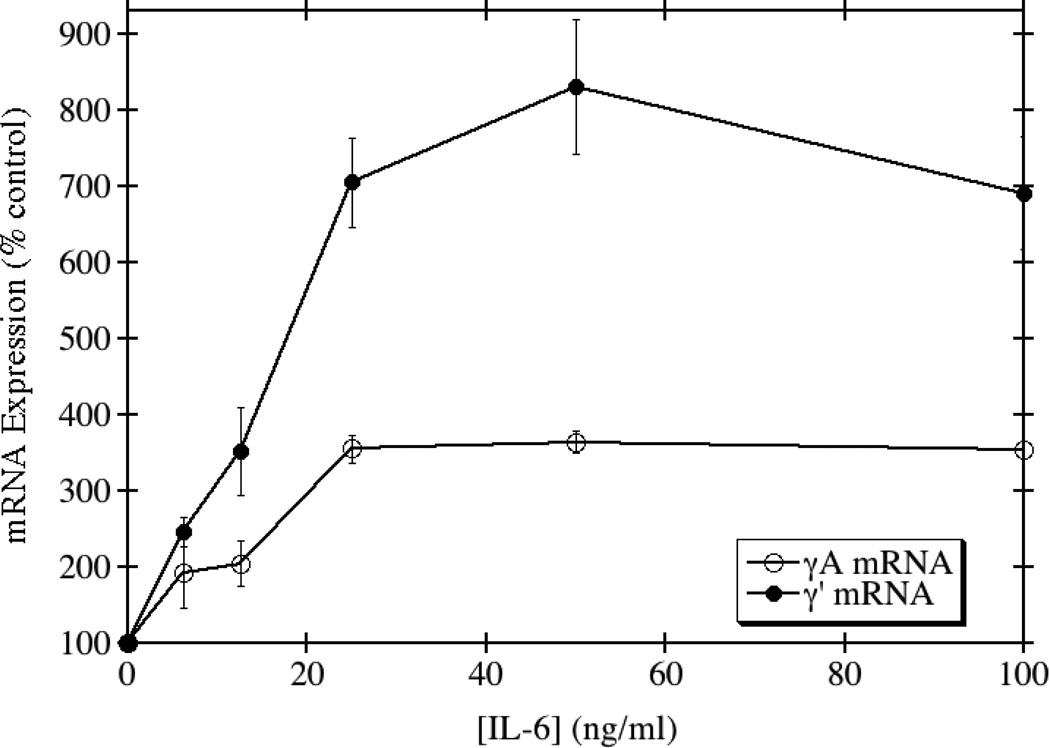
In contrast to the results obtained with IL-1β, TGF-β, and TNF-α, IL-6 induced a significant up-regulation of total and γ' fibrinogen synthesis at both the protein and mRNA levels. IL-6 is an acute phase reactant and known inducer of total fibrinogen synthesis in HepG2 cells [23, 24]. In agreement with several previous reports of its induction potential, we saw approximately a 2.3-fold maximum increase in the production of total fibrinogen when HepG2 cells were treated with IL-6 (Fig. 4). In contrast, γ' fibrinogen production increased by a maximum of 3.2-fold. This lead to an increase in the ratio of γ'/total fibrinogen at physiological concentrations of IL-6. The mRNA levels for the γA chain, which normally constitutes >90% of the γ chains in total fibrinogen, increased 3.6-fold, but the mRNA levels for the γ' chain increased by 8.3-fold (Fig. 5). Together, these results show that IL-6 is able to differentially up-regulate the production of total and γ' fibrinogen in HepG2 cells.
Fig. 4. IL-6 disproportionately increases the expression of γ' fibrinogen relative to total fibrinogen.
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of IL-6 for 24 hours. Total and γ' fibrinogen levels in the conditioned medium were assayed by ELISA. The average of triplicate experiments is shown ± SE.
Fig. 5. IL-6 disproportionately increases the expression of γ' chain mRNA relative to γA mRNA.
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of IL-6 for 24 hours. The cells were solubilized, and γA and γ' mRNA levels were quantitated by RT-PCR. The average of triplicate experiments is shown ± SE.
IL-6 treatment increases phosphorylation of MEK in HepG2 cells
Several intracellular signaling pathways have been implicated in the regulation of alternative splicing; therefore we investigated the phosphorylation of p38, MEK and Jun in HepG2 cells in response to IL-6 treatment. As seen in Fig. 6, phosphorylation of MEK at Ser217/221 increased with increasing concentration of IL-6 treatment in HepG2 cells. There was no reproducible change in the phosphorylation of p38 or Jun (data not shown). These results suggest that IL-6 may be regulating the alternative splicing of the fibrinogen mRNA transcript via signaling through the MEK-ERK pathway.
Fig. 6. Phosphorylation of MEK in response to IL-6.
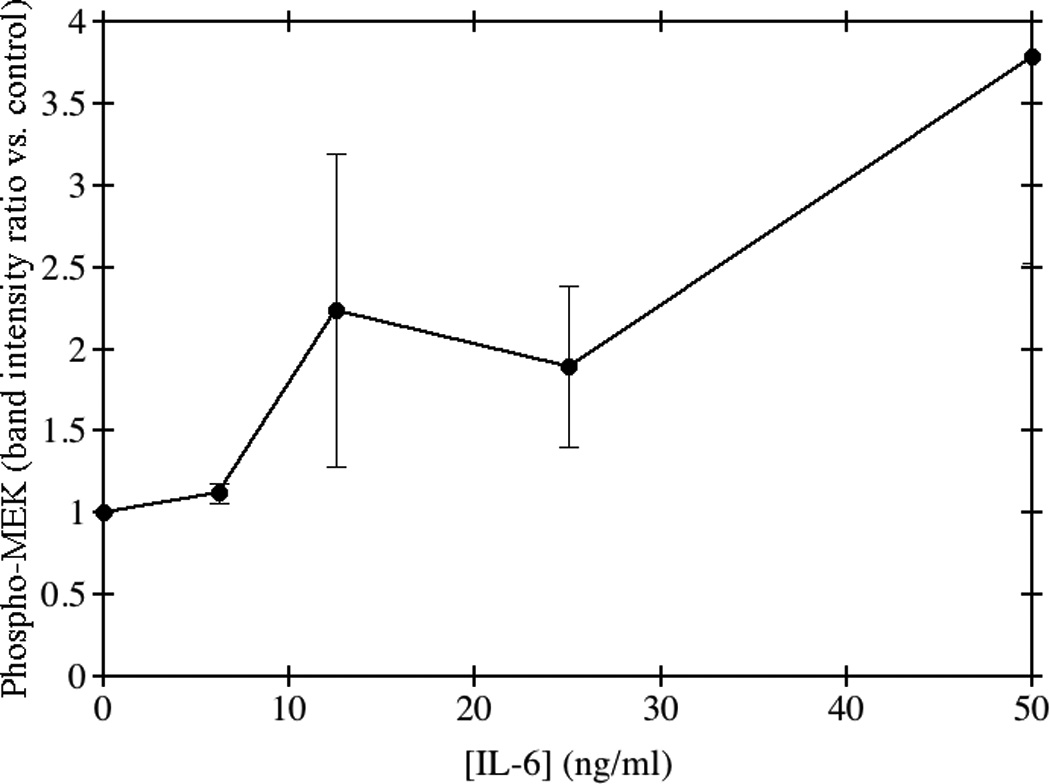
HepG2 cells were grown to confluence and incubated with medium containing the indicated concentrations of IL-6 for 24 hours. Total cellular protein was separated using 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed using a rabbit anti-human primary antibody to phospho MEK1/2 Ser217/221. Goat anti-rabbit HRP-conjugated secondary antibody was used and membranes were imaged by chemiluminescence. The average of triplicate experiments is shown ± SE.
Discussion
Total fibrinogen is a known acute phase reactant, responding to the actions of several cytokines generated during an acute phase response to injury or infection, but the role of γ' fibrinogen as an acute phase reactant was previously unknown. This study is the first to show that an inflammatory cytokine, namely IL-6, is able to differentially regulate the production of γ' fibrinogen, altering the γ'/total fibrinogen ratio. The γ' isoform of fibrinogen is a newly-emerging risk factor for cardiovascular disease [25, 26] that arises from alternative processing of the fibrinogen γ chain pre-mRNA [9, 10]. However, the events that regulate the alternative splicing event remain unknown. One report demonstrated that concentrations of γ' fibrinogen are higher in patients with ischemic stroke during the acute phase, as compared to the convalescent phase [5], suggesting that there is an increase in the alternative splicing event that produces γ' fibrinogen during this period. In addition, patients from the PAVE study who had both periodontal inflammation and a recent history of coronary artery blockage of 50% or greater had significantly increased levels of γ' fibrinogen.
IL-1β, TGF-β, and TNF-α all inhibited the production of γ' fibrinogen in HepG2 cells. IL-1β is also known to inhibit total fibrinogen synthesis, although this inhibition is caused by activation and nuclear translocation of NF-κB which directly inhibits the binding of STAT3 to the IL-6 response elements within the fibrinogen promoter [23, 24, 27, 28]. TGF-β has previously been shown to inhibit the production of total fibrinogen in a hepatocyte cell line [20], which is presumably mediated by Smads 3 and 4, downstream effector molecules of the TGF-β signaling pathway.
IL-6 is a well-studied inducer of total fibrinogen synthesis, due to the presence of several IL-6 response elements within the promoters of the three fibrinogen genes. Most notably, the fibrinogen γ chain promoter contains three such IL-6 response elements [23, 24, 27, 28], making it very responsive to IL-6 stimulation. In this case, we would expect to see a similar increase in the levels of total fibrinogen and γ' fibrinogen due to the fact that IL-6 acts at the promoter level. However, we saw a disproportionate increase in the levels of γ' fibrinogen relative to total fibrinogen (Fig. 4). Taken together, these studies suggest that inflammation plays a crucial role in regulating the process of alternative splicing of the fibrinogen γ gene, although the molecular mechanism behind this regulation remains unclear.
Extracellular signals have been known to alter the splicing of several genes by changing the phosphorylation patterns of proteins of the spliceosome and the polyadenylation pathway [29]. Specifically, IL-6 has been shown to alter the splicing pattern of Bcl-XL in a leukemia cell line, and this alteration in splicing requires different intronic sequences to be present in the downstream intron [30]. Additionally, IL-6 is known to activate the Ras-Raf-MEK-ERK pathway downstream of IL-6 receptor activation. The activation of the MEK-ERK pathway has been shown to affect the phosphorylation of proteins involved in alternative splicing [31]. In HepG2 cells, we see that treatment with IL-6 leads to increased phosphorylation of MEK1/2, a phenomenon observed in several other cell types and seen previously in hepatocytes [32]. Activation of the MAPK pathway has been implicated in the regulation of alternative pre-mRNA splicing in several cell types [29, 31] and MEK has been identified as both a positive and negative regulator of alternative splicing, with its effects dependent upon the stimulus and the cell type being studied [33, 34]. This potential role in alteration of splicing may provide a potential mechanism for the increase in γ' fibrinogen expression that is reported here, and may explain the link between increased γ' fibrinogen levels and inflammatory diseases.
Acknowledgement
This work was supported by NIH R21 HL097298 (to D.H.F.).
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CRP
C-reactive protein
- EBSS
Earle's balanced salt solution
- ELISA
enzyme-linked immunosorbant assay
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- MEM
minimal essential medium
- PBS
phosphate-buffered saline
- RT-PCR
real-time polymerase chain reaction
- SE
standard error of the mean
- TGF-β
transforming growth factor-β
- TMB
3,3′,5,5′-tetramethylbenzidine
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
OHSU and Dr. David Farrell have a significant interest in Gamma Therapeutics, a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU.
References
- 1.Lovely RS, Falls LA, Al-Mondhiry HA, Chambers CE, Sexton GJ, Ni H, Farrell DH. Association of γA/γ' fibrinogen levels and coronary artery disease. Thromb Haemost. 2002;88:26–31. [PubMed] [Google Scholar]
- 2.Drouet L, Paolucci F, Pasqualini N, Laprade M, Ripoll L, Mazoyer E, Bal dit Sollier C, Vanhove N. Plasma γ'/γ fibrinogen ratio, a marker of arterial thrombotic activity : A new potential cardiovascular risk factor? Blood Coagul Fibrinol. 1999;10(S1):128. [PubMed] [Google Scholar]
- 3.Mannila MN, Lovely RS, Kazmierczak SC, Eriksson P, Samnegård A, Farrell DH, Hamsten A, Silveira A. Elevated plasma fibrinogen γ' concentration is associated with myocardial infarction: Effects of variation in fibrinogen genes and environmental factors. J Thromb Haemost. 2007;5:766–773. doi: 10.1111/j.1538-7836.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheung EY, Vos HL, Kruip MJ, den Hertog HM, Jukema JW, de Maat MP. Elevated fibrinogen γ' ratio is associated with cardiovascular diseases and acute phase reaction but not with clinical outcome. Blood. 2009;114:4603–4604. doi: 10.1182/blood-2009-08-236240. [DOI] [PubMed] [Google Scholar]
- 5.Cheung EY, de Willige SU, Vos HL, Leebeek FW, Dippel DW, Bertina RM, de Maat MP. Fibrinogen γ' in ischemic stroke: A case-control study. Stroke. 2008;39:1033–1035. doi: 10.1161/STROKEAHA.107.495499. [DOI] [PubMed] [Google Scholar]
- 6.Alexander KS, Madden TE, Farrell DH. Association between γ' fibrinogen levels and inflammation. Thromb Haemost. 2011;105:605–609. doi: 10.1160/TH10-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovely RS, Kazmierczak SC, Massaro JM, D'Agostino RB, Sr, O'Donnell CJ, Farrell DH. γ' fibrinogen: evaluation of a new assay for study of associations with cardiovascular disease. Clin Chem. 2010;56:781–788. doi: 10.1373/clinchem.2009.138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung DW, Davie EW. γ and γ' chains of human fibrinogen are produced by alternative mRNA processing. Biochemistry. 1984;23:4232–4236. doi: 10.1021/bi00313a033. [DOI] [PubMed] [Google Scholar]
- 10.Fornace AJ, Cummings DE, Comeau CM, Kant JA, Crabtree GR. Structure of the human γ-fibrinogen gene. alternate mRNA splicing near the 3' end of the gene produces γA and γB forms of γ-fibrinogen. J Biol Chem. 1984;259:12826–12830. [PubMed] [Google Scholar]
- 11.Collet JP, Nagaswami C, Farrell DH, Montalescot G, Weisel JW. Influence of γ' fibrinogen splice variant on fibrin physical properties and fibrinolysis rate. Arterioscler Thromb Vasc Biol. 2004;24:382–386. doi: 10.1161/01.ATV.0000109748.77727.3e. [DOI] [PubMed] [Google Scholar]
- 12.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 13.Farrell DH, Mulvihill ER, Huang S, Chung DW, Davie EW. Recombinant human fibrinogen and sulfation of the γ' chain. Biochemistry. 1991;30:9414–9420. doi: 10.1021/bi00103a004. [DOI] [PubMed] [Google Scholar]
- 14.Mackiewicz A, Speroff T, Ganapathi M, Kushner I. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol. 1991;146:3032–3037. [PubMed] [Google Scholar]
- 15.Schorer AE, Moldow CF, Rick ME. Interleukin 1 or endotoxin increases the release of von Willebrand factor from human endothelial cells. Br J Haematol. 1987;67:193–197. doi: 10.1111/j.1365-2141.1987.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 16.Nachman R, Hajjar K, Silverstein R, Dinarello CA. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. J Exp Med. 1986;163:1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawroth PP, Handley DA, Esmon CT, Stern DM. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986;83:3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahni A, Guo M, Sahni SK, Francis CW. Interleukin-1β but not interleukin-1α binds to fibrinogen and fibrin and has enhanced activity in the bound form. Blood. 2004;104:409–414. doi: 10.1182/blood-2004-01-0126. [DOI] [PubMed] [Google Scholar]
- 19.Grainger DJ. TGF-β and atherosclerosis in man. Cardiovasc Res. 2007;74:213–222. doi: 10.1016/j.cardiores.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Hassan JH, Chelucci C, Peschle C, Sorrentino V. Transforming growth factor β (TGF-β) inhibits expression of fibrinogen and factor VII in a hepatoma cell line. Thromb Haemost. 1992;67:478–483. [PubMed] [Google Scholar]
- 21.Mackiewicz A, Ganapathi MK, Schultz D, Brabenec A, Weinstein J, Kelley MF, Kushner I. Transforming growth factor β1 regulates production of acute-phase proteins. Proc Natl Acad Sci U S A. 1990;87:1491–1495. doi: 10.1073/pnas.87.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riipi L, Carlson E. Tumor necrosis factor (TNF) is induced in mice by Candida albicans: Role of TNF in fibrinogen increase. Infect Immun. 1990;58:2750–2754. doi: 10.1128/iai.58.9.2750-2754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan HO, Simpson-Haidaris PJ. Functional analysis of interleukin 6 response elements (IL-6REs) on the human γ-fibrinogen promoter. J Biol Chem. 2003;278:41270–41281. doi: 10.1074/jbc.M304210200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Fuentes NL, Fuller GM. Characterization of the IL-6 responsive elements in the γ fibrinogen gene promoter. J Biol Chem. 1995;270:24287–24291. doi: 10.1074/jbc.270.41.24287. [DOI] [PubMed] [Google Scholar]
- 25.Uitte de Willige S, Standeven KF, Philippou H, Ariëns RA. The pleiotropic role of the fibrinogen γ' chain in hemostasis. Blood. 2009;114:3994–4001. doi: 10.1182/blood-2009-05-217968. [DOI] [PubMed] [Google Scholar]
- 26.Farrell DH. γ' fibrinogen as a novel marker of thrombotic disease. Clin Chem Lab Med. 2012 doi: 10.1515/cclm-2012-0005. In press. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Fuller GM. Interleukin 1β inhibits interleukin 6-mediated rat γ fibrinogen gene expression. Blood. 2000;96:3466–3472. [PubMed] [Google Scholar]
- 28.Mizuguchi J, Hu CH, Cao Z, Loeb KR, Chung DW, Davie EW. Characterization of the 5'-flanking region of the gene for the γ chain of human fibrinogen. J Biol Chem. 1995;270:28350–28356. doi: 10.1074/jbc.270.47.28350. [DOI] [PubMed] [Google Scholar]
- 29.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Chu JY, Yu JK, Huang XQ, Liu XJ, Shi L, Che YC, Xie JY. Regulation of alternative splicing of Bcl-x by IL-6, GM-CSF and TPA. Cell Res. 2004;14:473–479. doi: 10.1038/sj.cr.7290250. [DOI] [PubMed] [Google Scholar]
- 31.Weg-Remers S, Ponta H, Herrlich P, König H. Regulation of alternative premRNA splicing by the ERK MAP-kinase pathway. EMBO J. 2001;20:4194–4203. doi: 10.1093/emboj/20.15.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Baumann H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999;19:5326–5338. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalivendi SV, Yedlapudl D, Hillard CJ, Kalyanaraman B. Oxidants induce alternative splicing of alpha-synuclein: Implications for Parkinson's Disease. Free Radic Biol Med. 2010;48:377–383. doi: 10.1016/j.freeradbiomed.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins EW, Travanty EA, Yang K, Iczkowski KA. MAP kinase pathways and calcitonin influence CD44 alternate isoform expression in prostate cancer cells. BMC Cancer. 2008;8:260. doi: 10.1186/1471-2407-8-260. [DOI] [PMC free article] [PubMed] [Google Scholar]