Abstract
Background and Purpose
Over-assembly of synaptic glutamate receptors leads to excitotoxicity. The goal of this study is to investigate phosphorylation and assembly of AMPA and NMDA receptors after brain ischemia with reperfusion (I/R).
Methods
Rats were subjected to 15 min of global ischemia followed by 0.5, 4, and 24 h of reperfusion. Phosphotyrosine (Ptyr) peptides of glutamate receptors in synaptosomal fraction after I/R were identified and quantified by state-of-the-art immuno-affinity purification of Ptyr peptides followed by LC-MS/MS analysis (IAP-LC/MS/MS). Glutamate receptor phosphorylation and synaptic assembly after I/R were studied by biochemical methods.
Results
Numerous Ptyr sites of AMPA and NMDA were upregulated by about 2- to 37-fold after I/R. A core glutamate receptor kinase, Src kinase, was significantly activated. GluR2/3 and NR2A/B were rapidly clustered from extrasynaptic to synaptic membrane fractions after I/R. GluR2/3 was then translocated into the intracellular pool, whereas NR2A/B remained in the synaptic fraction for as long as 24 h. Consistently, trafficking-related phosphorylation of GluR2/3-S880 was significantly but transiently upregulated, whereas NR2A/B-Y1246 and -Y1472 were significantly and persistently upregulated after I/R.
Conclusions
Phosphorylation of glutamate receptors at synapses may lead to over-assembly of glutamate receptors, probably via activation of Src family kinases, after I/R. This study provides “global” proteomic information about glutamate receptor tyrosine phosphorylation after brain ischemia.
Keywords: Brain Ischemia, Glutamate Receptor, Tyrosine Phosphorylation, Src Family Kinases, Synapse, Mass Spectrometry, Proteomics
INTRODUCTION
An episode of complete ischemia with reperfusion (I/R) or incomplete ischemia in the penumbral area after focal ischemia produces minimal apparent initial damage, and does not result in acute neuronal death. Rather, these milder insults are associated with profound alterations in synaptic ultrastructure, molecular organization and neurological dysfunction.1, 2 Ultimately, functional recovery after brain ischemia requires precise synaptic communication and is probably the most important issue for stroke patients. For that reason, it may be important to investigate the effects of ischemia on synaptic glutamate receptor tyrosine phosphorylation, even in the brain regions where do not show any obvious lesion under the light microscopy. The rationales of such study may include that: (i) brain ischemia often leads to neuronal death in selective brain regions, (ii) our previous studies have shown that there are profound and long-lasting synaptic morphological and molecular composition changes in these brain areas where overt neuronal damage does not occurs after ischemia;1, 2 (iii) functional changes such as language representations, mood, and memory after ischemia may be correlated with synaptic connections in the brain area where neuronal death may not be necessarily to occur after ischemia;3 (iv) the most vulnerable area where neuronal death occurs such as CA1 after I/R may lose synaptic connections to the projecting cortical areas; and (v) postischemic neurons may modify existing synapses or form new synapses via changes in tyrosine phosphorylation-mediated signaling pathways for adapting ischemic brain injury.4 Therefore, studying of growth-related tyrosine phosphorylation of synaptic proteins after ischemia may provide more information about functional changes and neuroplasticity after brain ischemia.
Ionotropic glutamate receptors include N-methyl-D-aspartate (NMDA) receptors (NR1, NR2A-2D) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (GluR1–4). Both NMDA and AMPA receptors are not only located in the postsynaptic density (PSD)-associated membrane, but a significant proportion of them are distributed also to the perisynaptic membranes, and intracellular light membranes, known as extrasynaptic receptor and intracellular receptor pools, respectively. In response to changes in synaptic activities, NMDA and AMPA receptors are translocated rapidly among different pools under physiological conditions.5–7 Src family kinases (SFKs) have been suggested to act as a “core kinases” to integrate synaptic signalings to synaptic activities.8–11
In the present study, we identified numerous Ptyr sites of ionotropic glutamate receptors by a state-of-the-art IAP-LC-MS/MS analysis. Most of them were significantly upregulated as high as 37 fold after I/R. We also found that AMPA and NMDA receptors were significantly clustered at synapse during the reperfusion phase. The results provide new insights into phosphorylation and synaptic assembly of glutamate receptors after I/R.
MATERIALS AND METHODS
Animal Model
All procedures were approved by the Animal Care and Use Committee at the University of Maryland. Brain ischemia followed by reperfusion was produced using the 2-vessel occlusion (2VO) model in Wistar rats as described before.1 Blood gases were measured and adjusted to PaO2 > 90 mmHg, PaCO2 35–45 mmHg, and pH 7.35–7.45 by changing the tidal volume of the respirator, and brain temperature was maintained at 37°C before, during and after ischemia. A sham-operated control group and groups of 15 min of ischemia followed by 0.5, 4 and 24 h of reperfusion were used as described before.1 For histopathology, acid fuchsin and celestine blue-stained 8 µm brain sections were examined by light microscopy. For biochemical analysis, brains were frozen in situ with liquid nitrogen according to the method of Poten et al.11 Frozen brains were sliced into 1–2 mm coronal sections. The dorsal cortical areas above the rhinal fissure and between bregma +1.5 mm and bregma −2.5 mm, were dissected in a glove freezer box at −12°C. The cortical tissues was cut into very small pieces and mixed thoroughly to avoid unevenly sampling among different experimental groups.
Preparation of Synaptosomal Fraction
The synaptosomal fraction was isolated according to the procedure described previously.1 Briefly, cortical tissue homogenate was centrifuged at 10,000 g to obtain a pellet fraction. The pellet fraction was loaded onto a sucrose density gradient of 0.85M/1.0M/1.2M and centrifuged at 82,500 g for 2 h at 4°C. The synaptosomal fraction was collected from the 1.0M/1.2M sucrose interface. After washing with 1% Triton X-100, the synaptosomal pellet and synaptosomal supernatant fractions were collected by centrifugation. The synaptosomal pellet was dissolved and denatured in 8 M Urea lysis buffer (20 mM Hepes, 8 M Urea, 1 mM sodium vanadate, 2.5 mM sodium pyrophosphate, 1 mM b-glycerol-phosphate), reduced by adding DTT (4.5 mM), Alkylated by adding iodoacetamide (8.3 mM), and digested by trypsin. Protein digested was purified over C18 Sep-pak cartridge (Waters). Eluted peptides were lyophilized, and stored at −80°C.
Immunoaffinity purification (IAP)-coupled liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS) analysis of Ptyr peptides
IAP of phosphotyrosine peptides was carried out according to the method of Rush et al.12 using Phospho-tyrosine PhosphoScan® Kit (Cell Signaling Technology, Danvers, MA). LC-MS/MS analysis was performed with an LTQ Orbitrap Mass Spectrometer (Thermo Fisher Scientific) and a peptide mass accuracy of ±3 ppm was one of the filters used for peptide identification. Details were described previously.13 Sequest (Thermo Fisher Scientific) searches were done against the NCBI rat database, allowing for tyrosine phosphorylation (Y+80) and oxidized methionine (M+16) as differential modifications. The PeptideProphet probability threshold was chosen to give a false positive rate of 5% for the peptide identifications.14
Quantitative MS/MS analysis of Ptyr peptides
To quantify Ptyr peptides, the intensity value of the peak apex for each peptide was determined for each individual LC-MS/MS run and compared against other LC-MS/MS runs.13 For LC-MS/MS runs in which the peptide was not identified by MS/MS, peptide m/z and retention time (RT) values were used to search for peaks in the MS1 channel, which would correspond to the peptide ion. Similar to several previous studies13, about 30–40% peptide intensities were derived from MS1 channel. In order to reduce the selection of the incorrect peak when an MS/MS was not triggered, we used several criteria. For example, in cases where an MS/MS was not triggered for a peptide m/z (mass-to-charge ratio), we used peptide m/z values in common between runs to define retention time (RT) differences by simple linear regression. Once RT drift was defined, we required the m/z value to be present in a narrow RT window of 2 min. Within that RT window, we required a mass tolerance of 10 ppm. Peaks identified within the RT window and mass tolerance were further scrutinized for the correct charge state by requiring at least two C13-containing isotopes along with the monoisotopic m/z. Finally, only one peak was allowed being observed passing each of these criteria. If an identified peak passed each of these criteria, its intensity at the peak's apex was extracted and used for quantification.
Preparation of synaptic, extrasynaptic and intracellular light membrane fractions
Neocortical tissues were homogenized with a Dounce homogenizer (50 strokes) in a homo-buffer by the method described previously.1, 15 The homogenate was centrifuged at 10,000 g for 10 min at 4°C to obtain a pellet and a supernatant S2. The pellet was washed with the homo-buffer containing 2% Triton X-100 (TX100) and 500 mmol/L KCl, then centrifuged at 18,000 g for 20 min. The pellet, referred to as P(1+2)p or synaptic fraction (SYN), containing detergent- and salt-insoluble proteins tightly associated to PSDs.16 The supernatant was referred to as P(1+2)s or extrasynaptic fraction (EXTRA), containing detergent- and salt-soluble proteins not associated with PSDs.16 The S2 fraction was further centrifuged at 165,000 g for 60 min at 4°C to obtain a pellet or intracellular light membrane fraction (P3 or LM) and cytosol (S3 or CYTO). Protein concentration was determined by the micro-bicinchonic acid method (Pierce Biochemicals, Rockford, IL, USA).
Western blot analysis
Western blot analysis was carried out with 8% SDS-PAGE. Equal amounts of samples containing 30 µg protein from homogenate, P(1+2)s, S3 fractions and 50 µg from P(1+2)p and P3 fractions were subjected to SDS-PAGE and then transferred to Immobilon-P membranes (Millipore). After incubation with primary and then horseradish peroxidase-conjugated secondary antibodies, blots were developed with an ECL detection system (Amersham, Piscataway, NJ, USA). The films were scanned and the optical densities of the protein bands were quantified using NIH ImageJ software. One-way ANOVA followed by Dunnett’s post hoc test was employed to assess statistical significance (* p < 0.05 and ** p < 0.01).
RESULTS
Histology
No morphological changes in brain sections were seen under the light microscopy between sham-operated control rats and rats subjected to 15 min of ischemia followed by 4 h of reperfusion. Delayed neuronal death occurred after 48 h of reperfusion selectively in dorsal CA1 neurons. Only a few, if any, neocortical neurons were damaged at 7 days of reperfusion (Supplemental Figure S1 B, arrows). All these results are consistent with previous reports.17, 18 The objective of this study was to investigate synaptic NMDA and AMPA receptor assembly in brain tissues without apparent pathological damage. Therefore, neocortical tissues from sham-control and 4 h of reperfusion (I/R-4h) were employed in the IAP-LC-MS/MS analysis.
IAP-LC/MS/MS Analysis of Synaptic NMDAR and AMPAR Ptyr Motifs
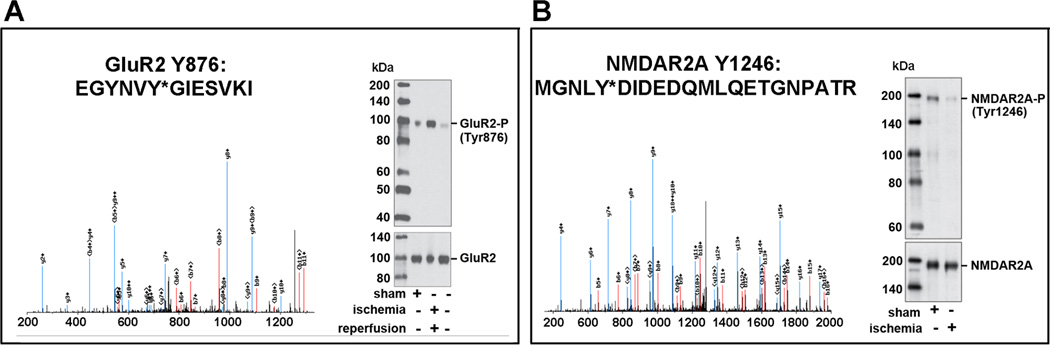
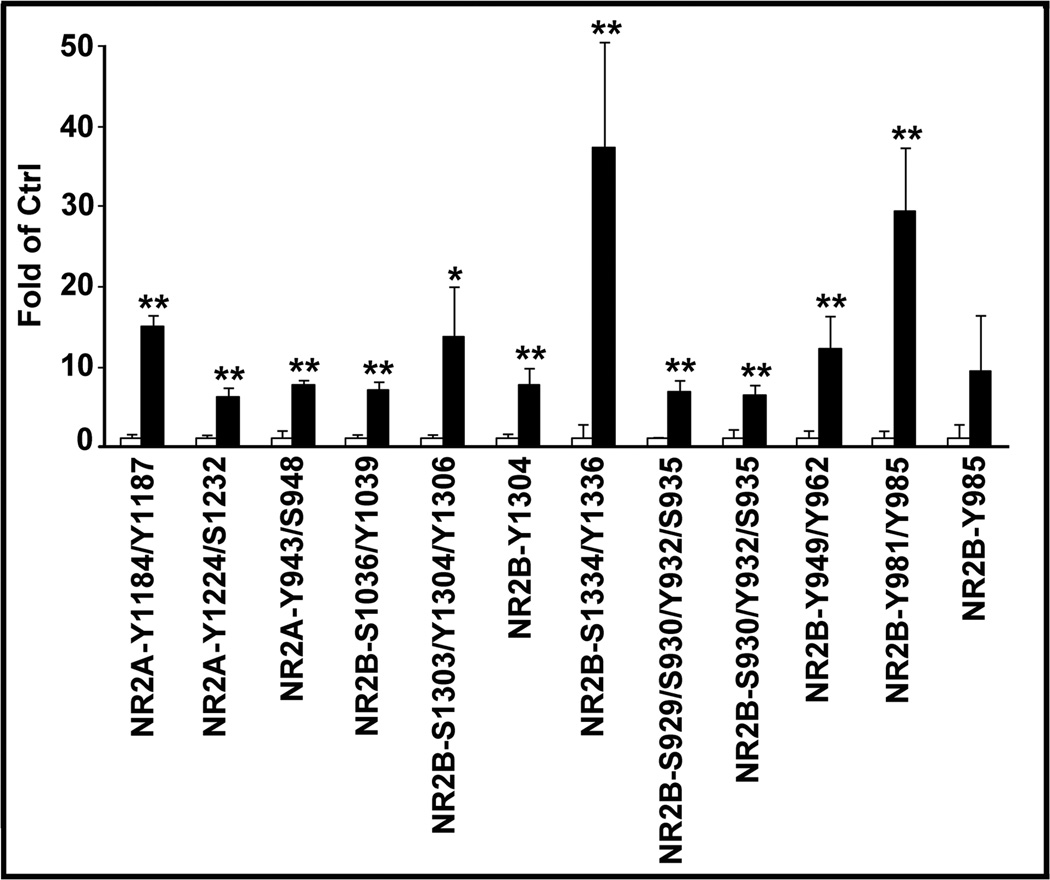
All Ptyr peptide sequences presented in this study were reproducibly captured at least three times. As demonstrated in recent studies, the IAP-LC-MS/MS analysis is highly specific for detecting Ptyr-peptides.12, 13 Fig. 1 shows representative MS spectra of GluR2 and NR2, respectively. Western blot validations revealed that phosphorylations of GluR2/3-Y867 and NR2B-Y1246 were significantly reduced in brain tissue owing to ATP depletion during ischemia, and were markedly increased at 4 h of reperfusion. Several Ptyr sites at C-termini of GluR2/3 were identified (Table 1 and Supplemental Table S2). In addition to the previously known GluR2-Y876, GluR3-Y871/881/869/Y873/Y874 sites were newly identified. Similarly, NR2A Ptyr-1184/1187/1246/1267 and NR2B Ptyr-1070/1155/1225/1304 sites were newly identified and they were mostly increased at 4 h of reperfusion (Table 1 and Supplemental Table S2). Fig. 2 shows the quantities of those Ptyr-sites with a more than 5-fold increase after ischemia.
Fig. 1.
IAP-LC-MS/MS identification of Ptyr sites of NMDA and AMPA receptors. Brain synaptosomal fractions were prepared from sham-operated control rats and rats subjected to 15 min of ischemia followed by 30 min of reperfusion (I/R). A: MS spectrum of GluR2-Y876 and its phosphorylation status after I/R. B: MS spectrum of NR2A-Y1246 and its phosphorylation status during ischemia.
Table 1.
Phosphotyrosine peptides analysis of synaptosomal fraction purified from rat cortical tissues after ischemia.
| Protein Name | Sites | Charge | Calc. m/z | Count in detail | Peptide | Fold of Control |
|---|---|---|---|---|---|---|
| Glutamate receptor 2 isoform 2 precursor | ||||||
| GluR2 | 869, 876 | 3 | 1284.2381 | 1 | NPQNINPSSSQNSQNFATY*KEGYNVY*GIESVKI | 3.07 |
| GluR2 | 876 | 2 | 775.8605 | 5 | EGYNVY*GIESVKI | 1.85 |
| Glutamate receptor 3 isoform 2 precursor | ||||||
| GluR3 | 871 | 3 | 794.0288 | 7 | NTQNFKPAPATNTQNY*ATYR | 1.82 |
| GluR3 | 871, 874 | 3 | 820.6842 | 1 | NTQNFKPAPATNTQNY*ATY*R | 4.50 |
| GluR3 | 873 | 3 | 794.0288 | 3 | NTQNFKPAPATNTQNYAT*YR | |
| GluR3 | 881 | 2 | 769.8424 | 1 | EGYNVY*GTESVKI | 1.38 |
| Glutamate receptor [NMDA] subunit epsilon-1 precursor | ||||||
| NMDAR2A | 1184, 1187 | 3 | 835.0279 | 6 | NPLHNEDGLPNNDQY*KLY*AK | 15.15 |
| NMDAR2A | 1187 | 3 | 808.3724 | 7 | NPLHNEDGLPNNDQYKLY*AK | 2.23 |
| NMDAR2A | 1224, 1232 | 3 | 830.6843 | 3 | SCLSNLPTY*SGHFTMRS*PFK | 6.18 |
| NMDAR2A | 1246 | 3 | 869.6961 | 11 | M#GNLY*DIDEDQMLQETGNPATR | 2.60 |
| NMDAR2A | 1267 | 3 | 779.0057 | 2 | EEVY*QQDWSQNNALQFQK | 2.95 |
| NMDAR2A | 943, 948 | 3 | 968.4397 | 1 | GSLIVDMVSDKGNLIY*SDNRS*FQGK | 7.67 |
| Glutamate receptor [NMDA] subunit epsilon-2 precursor | ||||||
| NMDAR2B | 1036, 1039 | 2 | 908.889 | 2 | HSQLS*DLY*GKFSFK | 7.01 |
| NMDAR2B | 1225 | 3 | 743.9951 | 6 | SCPSKLHNY*SSTVAGQNSGR | 2.61 |
| NMDAR2B | 1303, 1304, 1306 | 3 | 905.3853 | 1 | RQHS*Y*DT*FVDLQKEEAALAPR | 13.84 |
| NMDAR2B | 1304 | 3 | 852.0744 | 6 | RQHSY*DTFVDLQKEEAALAPR | 7.83 |
| NMDAR2B | 1334, 1336 | 3 | 915.0032 | 2 | FM#DGS*PY*AHM#FEMPAGESSFANK | 37.33 |
| NMDAR2B | 1472 | 2 | 745.309 | 5 | AFNGSSNGHVY*EK | 1.54 |
| NMDAR2B | 930, 932, 935 | 2 | 781.2541 | 3 | RESS*VY*DIS*EH | 6.53 |
| NMDAR2B | 949, 962 | 4 | 921.8638 | 2 | SFTHSDCKSY*NNPPCEENLFSDY*ISEVER | 12.30 |
| NMDAR2B | 981, 985 | 3 | 746.9516 | 6 | DSNVY*QDHY*HHHHRPH | 29.53 |
| NMDAR2B | 985 | 3 | 720.2962 | 15 | DSNVYQDHY*HHHHRPH | 9.53 |
Ptyr (pY) peptide sequences are presented along with the charge, mass-to-charge ratios (m/z). Increased phosphorylation is shown in fold of control relative to sham-operated control. Bold letters indicate novel Ptyr sites
Fig. 2.
Quantitative analysis of Ptyr sites of glutamate receptors. The same synaptosomal fractions as in Fig. 1 were analyzed by IAP-LC/MS/MS analysis. Those with more than 5-fold upregulation of Ptyr-sites after I/R are presented. Data are mean ± SD of fold of control (N=4). One-way ANOVA followed by Dunnett’s test was used for statistical analysis. *p<0.05 and **p<0.01 between sham-operated control and postischemic condition.
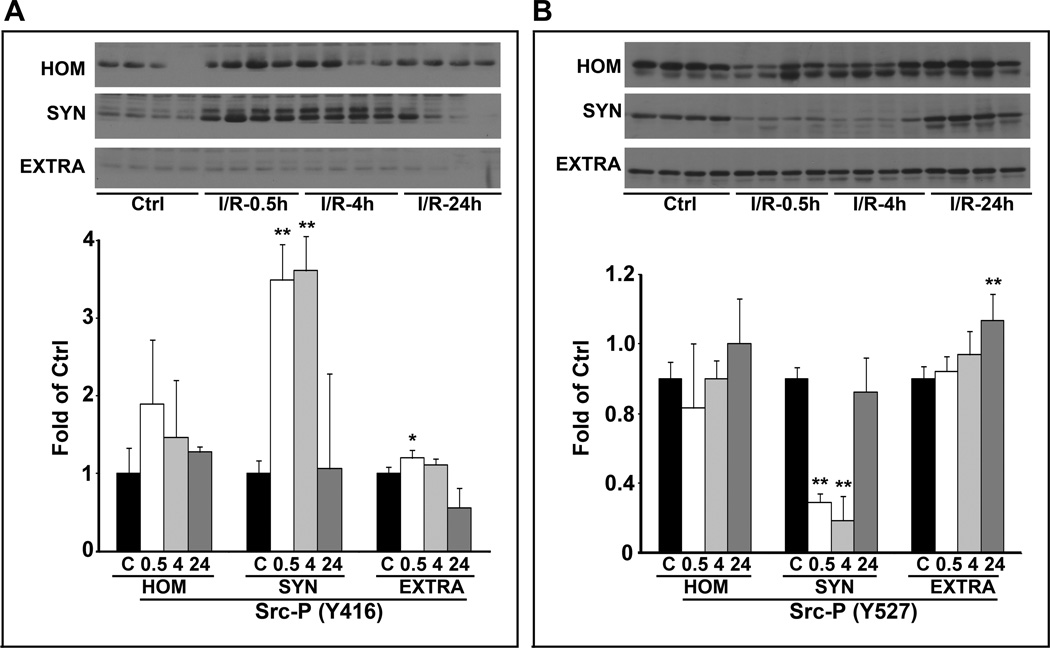
Activation of Synaptic Src Kinase after I/R
Activation of Src kinase requires phosphorylation of the active loop Y416 and/or dephosphorylation of the inhibitory site Y527.10 The level of phospho-Src Y416 was significantly upregulated at 30 min and 4 h, and then returned to the control level at 24 h of reperfusion (Fig. 3A). In contrast, phospho-Src Y527 was significantly downregulated at 30 min and 4 h, and then returned to the control level at 24 h of reperfusion (Fig. 3B). Up-regulation of the activation-site Y416 and down-regulation of the inhibitory-site Y527 were observed only in TX100-insoluble synaptic fractions (SYN), while no changes were detected in TX100-soluble extrasynaptic fractions (EXTRA) (Fig. 3). The data showed that Src was significantly activated only in the synaptic fraction after I/R.
Fig. 3.
Changes in Src-kinase phosphorylation after I/R. Neocortical subcellular fractions of homogenate (HOM), synaptic membranes (SYN), and extrasynaptic membranes (EXTRA) were obtained from sham-operated rats and rats subjected to 15 min of ischemia followed by 0.5, 4 and 24 h of reperfusion. Immunoblot membranes were labeled with antibodies to Src-Y416 (a) and Src-Y527 (b). Data are mean ± SD of fold of control (N=4). One-way ANOVA followed by Dunnett’s test was used. *p<0.05 and **p<0.01 between sham-operated control and postischemic conditions.
Synaptic Glutamate Receptor Clustering
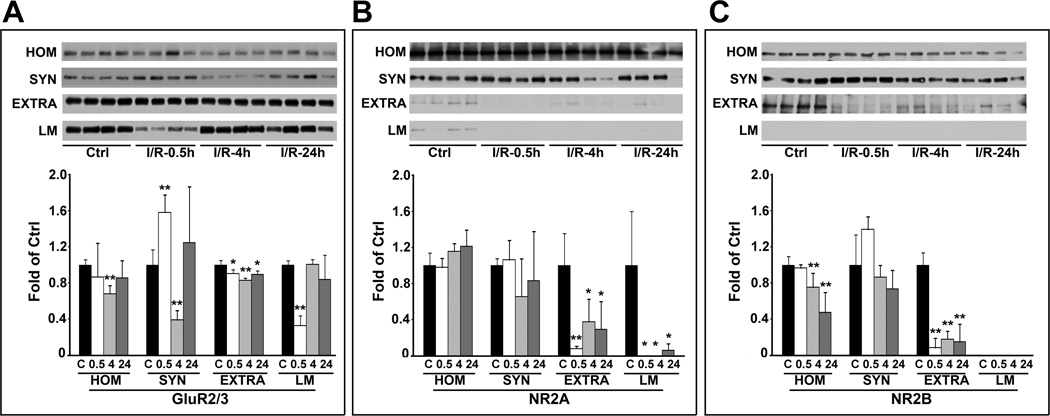
To understand glutamate receptor clustering after I/R, we prepared synaptic (SYN), extrasynaptic (EXTRA), and intracellular light membrane (LM) fractions (Supplemental Figure S3 A). β-actin, as a protein-loading control, was unchanged in all subcellular factions after I/R (Supplemental Figure S4). PSD93 and PSD95 are PSD proteins and thus located mainly in the synaptic fraction (SYN) and to a much lesser degree in EXTRA and LM fraction. Clathrin and Rab4 are early endosome markers. They are mainly located in the light membrane fraction (LM), and too a much lesser degree also in the cytosol (Supplemental Figure S3 B).
GluR2/3 was significantly increased in SYN and concomitantly decreased in EXTRA and LM fractions at 30 min of reperfusion. GluR2/3 was then reduced to below the control levels in SYN and EXTRA fractions but recovered in LM (intracellular pool) fraction (Fig. 4A). The data suggested that GluR2/3 was translocated from EXTRA and intracellular LM pools to synapses initially, and then returned to the intracellular pools, probably via the endocytic pathway.19–21
Fig. 4.
Redistribution of GluR2/3, NR2A and NR2B after I/R. GluR2/3, NR2A, and NR2B in the separated subcellular fractions as shown in Data supplement 3 were analyzed by Western blotting and were quantified with NIH ImageJ software. Data are fold of control (mean ± SD, n = 4). One-way ANOVA followed by Dunnett’s test were used for statistical analysis. *p<0.05 and **p<0.01 between sham-operated control and experimental groups.
Similarly, NR2A and NR2B tended to increase in SYN fractions, but significantly decreased in EXTRA and LM fractions for as long as 24 h of reperfusion after I/R (Fig. 4B and Fig. 4C).
Phosphorylation of GluR2-S880, NR2A-Y1246, and NR2B-Y1472
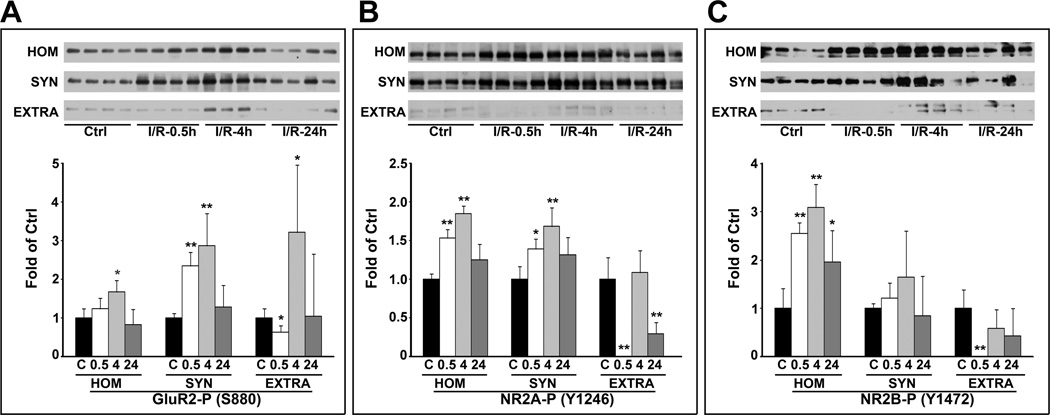
To verify further the proteomic MS data and synaptic clustering of glutamate receptors shown above, we studied all subcellular fractions with antibodies to GluR2-S880, NR2A-Y1246, and NR2B-Y1472. These sites are related to glutamate receptor trafficking.19–25 The Western blot data showed that the levels of GluR2-S880, NR2A-Y1246, and NR2B-Y1472 were significantly increased at 0.5 and 4 h of reperfusion in the synaptic (SYN) fractions after I/R (Fig. 5). In the perisynaptic (EXTRA) fraction, GluR2-S880, NR2A-Y1246, and NR2B-Y1472 were initially decreased at 0.5 h, transiently returned to or above the control level at 4 h of reperfusion, and then decreased again at 24 h of reperfusion (Fig. 5). These sites were not detectable in the intracellular LM fraction after I/R (data not shown). These results suggested that the glutamate receptor trafficking-related phosphorylation sites were highly upregulated in the synaptic fraction after I/R.
Fig. 5.
Phosphorylation of GluR2/3-S880, NR2A-Y1246 and NR2B-Y1472 after I/R. GluR2/3-S880, NR2A-Y1246 and NR2B-Y1472 in the separated subcellular samples were further analyzed by Western blotting and quantified with NIH ImageJ software. Data are mean ± SD of fold of control (n = 4). One-way ANOVA followed by Dunnett’s test were used for statistical analysis. *p<0.05 and **p<0.01 between sham-operated control and experimental groups.
DISCUSSION
The present study showed that I/R led to upregulation of phosphorylated glutamate receptors at multiple Ptyr-sites (see Table 1, Data supplement S2). Many novel Ptyr-sites were identified in this study. Consistently, a core glutamate receptor kinase, Src kinase, was activated after I/R. GluR2/3, as well as NR2A/B, were rapidly clustered from extrasynaptic to synaptic membranes after I/R. Synaptic GluR2/3 was then translocated into the intracellular LM pool, whereas NR2A/B remained in the synaptic fraction for as long as 24 h of reperfusion after I/R. Consistently, GluR2/3 trafficking-related phosphorylation site S880 was significantly but transiently upregulated after I/R, whereas NR2A and NR2B trafficking-related phosphorylation sites Y1246 and Y1472 were significantly and persistently upregulated after I/R. The results suggest that tyrosine phosphorylation of glutamate receptors at synapses may lead to over-assembly of glutamate receptors after I/R. This study provides “global” proteomic information about ionotropic glutamate receptor tyrosine phosphorylation after brain ischemia.
Identification of Glutamate Receptor Ptyr Sites by IAP-LC/MS/MS
Although most neocortical neurons will not die after a brief episode of brain ischemia, there are profound changes in ultrastructure and molecular composition at the synapses.1, 2, 15 In collaboration with Cell Signaling Technology (CST) (Danvers, MA), this study employed a state-of-the-art IAP-LC/MS/MS technology to study the overall glutamate receptor tyrosine phosphorylation status in the synaptic fractions after I/R. The selection of post-ischemic synaptic fractions is based on our previous observation that tyrosine phosphorylation in synaptic fractions is the most dramatic as well as the most selective among different subcellular fractions after brain ischemia.1, 15 A key reason may be that a brief episode of global ischemia induces overall depolarization of synapses, followed by repolarization upon reperfusion in the entire forebrain.18 Therefore, unlike physiological stimuli that activate a few or a group of neuronal networks, an episode of ischemia followed by reperfusion leads to overall activation of all forebrain synapses on a global scale.18 This may explain why changes in the Ptyr number and degree are so dramatic, in that all currently known Ptyr proteins are detected in post-ischemic synaptic fraction by the IAP-LC/MS/MS analysis. This is further supported by the fact that most, if not all, glutamate receptor-related synaptic events can be detected after I/R, including neurotransmitter release, induction of LTP, translocation of protein kinases, activation of the neurotrophin/receptor pathways, and activation of ERK kinases, and so forth.1, 15 This IAP-LC-MS/MS analysis of Ptyr-sites has tremendous advantages over more traditional methods in which a single protein or motif is usually characterized.26 Existing evidence proves that the IAP-LC-MS/MS analysis is highly efficient and specific for detecting Ptyr sites.12, 13
It should be pointed out that the synaptosomal fraction we used in this study contains mainly synaptic structures from neurons as demonstrated by the morphological assessment. However, this fraction might also contain small amount of membranes from non-neuronal cells. Therefore, the changes in glutamate receptor tyrosine phosphorylation is highly likely mainly neuronal, but may also occur in neuronal-to-astrocyte synapses after brain ischemia.27
Redistribution of Glutamate Receptors after I/R
AMPA and NMDA receptors are present in synaptic, extrasynaptic and intracellular pools of neurons.5–7 AMPA receptors are highly mobile, rapidly shuttling between these different pools. This process is regulated by phosphorylation of S880.19, 23, 25 Similarly, the synaptic translocation of NMDA receptors also depends on phosphorylation of NR2A-Y1246 and NR2B-Y1472. By using detergent-extraction and different receptor pool separation, this study analyzed glutamate receptor redistribution and showed that both GluR2/3 and NR2A/B were translocated to the synaptic fraction after I/R (see Fig. 4 and Fig. 5). These data are consistent with previous studies showing that synaptic localization of AMPA and NMDA receptors are tightly regulated by phosphorylation and synaptic activities.5, 19–25
Previous studies have shown that tyrosine phosphorylation of glutamate receptors by Src family kinases (SFKs) enhances their synaptic localization28. Inhibition of Src-kinase protects brain from ischemic injury.29–31 This is consistent with the fact that Src Family Kinases (SFKs) are responsible for GluR2/3 and NR2A/B synaptic clustering. In fact, many ischemia-upregulated phosphorylation sites of glutamate receptors listed in Table 1 (also in Data supplement S2) are Src kinase substrates. The evidence is also in agreement with the significant upregulation of Src active loop Y416 and down-regulation of Src inhibitory domain Y527 in a time course similar to that of NR2A/B tyrosine phosphorylation after I/R (see Fig. 3).
In summary, we have identified numerous Ptyr sites of ionotropic glutamate receptors by a state-of-the-art IAP-LC-MS/MS method. Most of them are Src-kinase substrates and are dramatically upregulated after I/R. Such upregulation is in accordance with GluR2/3 and NR2A/B synaptic clustering after I/R. These alterations provide an explanation of the synaptic dysfunction after brain ischemia.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING
This work was supported by National Institutes of Health grants NS36810 and NS030291, American Heart Association grant EIA 090042N.
Abbreviation
- I/R
ischemia and reperfusion
- Ptyr
phosphotyrosine
- Y
tyrosine
- S
Serine
- SFKs
Src family kinases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Hu B, Park M, Martone M, Fischer W, Ellisman M, Zivin J. Assembly of Proteins to Postsynaptic Densities after Transient Cerebral Ischemia. J. Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martone M, Jones Y, Young S, Ellisman M, Zivin J, Hu B. Modification of Postsynaptic Densities after Transient Cerebral Ischemia: A Quantitative and Three-Dimensional Ultrastructural Study. J Neurosci. 1999;19:1988–1997. doi: 10.1523/JNEUROSCI.19-06-01988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 4.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109:E2230–E2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petralia R, Wang Y, Hua F, Yi Z, Zhou A, Ge L, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardingham G, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nature Reviews Neurosciences. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tymianski M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nature Neuroscience. 2011;14:1369–1373. doi: 10.1038/nn.2951. [DOI] [PubMed] [Google Scholar]
- 8.Schumann J, Michaeli A, Yaka R. Src-protein tyrosine kinases are required for cocaine-induced increase in the expression and function of the NMDA receptor in the ventral tegmental area. J Neurochem. 2009;108:697–706. doi: 10.1111/j.1471-4159.2008.05794.x. [DOI] [PubMed] [Google Scholar]
- 9.Sinai L, Duffy S, Roder J. Src inhibition reduces NR2B surface expression and synaptic plasticity in the amygdala. Learn Mem. 2010;17:364–371. doi: 10.1101/lm.1765710. [DOI] [PubMed] [Google Scholar]
- 10.Salter M, Kalia L. Src kinase: a hub for NMNDA receptor regulation. Nature Reviews Neuroscience. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 11.Ponte´n U, Ratcheson R, Siesjo B. Metabolic changes in the brains of mice frozen in liquid nitrogen. J Neurochem. 1973;21:1211–1216. [PubMed] [Google Scholar]
- 12.Rush J, Moritz A, Lee K, Guo A, Goss V, Spek E, et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 13.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Deutsch E, Shteynberg D, Lam H, Sun Z, Eng J, Carapito C, et al. Trans-Proteomic Pipeline supports and improves analysis of electron transfer dissociation data sets. Proteomics. 2010;10:1190–1195. doi: 10.1002/pmic.200900567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B, Wieloch T. Tyrosine phosphorylation and activation of mitogen-activated protein kinase in the rat brain following transient cerebral ischemia. J Neurochem. 1994;62:1357–1367. doi: 10.1046/j.1471-4159.1994.62041357.x. [DOI] [PubMed] [Google Scholar]
- 16.Goebel-Goody S, Davies K, Alvestad Linger R, Freund R, Browning M. Phospho-regulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Hu B, Martone M, Jones Y, Liu C. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–3199. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjö B. Models for studying long-term recovery following forebrain ischemia in the rat. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. (1984). [DOI] [PubMed] [Google Scholar]
- 19.Fu J, deSouza S, Ziff EB. Intracellular membrane targeting and suppression of Ser880 phosphorylation of glutamate receptor 2 by the linker I-set II domain of AMPA receptor-binding protein. J Neurosci. 2003;23:7592–7601. doi: 10.1523/JNEUROSCI.23-20-07592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kropf M, Rey G, Glauser L, Kulangara K, Johnsson K, Hirling H. Subunit-specific surface mobility of differentially labeled AMPA receptor subunits. Eur J Cell Biol. 2008;87:763–778. doi: 10.1016/j.ejcb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Utvik JK, Haglerød C, Mylonakou MN, Holen T, Kropf M, Hirling H, et al. Neuronal enriched endosomal protein of 21 kDa colocalizes with glutamate receptor subunit GLUR2/3 at the postsynaptic membrane. Neuroscience. 2009;158:96–104. doi: 10.1016/j.neuroscience.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Roche K, Standley S, McCallum J, Dune Ly C, Ehlers M, Wenthold R. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 23.Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa T, Komai S, Watabe A, Kiyama Y, Fukaya M, Arima-Yoshida F, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.States BA, Khatri L, Ziff EB. Stable synaptic retention of serine-880-phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci. 2008;38:189–202. doi: 10.1016/j.mcn.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nada S, Shima T, Yanai H, Husi H, Grant S, Okada M, et al. Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem. 2003;278:47610–47621. doi: 10.1074/jbc.M303873200. [DOI] [PubMed] [Google Scholar]
- 27.Murai KK, Pasquale EB. Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia. 2011;59:1567–1578. doi: 10.1002/glia.21226. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Knox R, Pathipati P, Ferriero D. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci Lett. 2011;503:215–219. doi: 10.1016/j.neulet.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto R, Fujimaki K, Jeong MR, Christ L, Chuang DM. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity. FEBS Lett. 2003;538:145–148. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 30.Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlström H, Terént A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand. 2004;110:175–179. doi: 10.1111/j.1600-0404.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- 31.Hou XY, Liu Y, Zhang GY. PP2, a potent inhibitor of Src family kinases, protects against hippocampal CA1 pyramidal cell death after transient global brain ischemia. Neurosci Lett. 2007;420:235–239. doi: 10.1016/j.neulet.2007.03.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.