Abstract
Background
Dyslipidemia is a common complication among HIV-infected children after antiretroviral therapy (ART). However, HIV itself can cause abnormal lipid metabolism. There is limited information of lipid profile among Asian HIV-infected children naïve to ART.
Methods
274 HIV-infected ART-naïve Thai and Cambodian children 1–12 years of age with CD4 between 15–24% were included. Patients were fasted for ≥ 4 hours before blood was drawn. Abnormal lipid level was defined as triglyceride (TG) > 130 mg/dL, total cholesterol (TC) > 200 mg/dL, low density lipoprotein (LDL) > 130 mg/dL, and high density lipoprotein (HDL) ≤ 40 mg/dL.
Result
The mean (SD) was 76.6 (33.8) months for age and −1.3 (1.0) for weight Z-score. Mean (SD) CD4% was 19.9 (4.8) % and HIV RNA was 4.6 (0.6) log10 copies/ml. The median (SD) fasting time was 13.0 (2.7) hours. Mean (SD) for lipids were 116 mg/dl (62) for TG, 139 mg/dl (29) for TC, 73 mg/dl (29) for LDL and 45 mg/dl (19) for HDL. Overall 63.9% had dyslipidemia with hypertriglyceridemia and hypo HDL being the most common: 28% and 45% respectively, while 2% had hypercholesterolemia or hyper-LDL. After adjusting for age, having HIV RNA > 5 log10 copies/ml was associated with hypo-HDL with odds ratios of 8.1 (95% CI 2.7–24.3).
Conclusions
Up to two-third of ART-naïve, HIV-infected Asian children with mild to moderate immune suppression had dyslipidemia. Low HDL was the most common and was associated with high HIV viremia. The long term consequence of low HDL deserves further investigation in children.
Keywords: dyslipidemia, ART-naïve, HIV, children, Asian
Introduction
Lipid abnormalities have been reported in HIV infected children [1–4] and adults [5] before and after initiation of antiretroviral therapy (ART). Data from PA CTG219C, a large cohort of HIV-infected children receiving antiretroviral therapy from US and Puerto Rico, showed that 13% of HIV-infected children had hypercholesterolemia comparing to 4.8% among uninfected children.[4]. Lipids, lipoproteins, triglyceride (TG) clearance and cytokines are altered in HIV-infected persons particularly in those with advanced HIV. Early during the course of HIV infection, high density lipoprotein (HDL) levels decrease. As immune function declines, a decrease in the low density lipoproteins (LDL) level occurs, which is then followed by an increase in very low density lipoprotein (VLDL) particles and TG at the time of the transition to AIDS[6, 7]. Results from a large cohort of HIV-infected adults naïve to ART showed that patients who had AIDS-defining illness had high total cholesterol (TC), VLDL and TG along with low HDL[5, 8]. Dyslipidemia is clearly associated with cardiovascular risk in adults as shown in the US National Cholesterol Education Program III treatment guidelines which noted higher risks of cardiovascular risk as lipid levels increase.[9–11] Such risk might be even higher in children as they could be exposed to dyslipidemia for longer periods of time[12–14].
Abnormalities in lipid profiles are well recognized toxicities in HIV-infected children on ART[1–3]. TC, LDL, HDL and TG abnormalities tend to increase with longer exposure to ART. The favorable lipid changes demonstrated by the increase in HDL level and decrease in total cholesterol/HDL ratio are also demonstrated after ART initiation and associated with increased CD4% and decreases in proinflammatory cytokines [15]. Studies reporting abnormal lipid parameters in HIV-infected children are limited and the majority are among Black, Hispanic and Caucasian children whose dietary intakes are different from Asian children. [1–4] There are only few data from Asian children, especially those naïve to ART[16].
The objective of this study was to describe the prevalence of lipid abnormalities among ART naïve HIV infected Thai and Cambodian children. The secondary objective was to assess relationships between lipid parameters and growth, immunological and virological status.
Materials and Methods
This study used baseline data of children who enrolled in the Pediatric Randomized of Early vs. Deferred Initiation in Cambodia and Thailand (PREDICT) study (clinicaltrials.gov identification number NCT00234091) conducted at 7 hospitals in Thailand and 2 hospitals in Cambodia. PREDICT is a randomized clinical trial to compare the immunological criteria (CD4 < 15% vs. CD4 15–24%) for ART initiation in children. The inclusion criteria were ART-naïve, HIV-infected children aged 1–12 years who had CD4 between 15 to 24% without severe HIV symptoms or clinical AIDS. The study was approved by national and local institutional review boards in Thailand and in Cambodia. All guardians gave consent.
At the study visit, body weight and height were measured. Blood was drawn after at least 4 hours of fasting for laboratory testing including CD4%, HIV RNA, and lipid profiles including TG, TC and HDL. CD4% was measured by dual platform flow cytometry. HIV RNA was measured by using Roche Amplicor assay (Palo Alto, USA). TG, TC and HDL were measured using standard techniques with an automated chemistry analyzer. LDL values were calculated by the Friedwald equation as follows: LDL (mg/dL) = TC (mg/dL)-HDL-C (mg/dL)-TG/5 (mg/dL). Proficiency Testing from Accutest was performed and the results were reviewed by the US Safety Monitoring International Laboratory Evaluation (SMILE). All laboratories passed the US National Institute of Allergy and Infectious Diseases, Division of AIDS (DAIDS) assessment.
Due to limited data on normal lipid profile in Asian children, we used cutoff values described in published manuscripts[1, 12, 13, 17, 18]. The cutoff levels for abnormallipids were defined as follows: TG > 130 mg/dL, TC > 200 mg/dL, LDL > 130 mg/dL and HDL ≤ 40 mg/dL.
Statistical methods
The lipid levels were described and the proportion of patients with dyslipidemia was calculated. We assessed the relationship of gender, age, weight for age Z score (WAZ), height for age Z score (HAZ), CD4 percentage and HIV RNA with dyslipidemia by using logistic regression models, and factors with p<0.15 were adjusted for in multivariate models. Data analysis was performed with Stata Version 11 (Statacorp, College Station, Tx).
Results
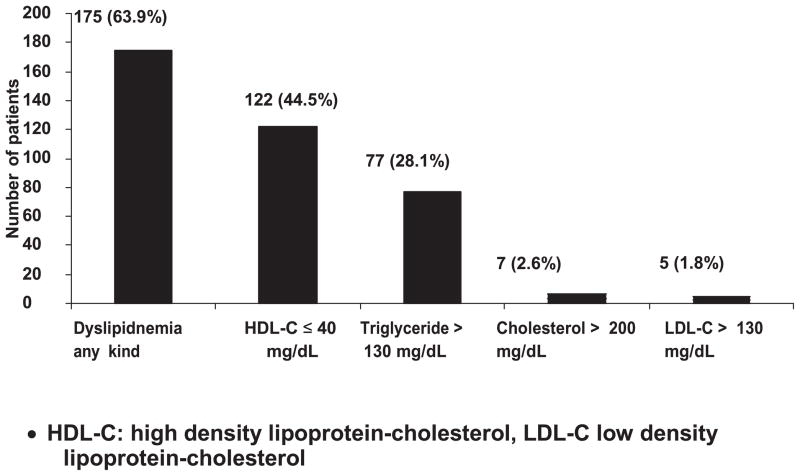
Between March 2006 to September 2008, 300 children were enrolled to the PREDICT study. The data from 274 patients were used in this analysis, 25 children were excluded due to fasting time less than 4 hours and 1 child withdrew from the study. The mean (SD) of fasting time was 13.0 (2.7) hours. The characteristics of the children are shown in Table 1. Most children had mild HIV symptoms. About one-third were underweight or stunted. Overall, 63.9% had dyslipidemia (Figure 1) with the most common abnormalities being hypo HDL (44.5%) and hypertriglyceridemia (28.3%). The proportion of children with dyslipidemia within different groups was explored (data not shown). There were no significant difference by gender, weight (≥ − 2 SD for WAZ), and CD4 percentage (CD4 < 20%).
Table 1.
Demographic characteristics of 274 antiretroviral naive HIV-infected children
| Patient characteristics | Values |
|---|---|
| Gender (M:F) | 115:159 |
| Mean age, months (SD) | 76.6 (33.8) |
| Mean weight for age Z score (SD) | −1.30 (1.04) |
| Mean height for age Z score (SD) | −1.64 (1.33) |
| Ethnicity Thai: Cambodian | 155:119 |
| CDC Category N:A:B | 5: 170: 99 |
| Body Mass Index (kg/m2) | 15.23 (1.68) |
| N (%) children who are underweight (< −2SD for WAZ) | 72(26.3) |
| N (%) children who are stunted (< − 2SD for HAZ) | 102(37.2) |
| N (%) children who are wasted (< − 2SD for WAH) | 12(4.4) |
| Mean CD4 % (SD) | 19.9 (4.8) |
| Mean Log10 copies/ml HIV RNA (SD) | 4.6 (0.6) |
Figure 1.
Proportion of dyslipidemias in 274 antiretroviral-naive Thai and Cambodian HIV infected children with mild to moderate immunosuppression
However, more children with HIV RNA > 5 log10 copies/ml had hypo-HDL (22 in 26, 84.6%) compared to those with HIV RNA ≤ 5 log10 copies/ml (100 in 248, 41.3%), p <0.05.
The relationship between clinical characteristics and different dyslipidemias is shown in Table 2. In a multivariate logistic regression model adjusting for age, having plasma HIV RNA > 5 log10 copies/ml was associated with hypo-HDL with odds ratios of 8.1 (95% CI 2.7–24.3). No multivariate models for TG, TC or LDL-C were developed, however there was a trend of higher triglyceride levels among children with WAZ score < −2 but this did not reach statistical significance.
Table 2.
Association of clinical characteristics of HIV-infected children with dyslipidemia
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Crude Odds Ratio | 95%CI | P | Adjusted Odds Ratio | 95%CI | P | |
| Triglyceride > 130 mg/dL | ||||||
| Male vs. Female | 1.28 | 0.74–2.19 | 0.37 | |||
| Age (per year increase) | 0.94 | 0.86–1.04 | 0.29 | |||
| Weight for age Z score < − 2sd | 1.63 | 0.95–2.78 | 0.08 | |||
| Height for age Z score < − 2sd | 1.09 | 0.64–1.85 | 0.75 | |||
| CD4% ≤20% | 0.74 | 0.29–1.91 | 0.54 | |||
| Plasma HIV Viral load > 5 log10 copies/ml | 1.28 | 0.74–2.19 | 0.37 | |||
| HDL-C ≤ 40 mg/dL | ||||||
| Male vs. Female | 1.29 | 0.79–2.09 | 0.30 | |||
| Age (per year increase) | 1.07 | 0.98–1.17 | 0.12 | 1.09 | 1.01–1.20 | 0.04 |
| Weight for age Z score < − 2sd | 0.85 | 0.49–1.47 | 0.57 | |||
| Height for age Z score < − 2sd | 0.91 | 0.56–1.49 | 0.72 | |||
| CD4% ≤20% | 0.87 | 0.54–1.41 | 0.59 | |||
| Plasma HIV Viral load > 5 log10 copies/ml | 8.14 | 2.72–24.34 | < 0.01 | 8.8 | 2.9–26.6 | <0.01 |
| Cholesterol >200 mg/dL | ||||||
| Male vs. Female | 0.96 | 0.21–4.39 | 0.96 | |||
| Age (per year increase) | 0.99 | 0.75–1.29 | 0.92 | |||
| Weight for age Z score < − 2sd | 2.15 | 0.47–9.86 | 0.70 | |||
| Height for age Z score < − 2sd | 1.27 | 0.28–5.80 | 0.44 | |||
| CD4% ≤20% | 0.48 | 0.09–2.48 | 0.38 | |||
| Plasma HIV Viral load > 5 log10 copies/ml | 0.98 | 0.01–6.81 | 0.98 | |||
| LDL-C >130 mg/dL | ||||||
| Male vs. Female | 4.97 | 0.67–10.15 | 0.36 | |||
| Age (per year increase) | 1.13 | 0.82–1.54 | 0.45 | |||
| Weight for age Z score < − 2sd | 0.70 | 0.08– 6.34 | 0.18 | |||
| Height for age Z score < − 2sd | 0.42 | 0.05–3.77 | 0.65 | |||
| CD4% ≤20% | 1.83 | 0.30–11.15 | 0.51 | |||
| Plasma HIV Viral load > 5 log10 copies/ml | 0.29 | 0.05–1.76 | 0.99 | |||
Discussion
Our data demonstrated that ART-naïve Asian children with mild to moderate immunosuppression had a high prevalence of dyslipidemia with almost half having low HDL and one-third having high TG. The long term follow-up for the change of lipid parameters over time and the effect on cardiovascular risk should be further investigated.
A pediatric study among 90 US children (P1010 study) reported that 30% had hypo-HDL. The higher prevalence of hypo-HDL in our study may be explained by more immunosuppression (mean CD4 19.9% versus 24.8%) and not exposed to ART (100% versus 24.8%) [2]. Data from a severely immunodeficient cohort of Thai children with mean CD4 6% showed that 94% of children had low HDL prior to initiating ART[16]. These data lend support to the conjecture that more advanced HIV disease is associated with a higher prevalence of hypo HDL.
In ART- naïve adult HIV patients, one study reported hypercholesterolemia in 12.4% and hypertriglyceridemia in 19.4%[19]. Several studies in adults have shown that dyslipidemia was observed in patients who had advanced HIV disease, low CD4 count, high HIV RNA and longer duration of HIV infection[8, 19, 20]. Our study adds to this body of literature that in children without advanced HIV disease, hypertriglyceridemia is common: about one-third of children had triglyceride > 130 mg/dL. However, unlike adult patients, only 2.6% of HIV-infected children had hypercholesterolemia. The limitation is using cut-off values based on published literature due to lack of normative data of lipid profiles among Asian children.
The cause of lipid abnormalities in HIV patients is not well understood. Large adult studies have shown evidence for both HIV [10] and long term ART [9] to be associated with dyslipidemia and cardiovascular risk. We found an association between low HDL and high HIV RNA. There is an association between HIV viremia, low HDL and risk of cardiovascular diseases[10]. In Thai adults, high HIV viremia correlated with high proinflammatory markers that may promote cardiovascular events such as s-VCAM-1, P-selectin, leptin and D-dimer, whereas mediators with anti-inflammatory properties such as adiponectin and IL-10 were decreased. These relationships reversed after ART[21]. It has been shown that after children received ART and have HIV RNA suppression, the HDL level is significantly improved to normal levels [16] together with decreased total cholesterol:HDL ratio and proinflammatory cytokines. [15] It is unknown if and how HDL and HIV viremia affects children’s risk for developing cardiovascular events in the longer term. The PREDICT study is ongoing and at the conclusion of the trial in 2011, the 3-year longitudinal data will allow us to assess the change of lipid profile during no ART (deferred arm) and after ART (immediate arm). It will be important to follow children to assess their risk of early cardiovascular events in adulthood.
PREDICT Study Group
CIP TH001: HIV Netherlands Australia Thailand (HIV-NAT) Research Collaboration, Thai Red Cross AIDS Research Center, Bangkok, Thailand; Dr. Kiat Ruxrungtham, Dr. Jintanat Ananworanich, Dr. Thanyawee Puthanakit, Dr. Chitsanu Pancharoen, Dr. Torsak Bunupuradah, Stephen Kerr, Theshinee Chuenyam, Sasiwimol Ubolyam, Apicha Mahanontharit, Tulathip Suwanlerk, Jintana Intasan, Thidarat Jupimai, Primwichaya Intakan, Tawan Hirunyanulux, Praneet Pinklow, Kanchana Pruksakaew, Oratai Butterworth, Nitiya Chomchey, Chulalak Sriheara, Anuntaya Uanithirat, Sunate Posyauattanakul, Thipsiri Prungsin, Pitch Boonrak, Waraporn Sakornjun, Tanakorn Apornpong, Jiratchaya, Ormrudee Rit-im, Nuchapong Noumtong, Noppong Hirunwadee, Chaiwat Ungsedhapand, Chowalit Phadungphon, Wanchai Thongsee, Orathai Chaiya, Augchara Suwannawat, Threepol Sattong, Niti Wongthai, Kesdao Nantapisan, Umpaporn Methanggool, Narumon Suebsri, Dr. Chris Duncombe, Taksin Panpuy, Chayapa Phasompa, Boonjit Deeaium, Pattiya Jootakarn
CIP TH003:Bamrasnaradura Infectious Disease Institute, Nonthaburi, Thailand; Dr. Jurai Wongsawat, Dr. Rujanee Sunthornkachit, Dr. Visal Moolasart, Dr. Natawan Siripongpreeda, Supeda Thongyen, Piyawadee Chathaisong, Vilaiwan Prommool, Duangmanee Suwannamass, Simakan Waradejwinyoo, Nareopak Boonyarittipat, Thaniya Chiewcharn, Sirirat Likanonsakul, Chatiya Athichathana, Boonchuay Eampokalap, Wattana Sanchiem.
CIP TH004:Srinagarind Hospital, Khon Kean University, Khon Kean, Thailand; Dr. Pope Kosalaraksa, Dr. Pagakrong Lumbiganon, Dr. Chulapan Engchanil, Piangjit Tharnprisan, Chanasda Sopharak, Viraphong Lulitanond, Samrit Khahmahpahte, Ratthanant Kaewmart, Prajuab Chaimanee, Mathurot Sala, Thaniita Udompanit, Ratchadaporn Wisai, Somjai Rattanamanee, Yingrit Chantarasuk, Sompong Sarvok, Yotsombat changtrakun, Soontorn kunhasura, Sudthanom Kamollert
CIP TH005:Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand; Dr. Wicharn Luesomboon, Dr. Pairuch Eiamapichart, Dr. Tanate Jadwattanakul, Isara Limpet-ngam, Daovadee Naraporn, Pornpen Mathajittiphun, Chatchadha Sirimaskul, Woranun Klaihong, Pipat Sittisak, Tippawan Wongwian, Kansiri Charoenthammachoke, Pornchai Yodpo.
CIP TH007:Nakornping Hospital, Chiang Mai, Thailand; Dr. Suparat Kanjanavanit, Dr. Maneerat Ananthanavanich, Dr. Penpak Sornchai, Thida Namwong, Duangrat Chutima, Suchitra Tangmankhongworakun, Pacharaporn Yingyong, Juree Kasinrerk, Montanee Raksasang, Pimporn Kongdong, Siripim Guadanant, Suphanphilat Thong-Ngao, Sangwan Paengta, Kasinee Junsom, Ruttana Khuankaew, Parichat Moolsombat, Duanpen Khuttiwung, Chanannat Chanrin, Sureeporn Sittilerts, Pramote Naveewongpanich.
CIP TH009:Chiangrai Regional Hopsital, Chiang Rai, Thailand; Dr. Rawiwan Hansudewechakul, Dr. Yaowalak Jariyapongpaiboon, Dr. Chulapong Chanta, Areerat Khonponoi, Chaniporn Yodsuwan, Warunee Srisuk, Pojjavitt Ussawawuthipong, Yupawan Thaweesombat, Polawat Tongsuk, Chaiporn Kumluang, Ruengrit Jinasen, Noodchanee Maneerat, Kajorndej Surapanichadul, Pornpinit Donkaew.
CIP TH010:National Pediatric Hospital, Phnom Penh, Cambodia; Dr. Sophann Vonthanak, Dr. Ung Vibol, Dr. Sam Sophan, Dr. Pich Boren, Dr. Kea Chettra, Lim Phary, Toun Roeun, Tieng Sunly, Mom Chandara, Chuop Sokheng, Khin Sokoeun, Tuey Sotharin.
CIP TH011:Social Health Clinic, Phnom Peng, Cambodia; Dr. Saphonn Vonthanak, Dr. Ung Vibol, Dr. Vannary Bun, Dr. Somanythd Chhay Meng, Dr. Kea Chettra, Sam Phan, Wuddhika In vong, Khuon Dyna.
CIP TH012:Prapokklao Hospital, Chantaburi, Thailand; Dr. Chaiwat Ngampiyaskul, Dr. Naowarat Srisawat, Wanna Chamjamrat, Sayamol Wattanayothin, Pornphan Prasertphan, Tanyamon Wongcheeree, Pisut Greetanukroh, Chataporn Imubumroong, Pathanee Teirsonsern.
Footnotes
Address for reprint is the same as that for corresponding authors
References
- 1.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. Aids. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–138. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 4.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C, Kotler DP, Hamadeh R, et al. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld C, Tien P. Difficulties in understanding the metabolic complications of acquired immune deficiency syndrome. Clin Infect Dis. 2003;37 (Suppl 2):S43–46. doi: 10.1086/375886. [DOI] [PubMed] [Google Scholar]
- 8.El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 9.Friis-Møller N, Reiss P, El-Sadr W, et al. Exposure to PI and NNRTI and Risk of Myocardial Infarction: Results from the D:A:D Study. 13th Conference on Retroviruses and Opportunistic Infections; 5–8 February 2006; Denver, Colorado. p. Abstract 144. [Google Scholar]
- 10.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 11.McComsey GA, O’Riordan M, Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. Aids. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP): highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 13.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 15.Cervia JS, Chantry CJ, Hughes MD, et al. Associations of proinflammatory cytokine levels with lipid profiles, growth, and body composition in HIV-infected children initiating or changing antiretroviral therapy. Pediatr Infect Dis J. 29:1118–1122. doi: 10.1097/INF.0b013e3181ed9f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurpibul L, Puthanakit T, Lee B, et al. Lipodystrophy and metabolic changes in HIV-infected children on non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Antivir Ther. 2007;12:1247–1254. [PubMed] [Google Scholar]
- 17.Botton J, Heude B, Kettaneh A, et al. Cardiovascular risk factor levels and their relationships with overweight and fat distribution in children: the Fleurbaix Laventie Ville Sante II study. Metabolism. 2007;56:614–622. doi: 10.1016/j.metabol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 19.Raghavan S, Chen L, El-Sadr W, et al. Prevalence and factors associated with fasting hypercholesterolemia, hypertriglyceridemia, and hyperglycemia in ethnically diverse antiretroviral naive patients (CPCRA 058) Int Conf AIDS. 2000 Jul 9–14;13:abstract no. WePeB4269. [Google Scholar]
- 20.Rose H, Woolley I, Hoy J, et al. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism. 2006;55:90–95. doi: 10.1016/j.metabol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Calmy A, Gayet-Ageron A, Montecucco F, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. Aids. 2009;23:929–939. doi: 10.1097/qad.0b013e32832995fa. [DOI] [PubMed] [Google Scholar]