Abstract
Aim
frailty is an emerging concept in primary care, which potentially can provide healthcare commissioners with a clinical focus for targeting resources at an ageing population. However, primary care practitioners need valid instruments that are easy to use. With that purpose in mind, we created a Frailty Instrument (FIt) for primary care based on the Survey of Health, Ageing and Retirement in Europe (SHARE). The aim of the present study was to compare the mortality prediction of the 5-item SHARE-FIt with that of a 40-item Frailty Index (FIx) based on comprehensive geriatric assessment (CGA).
Methods
the subjects were 15,578 women and 12,783 men from the first wave of SHARE. A correspondence analysis was used to assess the degree of agreement between phenotypic classifications. The ability of the continuous frailty measures (FIt score and FIx) to predict mortality (mean follow-up of 2.4 years) was compared using receiver operating characteristic (ROC) plots and areas under the curve (AUCs).
Results
in both sexes, there was significant correspondence between phenotypic categories. The two continuous measures performed equally well as mortality predictors (women: AUC-FIx = 0.79, 95% CI: 0.75 – 0.82, P < 0.001; AUC-FIt = 0.77, 95% CI: 0.73 – 0.81, P < 0.001. Men: AUC-FIx = 0.77, 95% CI: 0.74 – 0.79, P < 0.001; AUC-FIt = 0.76, 95% CI: 0.74 – 0.79, P < 0.001). Their equivalent performance was confirmed by statistical comparisons of the AUCs.
Conclusions
SHARE-FIt is simpler and more usable, and predicts mortality similarly to a more complex FIx index based on CGA.
Keywords: Frail Elderly, Severity of Illness Index, Longitudinal Study, Mortality, Validation studies
Introduction
Frailty in older adults is a key clinical concept characterized by dysregulation of multiple biological systems, accumulation of deficits, vulnerability to stressors and increased risk of adverse outcomes such as falls, disability, hospitalization, institutionalization and death 1–3. Although there is no international consensus on the definition of frailty 4,5, two popular operationalizations are the phenotypic and one defined by accumulation of deficits 6.
According to the phenotypic approach, frailty is defined as a clinical syndrome in which three or more of the following criteria are present: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity 7,8. Surrogates for individual criteria are possible 9–12. The phenotypic approach defines two additional states: pre-frail (i.e. one or two criteria present) and non-frail (i.e. none of the criteria present) 8. An advantage of this approach is that it requires the measurement of only five variables, which makes the frailty assessment relatively quick.
Another way to operationalize frailty is by counting in an individual the number of deficits that he/she has accumulated from a given list (of usually 40 or more potential deficits) 13. The number of counted deficits divided by the number of deficits considered results in a score called frailty index (FIx), which ranges from 0 (i.e. none of the deficits present) to 1 (i.e. all deficits present). The FIx approach is usually based on comprehensive geriatric assessment (CGA) 14. Therefore, compared to the phenotypic approach, the FIx approach is more time consuming.
Frailty is an emerging concept in primary care 15, which potentially can provide healthcare commissioners with a clinical focus for targeting resources at an ageing population 16,17. However, in order to fulfil that purpose, family physicians and community practitioners need valid instruments for frailty that are easy to use 18. With that purpose in mind, we created and validated a Frailty Instrument (FIt) for primary care based on the Survey of Health, Ageing and Retirement in Europe (SHARE) 19. This FIt is based on a modified phenotypic approach and includes two web-based frailty calculators (one for each gender) that are freely accessible on BMC Geriatrics (http://www.biomedcentral.com/1471-2318/10/57/additional). Their use is intended for community-dwelling adults aged 50 and over. Translated versions of the calculators can be accessed on https://sites.google.com/a/tcd.ie/share-frailty-instrument-calculators/. We have validated the SHARE FIt against incident disability 20 and mortality 21.
The English NHS Evidence Adoption Centre has rated SHARE-FIt as a potentially useful and highly usable tool for the individual assessment of frailty 22. Furthermore, a recent systematic review of frailty screening tools in primary care identified SHARE-FIt as a promising measure in the primary care setting 23. However, in the latter publication it was pointed out that there is no comparison of the tool with a reference geriatric assessment 23. In that regard, we recently operationalized a FIx on SHARE based on 40 potential deficits 24, including the five defining SHARE-FIt. We found that SHARE-FIx was a powerful predictor of mortality 24, but we did not know the extent to which the addition of a considerably higher number of measurements would improve the mortality prediction vis-à-vis the more user-friendly SHARE-FIt. In order to answer that question, the aim of the present study was to compare the performance of these two tools to classify the SHARE sample and predict mortality.
Materials and methods
Subjects
15,578 women and 12,783 men from the first wave of the Survey of Health, Aging and Retirement in Europe (http://www.share-project.org/), including representative samples of 12 European countries: Austria (n = 1,620), Germany (n = 2,746), Sweden (n = 2,852), Netherlands (n = 2,777), Spain (n = 2,212), Italy (n = 2,304), France (n = 2,784), Denmark (n = 1,637), Greece (n = 2,581), Switzerland (n = 956), Belgium (n = 3,663) and Israel (n = 2,229). All subjects had information for both FIt and FIx.
Creation of SHARE-FIt
As detailed in our main study 19, SHARE-FIt was created via estimation of a discrete factor (DFactor) model based on five adapted phenotypic frailty items 12 (i.e. grip strength and four self-reported items: fatigue, loss of appetite and/or eating less than usual, difficulties climbing stairs and/or walking 100 metres, and low level of physical activity), using the LatentGOLD® statistical package. A DFactor with three ordered levels or latent classes (non-frail, pre-frail and frail) was obtained for each sex. When data is entered into the web-based calculator (http://www.biomedcentral.com/1471-2318/10/57/additional), the tool provides a continuous frailty score (i.e. the ‘predicted discrete factor score’, whose formulae are given in the paper) and enables classification into phenotypic frailty categories: non-frail, pre-frail and frail 19.
Creation of SHARE-FIx
Based on the first wave of SHARE, a 40-item FIx was created as per standard procedure 13. Each of the 40 deficit variables was scored such that 0 = deficit absent and 1 = deficit present. The scores were added and divided by the total number of deficits evaluated (i.e. 40), to produce a FI between 0.0 (i.e. no deficits present) and 1.0 (i.e. all deficits present). Table 1 shows the FIx deficit variables and cut-off points. Primarily, the FIx is a continuous variable, but it can be graded to be equivalent to the phenotypic definition: non-frail (FIx < 0.08), pre-frail (0.08 ≤ FIx < 0.25) and frail (FIx ≥ 0.25) 25.
Table 1.
Health variables and cut-points for the SHARE Frailty Index (FIx). The phenotypic variables underlying the SHARE Frailty Instrument (FIt) are highlighted in bold.
| SHARE code | Variable | Cut-point | Reference |
|---|---|---|---|
| ph049d3 | Difficulties: bathing or showering | Yes = 1, No = 0 | |
| ph049d1 | Difficulties: dressing, including shoes and socks | Yes = 1, No = 0 | |
| ph048d3 | Difficulties: getting up from chair | Yes = 1, No = 0 | |
| ph049d2 | Difficulties: walking across a room | Yes = 1, No = 0 | |
| ph049d4 | Difficulties: eating, cutting up food | Yes = 1, No = 0 | |
| ph048d7 | Difficulties: reaching or extending arms above shoulder | Yes = 1, No = 0 | |
| ph049d6 | Difficulties: using the toilet, including getting up or down | Yes = 1, No = 0 | |
| ph048d5 | Difficulties: climbing one flight of stairs | Yes = 1, No = 0 | |
| ph048d9 | Difficulties: lifting or carrying weights over 5 kilos | Yes = 1, No = 0 | |
| ph049d9 | Difficulties: shopping for groceries | Yes = 1, No = 0 | |
| ph049d12 | Difficulties: doing work around the house or garden | Yes = 1, No = 0 | |
| ph049d8 | Difficulties: preparing a hot meal | Yes = 1, No = 0 | |
| ph049d11 | Difficulties: taking medications | Yes = 1, No = 0 | |
| ph049d13 | Difficulties: managing money | Yes = 1, No = 0 | |
| ph048d1 | Difficulties: walking 100 metres | Yes = 1, No = 0 | |
| ph049d5 | Difficulties: getting in or out of bed | Yes = 1, No = 0 | |
| Phactiv | Moderate or vigorous physical activity: hardly ever, or never | Yes = 1, No = 0 | |
| mh011_, mh012_ | Diminution in the desire for food and/or eating less than usual | Yes = 1, No = 0 | |
| Spheu | Self-perceived health | Very bad = 1, Bad = 0.75, Fair = 0.5, Good = 0.25, Very good = 0 | (13) |
| ph004_ | Long-term illness | Yes = 1, No = 0 | |
| mh013_ | Fatigue | Yes = 1, No = 0 | |
| mh002_ | Sad or depressed | Yes = 1, No = 0 | |
| mh016_ | Lack of enjoyment | Yes = 1, No = 0 | |
| mh003_ | Hopelessness | Yes = 1, No = 0 | |
| ph006d2 | Doctor told you had: high blood pressure or hypertension | Yes = 1, No = 0 | |
| ph006d1 | Doctor told you had: heart attack | Yes = 1, No = 0 | |
| ph006d4 | Doctor told you had: stroke | Yes = 1, No = 0 | |
| ph006d10 | Doctor told you had: cancer | Yes = 1, No = 0 | |
| ph006d5 | Doctor told you had: diabetes or high blood sugar | Yes = 1, No = 0 | |
| ph006d8 | Doctor told you had: arthritis | Yes = 1, No = 0 | |
| ph006d6 | Doctor told you had: chronic lung disease | Yes = 1, No = 0 | |
| ph006d9 | Doctor told you had: osteoporosis | Yes = 1, No = 0 | |
| ph006d14 | Doctor told you had: hip fracture or femoral fracture | Yes = 1, No = 0 | |
| Orienti | Impaired orientation to date, month, year and day of week (i.e. less than good) | Yes = 1, No = 0 | |
| Bmi | Body mass index (Kg/m2) deficit | <18.5 or ≥30 = 1 25 to <30 = 0.5 18.5 to <25 = 0 |
(13) |
| ph010d3 | Bothered by: breathlessness | Yes = 1, No = 0 | |
| ph010d7 | Bothered by: falling down | Yes = 1, No = 0 | |
| ph010d8 | Bothered by: fear of falling down | Yes = 1, No = 0 | |
| ph010d9 | Bothered by: dizziness, faints or blackouts | Yes = 1, No = 0 | |
| Maxgrip | Grip strength (Kg) deficit |
Men:
|
(13) |
SHARE-FIt and SHARE-FIx: common variables
As Table 1 shows, the phenotypic variables that were used to construct SHARE-FIt 19 were included as deficits in SHARE-FIx 24. Thus, SHARE-FIx is based on the SHARE-FIt measurements plus 34 additional measurements.
Mortality prediction
Wave 2 established whether wave 1 participants had died, were still alive, or had been lost to follow-up. For those who had died, the exact time to death since the initial interview was not collected. Wave 1 data were collected between 2004–2006 and wave 2 between 2006–2007. The mean individual follow up period between Wave 1 and Wave 2 was 2.4 years.
Statistical analyses
Unless otherwise indicated, statistics were computed with SPSS 16.0, separately for each sex. The level of significance was established at 0.01 throughout.
The correlation between the two continuous frailty measures, the FIt score and the FIx, was assessed with the Spearman’s rank correlation coefficient (the normality of the variables was previously checked with the one-sample Kolmogorov-Smirnov test, and they were found non-normally distributed in both sexes). Correspondence analysis (with symmetrical normalization) was used to assess the degree of agreement between the two phenotypic classifications (FIt and FIx categories); correspondence analysis examines the relationship between two categorical variables graphically in a multidimensional space, and produces plots based on the scores. Categories that are similar to each other appear close to each other in the plots. In this way, it is easy to see which categories of a variable are similar to each other or which categories of the two variables are related.
To compare the performance of the phenotypic classifications (i.e. FIt and FIx categories) to predict mortality, Chi-square cross-tabulations were used, with the Phi statistic as measure of association (data were non-parametric). To compare the ability of the two continuous frailty measures (i.e. FIt score and FIx) to predict mortality, we used receiver operating characteristic (ROC) plots. The pairwise comparison of the ROC curves was undertaken using the MedCalc® 12.3 statistical package (by selecting the ‘comparison of ROC curves’ procedure; the DeLong comparison method was selected).
Results
15,578 women and 12,783 men from the first wave of SHARE had information for both FIt and FIx. Two hundred and sixty-seven women and 361 men had died at follow-up.
Correlation between FIt-frailty score and FIx
In women, the bivariate correlation between the FIt-frailty score and the FIx was strong and highly significant (two-tailed Spearman’s rank correlation coefficient: 0.70, P < 0.001). The same applied to men (two-tailed Spearman’s rank correlation coefficient: 0.59, P < 0.001).
FIt and FIx categories: correspondence analysis
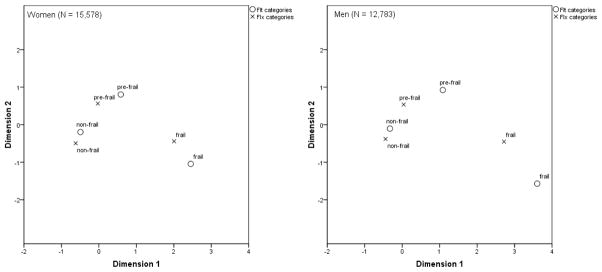
In women, there was a significant correspondence between FIt and FIx categories (inertia = 0.55, Chi-square = 8517.51, df = 4, P < 0.001). Table 2 shows the correspondence table and Figure 1 shows the correspondence biplot.
Table 2.
Correspondence between FIt and FIx categories.
| FIt categories | FIx categories
|
|||
|---|---|---|---|---|
| non-frail | pre-frail | frail | Active Margin | |
|
| ||||
| Women
| ||||
| non-frail | 5935 | 4349 | 136 | 10420 |
| pre-frail | 391 | 2669 | 965 | 4025 |
| frail | 0 | 179 | 954 | 1133 |
| Active Margin | 6326 | 7197 | 2055 | 15578 |
|
| ||||
| Men
| ||||
| non-frail | 6271 | 4121 | 125 | 10517 |
| pre-frail | 157 | 1212 | 502 | 1871 |
| frail | 0 | 49 | 346 | 395 |
| Active Margin | 6428 | 5382 | 973 | 12783 |
Figure 1.
Correspondence analysis between FIt and FIx categories.
In men, there also was a significant correspondence between FIt and FIx categories (inertia = 0.48, Chi-square 6164.49, df = 4, P < 0.001). Table 2 shows the correspondence table and Figure 1 shows the correspondence biplot.
FIt and FIx categories: prospective mortality rates
Table 3 shows the prospective mortality information (mean follow-up: 2.4 years) for FIt and FIx categories, by gender. In women, the association between FIt categories and mortality was statistically significant (Chi-square = 281.05, df = 2, P < 0.001; Phi = 0.164, P < 0.001); and the association between FIx categories and mortality was also significant (Chi-square = 520.80, df = 2, P < 0.001; Phi = 0.214, P < 0.001).
Table 3.
FIt and FIx: prospective mortality rates.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| FIt | FIx | FIt | FIx | |||||
| N total | N dead (%) | N total | N dead (%) | N total | N dead (%) | N total | N dead (%) | |
| Continuous measure | 15578 | 181 (1.7) | 15578 | 181 (1.7) | 12783 | 296 (3.4) | 12783 | 296 (3.4) |
| Non-frail | 10420 | 51 (0.7) | 6326 | 15 (0.4) | 10517 | 142 (2.0) | 6428 | 51 (1.1) |
| Pre-frail | 4025 | 67 (2.6) | 7197 | 74 (1.5) | 1871 | 102 (8.8) | 5382 | 152 (4.2) |
| Frail | 1133 | 63 (9.2) | 2055 | 92 (7.0) | 395 | 52 (22.6) | 973 | 93 (16.0) |
In men, the association between FIt categories and mortality was statistically significant (Chi-square = 402.37, df = 2, P < 0.001; Phi = 0.216, P < 0.001); and the association between FIx categories and mortality was also significant (Chi-square = 520.34, df = 2, P < 0.001; Phi = 0.238, P < 0.001).
Mortality prediction by FIt score and FIx: ROC analyses
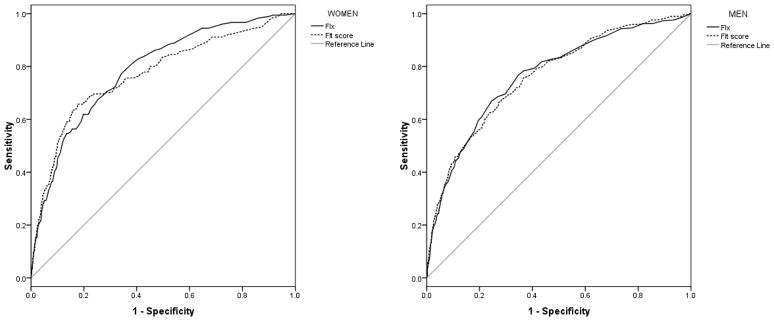
In women, the FIx had an area under the curve (AUC) of 0.79 (95% confidence interval, CI: 0.75 – 0.82; standard error, SE = 0.02; P < 0.001). The FIt score had an AUC of 0.77 (95% CI: 0.73 – 0.81; SE = 0.02; P < 0.001). Figure 2 shows the ROC curves. The pairwise comparison of the ROC curves showed the following results: Z statistic = 1.18, P = 0.238 (the non-significant P value indicates that the AUCs were not significantly different).
Figure 2.
Mortality prediction by FIt score and FIx: Receiver operating characteristic (ROC) curves, by gender.
In men, the FIx had an AUC of 0.77 (95% CI: 0.74 – 0.79; SE = 0.01; P < 0.001), and the FIt score had an AUC of 0.76 (95% CI: 0.74 – 0.79; SE = 0.01; P < 0.001). Figure 2 shows the ROC curves. The pairwise comparison of the ROC curves showed the following results: Z statistic = 0.22, P = 0.826 (the AUCs were not significantly different).
Discussion
The present study compared the ability of SHARE-FIt 19 and SHARE-FIx 24 to predict mortality over a mean follow-up of 2.4 years in a large European population-based sample. Whilst the use of SHARE-FIx is unlikely to be feasible in a primary care context given the high number of measurements required, SHARE-FIt only requires five simple measurements to be entered in an open-access online calculator.
Overall, in both women and men, there was a strong direct correlation between the FIt score and FIx. There was also a significant correspondence between the phenotypic categories. As Figure 1 shows, non-frail categories showed the highest degree of closeness; and pre-frail and frail categories were somewhat closer in women than in men. The frail definition by FIt was more ‘restrictive’ than the one by FIx, as supported by the fact that none of those classified as frail by FIt were classified as non-frail by FIx; however, 136 frail women and 125 frail men by FIx were non-frail by FIt (Table 2). This means that, in practice, FIt may be more specific (and perhaps less sensitive) than FIx for the phenotypic diagnosis of frailty. However, the phenotypic FIx cut-offs used in the present study 25 are of arbitrary nature, and alternative FIx cut-offs have been proposed 26 that could lead to difference correspondences with the FIt categories. In contrast, the FIt cut-offs are non-arbitrary and derive from discrete factor analysis 19.
The two continuous measures, the FIt score and the FIx, performed equally well as mortality predictors in the ROC analyses, as evidenced by the overlap of 95% CIs for the AUC, both in women and in men (Figure 2). This suggests that the measurement of 34 additional variables with FIx does not translate, in terms of mortality prediction, into any marginal benefit. The simpler combined measurement of exhaustion, appetite, two functional difficulties, physical activity and handgrip strength predicted mortality just as well as a 40-item FIx. Hence, it is possible that not all the variables defining the FIx have the same relative importance, as far as prospective mortality is concerned, and some of them could be redundant. On the other hand, results may indicate that the variables defining the frailty phenotype, which are based on biological theory (i.e. the cycle of frailty) 8, are in fact a good ‘summary’ of the multiple system dysregulation that occurs in frailty, at least as far as mortality prediction goes.
As in the present study, the focus of previous studies has been the comparison of the ability of different frailty tools to predict frailty-related outcomes and, in general, they have also found similar predictive properties. For example, in the Conselice Study of Brain Aging, a 43-item FIx and a 7-item Brain Aging Score were strongly correlated with each other and each was independently predictive of death in a multivariate model 27. In the Canadian Study of Health and Aging, the comparison of a 47-item FIx, a Clinical Frailty Score and the Fried frailty phenotype showed that all frailty measures allowed quantification of individual vulnerability and predicted both cognitive changes and mortality 28. A recent multi-centre study compared the prognostic accuracy for all-cause mortality of various frailty instruments and found that all the frailty indexes considered were significantly associated with one-month and one-year all-cause mortality 29. Other studies comparing phenotypic-based frailty tools have also found that simpler tools predict outcomes as well as more complex ones 30, with the advantage that the former may be more usable in a clinical setting 22.
SHARE-FIt is easy to use, freely accessible online (http://www.biomedcentral.com/1471-2318/10/57/additional) and may contribute to quality in primary care by helping assess and monitor frailty in community dwelling people over the age of 50, and prioritize their access to resources. Importantly, anyone using the online SHARE-FIt calculators should be aware that he/she is comparing him/herself to the European reference population as a whole, and not to the population of a specific participating country. The construction of SHARE-FIt was based on population-representative samples of 12 European countries, but the prevalences of frailty in the various participating countries have been noted to differ significantly 12, perhaps owing to European socio-economic gradients or even cultural differences in the interpretation of the subjective (i.e. self-reported) items 19. In our original study 19, we argued that the construction of country-specific SHARE-FIt calculators was at risk of underpower; for the same reason, the comparison of FIx and FIt at individual country levels was not undertaken, due to the small numbers of mortality events.
In conclusion, the present study provided further validation of the SHARE Frailty Instrument by comparing it with a Frailty Index based on comprehensive geriatric assessment, and found that their mortality prediction is very similar. SHARE-FI may contribute to quality in primary care by offering a readily accessible, simple and reliable way to assess and monitor frailty in community-dwelling adults over the age of 50, prioritize their access to resources and serve as a tool for audit and research.
Acknowledgments
This paper uses data from SHARE release 2.3.0, as of November 13th 2009 (SHARE-FIt) and 2.5.0, as of May 24th 2011 (SHARE-FIx). The SHARE data collection has been primarily funded by the European Commission through the 5th framework programme (project QLK6-CT-2001- 00360 in the thematic programme Quality of Life), through the 6th framework programme (projects SHARE-I3, RII-CT- 2006-062193, COMPARE, CIT5-CT-2005-028857, and SHARELIFE, CIT4-CT-2006-028812) and through the 7th framework programme (SHARE-PREP, 211909 and SHARE-LEAP, 227822). Additional funding from the U.S. National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, Y1-AG-4553-01 and OGHA 04-064, IAG BSR06-11, R21 AG025169) as well as from various national sources is gratefully acknowledged (see www.share-project.org for a full list of funding institutions).
Footnotes
Disclosure
The author has no conflict of interest.
References
- 1.Heuberger RA. The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr. 2011;30:315–68. doi: 10.1080/21551197.2011.623931. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–38. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 3.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collard RM, Oude Voshaar RC. Frailty; a fragile concept. Tijdschr Psychiatr. 2012;54:59–69. [PubMed] [Google Scholar]
- 5.Mohandas A, Reifsnyder J, Jacobs M, Fox T. Current and future directions in frailty research. Popul Health Manag. 2011;14:277–83. doi: 10.1089/pop.2010.0066. [DOI] [PubMed] [Google Scholar]
- 6.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. 2011;23:209–16. doi: 10.1007/bf03324962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solfrizzi V, Scafato E, Frisardi V, et al. Frailty syndrome and all-cause mortality in demented patients: the Italian Longitudinal Study on Aging. Age (Dordr) 2012;34:507–17. doi: 10.1007/s11357-011-9247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochat S, Cumming RG, Blyth F, et al. Frailty and use of health and community services by community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2010;39:228–33. doi: 10.1093/ageing/afp257. [DOI] [PubMed] [Google Scholar]
- 12.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64:675–81. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Lacas A, Rockwood K. Frailty in primary care: a review of its conceptualization and implications for practice. BMC Med. 2012;10:4. doi: 10.1186/1741-7015-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Lepeleire J, Iliffe S, Mann E, Degryse JM. Frailty: an emerging concept for general practice. Br J Gen Pract. 2009;59:e177–82. doi: 10.3399/bjgp09X420653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drey M, Wehr H, Wehr G, et al. The frailty syndrome in general practitioner care: a pilot study. Z Gerontol Geriatr. 2011;44:48–54. doi: 10.1007/s00391-010-0136-3. [DOI] [PubMed] [Google Scholar]
- 18.De Lepeleire J, Degryse J, Illiffe S, Mann E, Buntinx F. Family physicians need easy instruments for frailty. Age Ageing. 2008;37:484. doi: 10.1093/ageing/afn116. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Ortuno R, O’Shea D, Kenny RA. The SHARE frailty instrument for primary care predicts incident disability in a European population-based sample. Qual Prim Care. 2011;19:301–9. [PubMed] [Google Scholar]
- 21.Romero-Ortuno R. The Frailty Instrument of the Survey of Health, Ageing and Retirement in Europe (SHARE-FI) predicts mortality beyond age, comorbidities, disability, self-rated health, education and depression. Eur Geriatr Med. 2011;2:323–6. doi: 10.1016/j.eurger.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh R. Does targeted case-finding of the frail elderly result in improved QIPP outcomes? A literature review (Cambridge: NHS Evidence Adoption Centre East of England) [serial on the internet] 2012 Jun; [cited 2012 June 10]. Available from: http://www.eac.cpft.nhs.uk/viewResource.aspx?id=18686.
- 23.Pialoux T, Goyard J, Lesourd B. Screening tools for frailty in primary health care: a systematic review. Geriatr Gerontol Int. 2012;12:189–97. doi: 10.1111/j.1447-0594.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. 2012 doi: 10.1093/ageing/afs051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–94. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucicesare A, Hubbard RE, Fallah N, et al. Comparison of two frailty measures in the Conselice Study of Brain Ageing. J Nutr Health Aging. 2010;14:278–81. doi: 10.1007/s12603-010-0061-6. [DOI] [PubMed] [Google Scholar]
- 28.Mitnitski A, Fallah N, Rockwood MR, Rockwood K. Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J Nutr Health Aging. 2011;15:863–7. doi: 10.1007/s12603-011-0066-9. [DOI] [PubMed] [Google Scholar]
- 29.Pilotto A, Rengo F, Marchionni N, et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One. 2012;7:e29090. doi: 10.1371/journal.pone.0029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]