Abstract
Risk adapted therapy has been the cornerstone of treatment for pediatric B-precursor acute lymphoblastic leukemia (B-ALL). Recently, age (>13 years at diagnosis) has been identified as a very high-risk feature for chemotherapy treated pediatric B-ALL patients. Whether age at time of transplant is associated with poor outcomes in adolescents and young adults (AYA) is unknown. We hypothesized AYA receiving allogeneic hematopoietic cell transplantation (allo-HCT) would have greater relapse and inferior survival. We reviewed the outcomes in 136 consecutive patients (ages 0 to 30 years) with B-ALL who received myeloablative allo-HCT at our institution. Fifty-eight percent (n=79) were children <13 years of age and 42% (n=57) were AYA 13 to 30 years of age. Overall survival at 5-years was significantly lower in the AYA group [HR 1.74, 95% CI: 1.04–2.95; p=0.03]. In addition, AYA patients had a greater risk of transplant related mortality (TRM) at 1-year [HR 2.23, 95% CI: 1.01–4.90, p=0.05] but no difference in relapse [RR 0.85, 95% CI 0.41–1.76; p=0.66]. Based on this analysis, AYA patients undergoing allo-HCT for B-ALL have significantly inferior survival and greater TRM compared to children <13 years but no difference in relapse suggesting allo-HCT may overcome relapse in AYA. Further improvements in peri-transplant care are needed to limit complications in AYA patients.
Keywords: ALL, adolescent and young adult, transplantation
INTRODUCTION
Advances in the treatment of patients with pediatric B-precursor acute lymphoblastic leukemia (B-ALL) have led to dramatic improvements in both overall survival (OS) and disease free survival (DFS).(1) Despite these advances, cooperative group trials continue to identify subgroups of B-ALL patients with a greater risk for relapse following chemotherapy.(2, 3) These findings have resulted in intensification of therapy for patients deemed at high or very high-risk of relapse and conversely, de-escalation of therapy for those with a lower risk for relapse. Older age (≥10 years) at diagnosis has long been associated with an increased risk of B-ALL relapse (4, 5) and recently the Children's Oncology Group (COG) has identified age ≥13 years at the time of diagnosis as a very high-risk feature. On the CCG 1961 study, multimodal chemotherapy regimens in older (10–15 year old and >16 years) patients resulted in 3-year post-relapse survival of 35.4% and 14.7%, respectively compared to 48.6% for patients <10 years (p=0.001).(6)
Worse outcomes in chemotherapy treated older children and young adults with B-ALL were reported by several studies (7–9), leading to recognition that the adolescent and young adult (AYA) patient group has unique needs and challenges. There are a number of possible explanations to account for the inferior outcomes seen in AYA patients. These include poor chemotherapy compliance (10, 11) and a greater proportion of AYA patients with adverse risk factors, such as the Philadelphia chromosome (Ph+) or newly recognized high-risk mutations in IKAROS (12), JAK (13) and/or CRLF2 mutations/rearrangements. (14, 15) Whether these factors translate into inferior transplant outcomes is unknown. At present, there is a lack of data on the impact of age on allo-HCT outcomes for pediatric/AYA B-ALL. The AYA group has been variably defined with ages spanning from 13–15 years up to 25–40 years.(16–19) Here, we tested the hypothesis that AYA patients (defined between ages 13–30) would be at higher risk for relapse following allo-HCT compared to those younger than age 13.
PATIENTS AND METHODS
Study Design and Patient Characteristics
One hundred twenty-seven patients, ages 0 to 30 years, diagnosed with B-ALL underwent myeloablative allo-HCT at the University of Minnesota between 1995 and 2010. Patients who received a prior allo-HCT were excluded from this analysis. All patients and/or their parents or guardians signed consent to participate on institutional review board approved transplant protocols and outcomes were subsequently reviewed retrospectively. For the purpose of this analysis, we defined the adolescent and young adult (AYA) group as ages 13 to 30 years. See Table 1 for complete patient characteristics.
Table 1.
Patient Characteristics
| Factor | Age<13 | Age 13–30 | p-value | |
|---|---|---|---|---|
| N | 127 | 74 | 53 | |
| Age | <.01 | |||
| Median (range) | 9.97 (0.47–29.37) | 5.74 (0.47–12.53) | 17.70 (13.05–29.37) | |
| Gender | 0.82 | |||
| Male | 80 | 46 (62%) | 34 (65%) | |
| Donor source | 0.96 | |||
| Bone Marrow | 55 | 34 (46%) | 21 (40%) | |
| Related Donor | 32 | 17 (50%) | 15 (71%) | 0.08 |
| MURD | 19 | 15 (44%) | 4 (19%) | |
| MMURD | 4 | 2 (6%) | 2 (10%) | |
| Related PBSC | 7 | 2 (3%) | 5 (10%) | |
| Umbilical Cord Blood | 65 | 38 (51%) | 27 (51%) | |
| Single | 42 | 33 (87%) | 9 (33%) | <.01 |
| Double | 23 | 5 (13%) | 18 (67%) | |
| CR Status | 0.22 | |||
| CR1 | 42 | 20 (27%) | 22 (42%) | |
| CR2 | 70 | 45 (60%) | 25 (47%) | |
| CR3 | 15 | 9 (12%) | 6 (11 %) | |
| Recipient CMV | 0.01 | |||
| Negative | 66 | 46 (62%) | 20 (38%) | |
| Positive | 61 | 28 (38%) | 33 (62%) | |
| Ph + | .33 | |||
| Yes | 19 | 13 (18%) | 6 (11 %) | |
| No | 108 | 61 (82%) | 47 (89%) | |
| MLL-R | .79 | |||
| Yes | 11 | 6 (8%) | 5 (9%) | |
| No | 116 | 68 (92%) | 48 (91%) | |
| GVH prophylaxis | .18 | |||
| CSA | 3 | 3 (4%) | 0 | |
| CSA/MMF | 44 | 21 (28%) | 23 (43%) | |
| CSA/MTX | 56 | 32 (43%) | 24 (45%) | |
| CSA/PD/ATG | 19 | 15(20%) | 4 (8%) | |
| ELUTRIATION | 5 | 3 (4%) | 2 (4%) |
Seventy-four (58%) patients were younger than 13 years of age at the time of allo-HCT and 53 (42%) patients were 13–30 years old. The median age at allo-HCT for those <13 years was 5.74 (range, 0.47 – 12.53) years compared to 17.70 (range, 13.05 – 29.37) years for the AYA. The majority of patients were male for both groups. The median follow-up was 5.96 (range, 1.0 – 13.61) years for patients <13 years and 4.16 (1.0 – 14.07) years for the AYA. Prior to transplant, 20 (27%) patients <13 years were in first complete remission (CR1), 45 (60%) were in second complete remission (CR2) and 9 (12%) were in third complete remission (CR3), compared to 22 (42%) in CR1, 25 (47%) in CR2 and 6 (11%) in CR3 for the AYA group (p=0.22). There was a trend toward a shorter duration of first remission (CR1) with more early relapses (<36 months from diagnosis) in patients <13 years (n=32/45) compared to AYA patients (n=13/25) (p=0.06). For patients transplanted in CR2, there was no difference in time from relapse to transplant between the two age groups (p=0.19).
Time from initial diagnosis to transplant was similar between the two groups, with a median of 963.5 (range, 93 – 3711) days for patients <13 years and 593 (range, 80 – 4832) days for the AYA (p=0.43). Not surprisingly, patients <13 years of age were less likely to have been exposed to cytomegalovirus (CMV) prior to transplant compared to those ≥13 years (38% vs. 62%, p=0.01). Thirteen patients (18%) <13 years had a malignant clone with a Philadelphia chromosome rearrangement (Ph+; 9;22 translocation) compared to 6 (11%) of the AYA and 6 patients (8%) <13 years had mixed lineage leukemia rearrangements (MLL-R) compared to 5 (9%) of the AYA. Routine testing for other high-risk mutations, such as IKAROS, JAK and CRLF2 were not performed in these 127 patients.
Donor selection and Conditioning Regimens
Stem cell sources included HLA matched related donor (MRD) bone marrow, matched unrelated peripheral blood stem cell (PBSC), matched or mismatched unrelated donor (MURD or MMURD) bone marrow, and unrelated umbilical cord blood (UCB). Half of the patients received unrelated UCB with the remaining receiving either matched related or unrelated bone marrow grafts (n=34, 46% and n=21, 40%, respectively) or PBSC (n=2, 3% and n=5, 10%, respectively) (Table 1). More AYA patients received two umbilical cord blood units (p≤0.01). This difference is consistent with our intuitional cord blood selection criteria, where older patients are more likely to weigh more and require greater cell doses provided by two cord blood units.
All patients received myeloablative conditioning consisting of cyclophosphamide (120 mg/kg) +/− fludarabine (75 mg/m2) and total body irradiation (TBI; 1320 cGy) in 96%. The remaining 4% received busulfan/cyclophosphamide/fludarabine or etoposide/TBI. Graft-versus-host-disease (GVHD) prophylaxis was primarily cyclosporine based (n=122, 95%), with T cell depletion by elutriation in five patients.
Statistical Methods
Five outcomes were studied: OS, DFS, transplant related mortality (TRM), GVHD and relapse. The Kaplan-Meier method was used to estimate OS and DFS, while cumulative incidence was used to estimate TRM, GVHD and relapse.(20, 21) Cox multiple regression models were conducted for OS and DFS. Competing risk regression was employed for TRM, GVHD and risk of relapse. Age (<13 years versus ≥13 years) was the primary factor being considered for each endpoint in both univariate and multivariate regression. Other covariates used in the models included: number of prior remissions at the time of transplantation, gender, CMV status, bone marrow versus UCB, HLA matching (in the case of double cord transplant, the matching of the engrafting cord was used), year of transplant, presence of Philadelphia chromosome (Ph+) and MLL-R mutations. The backward method was used to determine the final model with a p-value of ≤0.05 considered significant in all statistical tests. Statistical analysis was performed with Statistical Analysis System statistical software version 9.2 (SAS Institute).
RESULTS
Neutrophil Engraftment and Survival
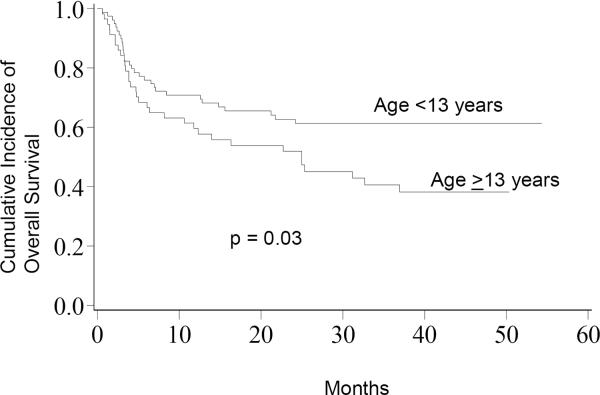
Engraftment by day 42 post-HCT (defined as 3 consecutive days with an ANC>500/μl) occurred in 70 (95%) patients <13 years and 48 (91%) AYA patients. In univariate analysis, patients <13 years had a significantly greater OS at 5-years compared to the AYA (62%, 95% CI 50–70% vs. 39%, 95% CI 26–53%; p=0.03, Figure 1). This finding was confirmed in a multivariate analysis that demonstrated a significant reduction in OS for the AYA group (HR 1.74, 95% CI: 1.04–2.95; p=0.03, Table 2). In contrast, 5-year DFS was not significantly different between patients <13 years compared to the AYA in either univariate (52%, 95% CI 40–63% vs. 40%, 95% CI 26–53%; p=0.18) or multivariate analyses (RR 1.39, 95% CI 0.85–2.25; p=0.18).
Figure 1.
Overall Survival; The cumulative incidence of overall survival for patients <13 years was 62% compared to 39% for patients ≥13 years.
Table 2.
Multivariate Analysis AYA vs. <13 years
| Factor | Relative Risk 95% CI | p-value |
|---|---|---|
| Overall Survival | 1.74 (1.04–2.95) | 0.03 |
| Disease-Free Survival | 1.39 (0.85–2.25) | 0.18 |
| Transplant Related Mortality | 2.23 (1.01–4.90) | 0.05 |
| Relapse | 0.80 (0.39–1.67) | 0.56 |
| Acute GVHD (Grade II–IV) | 1.38 (0.76–2.50) | 0.28 |
| Acute GVHD (Grade III–IV) | 0.71 (0.23–2.22) | 0.56 |
| Chronic GVHD | 2.73 (0.93–7.96) | 0.07 |
Treatment Related Mortality and GVHD
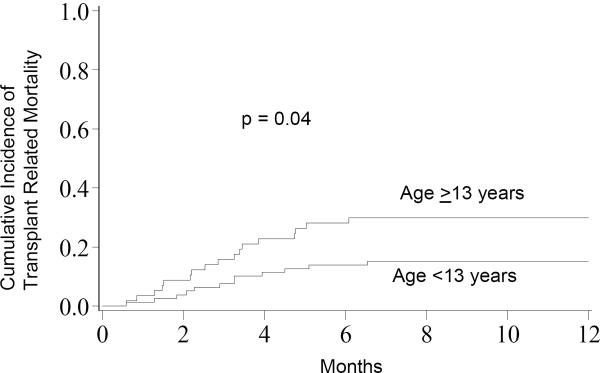
In multivariate analysis, the cumulative incidence of TRM at 1-year was higher in AYA patients compared to those <13 years (28%, 95% CI 16–41% vs. 14%, 95% CI 6–21%; p=0.04, Figure 2). As well, in the multivariate analysis older age was associated with a 2-fold increase in TRM (RR 2.23, 95% CI: 1.01–4.90, p=0.05, Table 2) and HLA 6/6 UCB matching lead to 3-fold decrease in TRM compared to 4/6 matched UCB transplants (RR 0.31, 95% CI: 0.13–0.78; p=0.01). TRM did not differ in the two groups based on the number of complete remissions (CR) prior to transplant. There were no significant differences in TRM based on gender, graft source (marrow vs. UCB), presence of GVHD, or for CMV seropositivity prior to HCT. CMV-positive AYA patients had a non-significant increase in TRM compared to those <13 years who were CMV-positive (36%, 95% CI 20–53% vs. 14%, 95% CI 2–27%, p=0.07) whereas there was no difference in TRM for CMV-negative AYA and younger patients (15%, 95% CI 0–30% vs. 13%, 95% CI 3–23%, p=0.82).
Figure 2.
Transplant Related Mortality; The cumulative incidence of transplant related mortality for patients <13 years was 14% compared to 28% for patients ≥13 years.
Comparing TRM among the two primary graft sources separately (bone marrow and UCB), revealed significantly greater TRM at 1-year in bone marrow recipients who were AYA compared to patients <13 years of age (41%, 95% CI 22–60% vs. 13%, 95% CI 3–24%, p=0.01). There was no significant difference in TRM between AYA patients and those <13 years who received UCB (15%, 95% CI 2–29% vs. 14%, 95% CI 3–25%, p=0.86). Age (<13 versus ≥13 years) was not a factor in the development of either grade II–IV (31%, 95% CI 21–42% vs. 41%, 95% CI 27–55%; p=0.26) or III–IV (11%, 95% CI 4–18% vs. 8%, 95% CI 1–15%; p=0.59) acute GVHD or chronic GVHD (7%, 95% CI 1–12% vs. 15%, 95% CI 5–25%; p=0.06) in univariate or multivariate analyses (Table 2).
Analyzing TRM based on the year of allo-HCT revealed no difference between AYA patients and those <13 years who received HCT between 1990 and 2000 (27%, 95% CI 5–48% vs. 21%, 95% CI 5–37%, p=0.63). Patients who were transplanted more recently (between 2000 and 2005) showed a significant difference in TRM between younger patients compared to AYA (10%, 95% CI 2–18% vs. 29%, 95% CI 14–43%, p=0.03) reflecting an overall decline in TRM in the past several years for a children <13 years, but no change for AYA patients.
Causes of TRM for the AYA patients (n=18) included organ failure (n=5), infection (n=5), GVHD (n=4), graft failure (n=2), hemorrhage (n=1) and veno-occlusive disease (VOD) (n=1) compared to GVHD (n=4), infection (n=4), organ failure (n=2), hemorrhage (n=1) for the patients <13 years of age (n=11).
Relapse
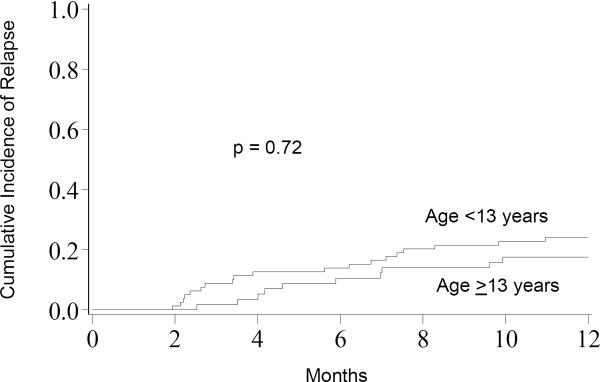
There was no significant difference in relapse at one year between patients <13 years and AYA (24%, 95% CI 14–34% vs. 19%, 95% CI 8–30%; p=0.72, Figure 3). In univariate analysis, patients who received allo-HCT in CR1 (n=42) had a lower incidence of relapse at 1-year (10%, 95% CI 1–18%) compared to those in CR2 (n=70; 30%, 95% CI 19–41%) or CR3 (n=15; 20%, 95% CI 0–40%) (p=0.05). Keeping age in the statistical model (<13 years vs. AYA), disease status (CR1 vs. ≥ CR2) remained the most significant risk factor for relapse in multivariate analysis (RR 5.72, 95% CI: 1.37–23.81; p=0.02). In addition, relapse was significantly less in non-MLL-R patients (RR 0.18, 95% CI: 0.04–0.76; p=0.02).
Figure 3.
Relapse; The cumulative incidence of relapse for patients <13 years was 24% compared to 19% for patients ≥13 years.
DISCUSSION
Although risk stratification for chemotherapy treatment in pediatric B-ALL has identified higher risk patients, the impact of age on transplant outcomes in pediatric and young adults with B-ALL undergoing allografting is unknown. In this analysis of 127 patients with B-ALL who received an allo-HCT at a single center, we identified AYA patients as having a reduced OS likely due to increased TRM. This is the first report to identify the age, <13 versus ≥13 to 30 years, as having inferior HCT outcomes in B-ALL with patients over age 13 having a 2-times greater transplant related mortality. As well, age ≥13 years has recently been recognized by the COG as a very high-risk factor associated with a higher relapse rate and worse DFS following chemotherapy treatment in newly diagnosed patients with B-ALL compared to younger patients. (Meenakshi Devidas, personal communication). In contrast to this finding in newly diagnosed patients, our results on allo-HCT for pediatric/AYA B-ALL show that the relapse rate following myeloablative conditioning is similar and independent of patients' age.
Myeloablative allo-HCT for pediatric leukemia has been associated with a risk of TRM ranging between 10–30% depending on recipient factors (remission status and performance status), donor type and conditioning regimen.(22, 23) As advancements have occurred in donor selection, conditioning regimens, infection monitoring/prophylaxis, and immunosuppression, rates of TRM have declined in pediatric patients.(23, 24) This increased risk of TRM in AYA is not likely the direct result of more treatment since remission status prior to transplantation was similar between the two groups. As well, these two groups had a similar number of patients with early versus late relapses, and the time from diagnosis to transplant was relatively shorter in the AYA group. Whether the increased TRM is also related to unique biologic factors present in the AYA group or due to factors not identified in this analysis is not clear and thus will require continued investigation into methods of minimizing transplant related toxicities.
Consistent with prior studies, disease status (CR1 vs. CR2) at the time of HCT, irrespective of age dramatically increased the risk of future relapse.(25–29) We also found a significantly greater relapse rate in patients with MLL-R. Abnormalities of 11q23 in B-ALL have been associated with high-risk of relapse in infants (30), but there continues to be uncertainty about the prognostic significance of 11q23 identified in non-infant B-ALL.(31, 32) A large international study incorporating 11 cooperative groups and single institutions cited outcomes for non-infant MLL-R B-ALL ranging in EFS from 33 to 100% based on a variety of clinical features.(32) In our cohort, there was no significant difference between the two age groups regarding presence of either Ph+ or MLL-R and thus neither cytogenetic abnormality can likely account for the inferior outcomes seen in our AYA cohort.
In summary, we identified that AYA patients with B-ALL have significantly inferior OS compared to children due to greater TRM. This identifies a new subgroup of B-ALL patients in the AYA population in need of further improvements in their peri-transplant care. Our data support the allotransplant graft-versus-leukemia effect mediated relapse protection and provide rationale for investigations to identify new pre-transplant risk factors that may allocate older age patients to allo-HCT in CR1 or CR2, rather than CR3 where outcomes are found to be significantly worse.(6, 25)
Acknowledgments
The NCI CA96028 (M.J.B.), Children's Cancer Research Fund (M.J.B., M.R.V.), American Cancer Society RSG-08-181 (M.R.V.), and the University of Minnesota Pediatric Leukemia Program supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest to disclose.
Authors' Contributions MJB: conceived the study, reviewed data and writing of the manuscript; NG: reviewed data and writing of the manuscript; JEW: reviewed data and writing of the manuscript; ARS: reviewed data and writing of the manuscript; VB: reviewed data and writing of the manuscript; QC: statistical analysis; MLM: reviewed data and writing of the manuscript; HSS: reviewed data and writing of the manuscript; DJW: reviewed data and writing of the manuscript; MRV: reviewed data and writing of the manuscript.
REFERENCES
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Sallan S, Relling MV, Masera G, Evans WE. International Childhood Acute Lymphoblastic Leukemia Workshop: Sausalito, CA, 30 November-1 December 2000. Leukemia. 2001;15:707–715. doi: 10.1038/sj.leu.2402111. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 6.Freyer DR, Devidas M, La M, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Group. Blood. 2011;117:3010–3015. doi: 10.1182/blood-2010-07-294678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock W. Adolescents and young adults with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:21–29. doi: 10.1182/asheducation-2010.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2008;15:400–407. doi: 10.1097/MOH.0b013e3283034697. [DOI] [PubMed] [Google Scholar]
- 10.Festa RS, Tamaroff MH, Chasalow F, Lanzkowsky P. Therapeutic adherence to oral medication regimens by adolescents with cancer. I. Laboratory assessment. J Pediatr. 1992;120:807–811. doi: 10.1016/s0022-3476(05)80256-2. [DOI] [PubMed] [Google Scholar]
- 11.Landier W, Hughes CB, Calvillo ER, et al. A grounded theory of the process of adherence to oral chemotherapy in Hispanic and caucasian children and adolescents with acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2011;28:203–223. doi: 10.1177/1043454211409582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison CJ. Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br J Haematol. 2009;144:147–156. doi: 10.1111/j.1365-2141.2008.07417.x. [DOI] [PubMed] [Google Scholar]
- 16.Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2011 doi: 10.1002/cncr.26634. [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of Allogeneic Hematopoietic Cell Transplantation for Adolescent and Young Adults Compared with Children and Older Adults with Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen-Gogo S, Marioni G, Laurent S, et al. End of life care in adolescents and young adults with cancer: experience of the adolescent unit of the Institut Gustave Roussy. Eur J Cancer. 2011;47:2735–2741. doi: 10.1016/j.ejca.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard S, Cuvelier G, Harlos M, Barr R. Palliative care in adolescents and young adults with cancer. Cancer. 2011;117:2323–2328. doi: 10.1002/cncr.26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998;102:423–438. doi: 10.1046/j.1365-2141.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith AR, Baker KS, Defor TE, Verneris MR, Wagner JE, Macmillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck JC, Cao Q, Trotz B, et al. Allogeneic hematopoietic cell transplantation outcomes for children with B-precursor acute lymphoblastic leukemia and early or late BM relapse. Bone Marrow Transplant. 2011;46:950–955. doi: 10.1038/bmt.2010.217. [DOI] [PubMed] [Google Scholar]
- 26.Borgmann A, Baumgarten E, Schmid H, et al. Allogeneic bone marrow transplantation for a subset of children with acute lymphoblastic leukemia in third remission: a conceivable alternative? Bone Marrow Transplant. 1997;20:939–944. doi: 10.1038/sj.bmt.1701013. [DOI] [PubMed] [Google Scholar]
- 27.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chessells JM, Veys P, Kempski H, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 29.Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24:5750–5762. doi: 10.1200/JCO.2006.07.1225. [DOI] [PubMed] [Google Scholar]
- 30.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:214–222. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 32.Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17:700–706. doi: 10.1038/sj.leu.2402883. [DOI] [PubMed] [Google Scholar]