Abstract
Influenza vaccination is generally recommended for non-Hodgkin’s lymphoma (NHL) patients, but no data are available about the activity of this vaccine after treatment with rituximab-containing regimens. We evaluated the humoral response to the trivalent seasonal influenza vaccine in a group of NHL patients in complete remission for ≥6 mo (median, 29 mo) after treatment with rituximab-containing regimens (n = 31) compared with age-matched healthy subjects (n = 34). B cell populations and incidence of influenza-like illness were also evaluated. For each viral strain, the response was significantly lower in patients compared with controls and was particularly poor in patients treated with fludarabine-based regimens. In the patient group, the response to vaccination did not fulfill the immunogenic criteria based on the European Committee for Medicinal Products for Human Use requirements. Among the patients, CD27+ memory B cells were significantly reduced, and their reduction correlated with serum IgM levels and vaccine response. Episodes of influenza-like illness were recorded only in patients. These results showed that NHL patients treated with rituximab-containing regimens have persisting perturbations of B cell compartments and Ig synthesis and may be at particular risk for infection, even in long-standing complete remission.
Influenza is an important cause of morbidity and mortality worldwide; in onco-hematological patients, case fatalities are reported to vary from 11–33% (1). Vaccination is recommended for individuals belonging to high-risk categories who may develop serious complications and death, including the elderly, those with chronic diseases or malignancies, and those who receive immunosuppressive medications (2, 3). Importantly, the same immune dysfunction that increases the risk for, and consequences of, influenza infection might also compromise vaccine responses and effectiveness (1, 4).
Few data are available about the efficacy/effectiveness of influenza vaccination in lymphoma patients, in whom this vaccine seems to elicit weaker humoral responses compared with healthy controls (5, 6), but it seems to confer sufficient immunoprotection in chemotherapy-naive or recently treated non-Hodgkin’s lymphoma (NHL) patients (6, 7). However, even in the more recent studies, the patients enrolled had been treated prior to the extensive use of mAbs in clinical practice (6, 7).
Rituximab is a mAb with specificity for CD20, a surface-membrane Ag expressed on B cells from early maturation up to their final differentiation steps into plasma cells. CD20 is found on the surface of malignant cells from most lymphoproliferative disorders (8, 9). Sustained B cell depletion occurs after rituximab administration, lasting up to 6 mo after treatment with subsequently increasing B cell numbers, leading to complete recovery in the majority of patients after ~1 y (8). Nonetheless, long-term persisting depletion of circulating B cells may be observed (10, 11). Late immunologic-related adverse events have been described, including hepatitis B virus reactivation (12, 13), persistent hypogammaglobulinemia, and/or recurrent infections (10, 14, 15). These sporadic events (10, 14, 15) are being reported with increasing frequency, especially when rituximab treatment is associated with autologous stem cell transplantation procedures or with fludarabine administration. In addition, decreased humoral responses against tetanus- and poliovirus-recall Ags were described in NHL patients treated with this mAb (4).
The above considerations led us to investigate whether NHL patients in complete remission (CR) after treatment with rituximab-based chemotherapy regain an immune competence, allowing humoral responses upon influenza vaccination comparable to healthy subjects.
Materials and Methods
Patients and controls
We studied subjects recommended to receive influenza vaccination according to the Italian health care program (16). Sequential patients and healthy persons presenting for routine seasonal influenza vaccination were screened and recruited, after providing full informed written consent, if they met the inclusion criteria. The study was approved by the Ethics Committee of the National Cancer Research Institute of Genoa (no. MI08.001). Patient inclusion criteria were age ≥18 y, a biopsy-proven diagnosis of NHL treated with chemotherapy and rituximab (with or without radiation therapy) completed ≥6 mo before the vaccination date, achievement of CR, and no evidence of disease at the time of vaccination (17, 18). Patient exclusion criteria included autologous or allotransplantation, other severe comorbidity, and Ig infusion ≤30 d following influenza vaccination. Age-matched healthy volunteers aged ≥18 y, recruited from health care workers, and healthy subjects aged ≥65 y served as controls. Additional exclusion criteria for all subjects/patients were immune-mediated disease, active or recent treatment with immunosuppressive drugs, severe chronic infections, or other scheduled vaccination ≤30 d following influenza vaccination.
Vaccination
Influenza vaccination consisted of one dose of a commercially available trivalent virosomal subunit vaccine administered i.m. (Inflexal V, Crucell, The Netherlands), containing 15 μg each of the influenza strains recommended for the northern hemisphere 2008–2009 influenza season: A/Brisbane/10/2007 (H3N2), A/Brisbane/59/2007 (H1N1), and B/Florida/4/2006 (3).
Evaluations
Disease status
Only patients whose clinical conditions fulfilled CR criteria were considered for eligibility (17, 18). Patients suspected of progressive disease or without clear evidence of CR were evaluated, as appropriate (18). Time after treatment was considered the time elapsed between the last day of chemotherapy and/or rituximab administration (whichever came later) and the assessment visit.
Sample collection and flow cytometry
Fifteen milliliters of peripheral blood were collected just before vaccination (T0) and 28 ± 2 d later (T1). Sera were collected and stored at −20°C until assayed. Complete blood count was performed at T0. Fifteen milliliters of peripheral blood were also collected in heparinized tubes at T0 and at T1 and immediately processed for flow cytometry analysis. PBMCs were obtained by density-gradient separation (Biochrom, Berlin, Germany). Three-color flow cytometric immunophenotyping of circulating B cell subpopulations (CD45+CD19+ CD27+ B memory cells and CD45+CD19+CD27− naive B cells) (19) was performed using the following fluorochrome-conjugated mAbs: FITC-CD19, allophycocyanin-CD45, and PE-CD27 (Becton Dickinson & Co., Milan, Italy). A total of 30,000 events was acquired on a FACSCalibur flow cytometer and analyzed using CellQuest software (both from Becton Dickinson & Co.). CD27+ memory B cells were identified by forward versus side scatter gating in combination with CD19+ B cell gating (20).
D.B., G.Z., and A.D.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Influenza serology and clinical monitoring
The hemagglutinin inhibition (HI) assay was used to study the Ab response. The HI assay was performed following the World Health Organization criteria, as previously described (21, 22). The obtained Ab titer was expressed as the reciprocal of the highest dilution of serum inhibiting hemagglutination. Any HI result <10 (undetectable) was expressed as 5. Tests were performed in duplicate. Immunogenicity for each strain was determined by the following parameters: geometric mean titer evaluated with HI assay (GMT) prevaccination: geometric mean HI titers at T0; GMT postvaccination: geometric mean HI titers at T1; mean fold increase (MFI) (T1/T0 vaccination titer ratio); seroprotection rate: the percentage of subjects achieving an HI titer ≥40; and seroconversion rate: the percentage of subjects with ≥4-fold increase in HI titer from a nonnegative pre-vaccination titer (i.e., ≥40) or increase from <10 to ≥40 in previously seronegative subjects. These parameters were compared between patients and controls. Results were matched to the European Committee for Medicinal Products for Human Use (CHMP) requirements for annual evaluation of the influenza vaccine in the elderly (age >60 y) (i.e., serocon-version rate >30%, seroprotection rate >60%, MFI >2.0) (23). Evaluation of CHMP criteria after age stratification was not carried out because the small number of enrolled subjects aged ≤60 y would have caused excessive fragmentation, preventing appropriate analysis.
HI titers were transformed into binary logarithms, and postvaccination data were corrected for prevaccination status [corrected T1 titer, as described by Beyer et al. (24) and Ansaldi et al. (25)]. They were confirmed to be normally distributed by the D’Agostino–Pearson normality test and are expressed as mean titers ± SD.
After vaccination, seasonal clinical monitoring of patients and controls was performed (November 2008 to April 2009) to record the occurrence of any episode of influenza-like illness (ILI), as defined by the Centers for Disease Control and Prevention: temperature ≥37.8°C with cough or sore throat, in the absence of a known cause other than influenza (26). Patients were contacted monthly and were instructed to promptly report by phone any episode of fever.
Statistical analysis
Seroprotection and seroconversion rates of patients and controls were compared using the χ2 test or the Fisher exact test, as appropriate. The GMTs between patients and controls were compared with the Mann–Whitney U test, whereas the Wilcoxon test was used to compare T1 versus T0 GMTs within each group. Corrected T1 titers were analyzed using Student t tests. Circulating B cells populations and Ig serum levels were analyzed using nonparametric tests because the data were not distributed normally (Mann–Whitney U test, Wilcoxon test). The Spearman rank correlation coefficient (ρ) test was used to correlate B cell populations, Ig serum level, and corrected T1 titer. To identify risk-defining clinical parameters, seroconversion/seroprotection rate and corrected T1 titers were correlated with age (≤60 versus >60 y); previous influenza vaccination (yes versus no); lymphoma histotype (aggressive versus indolent); type of administered chemotherapy (fludarabine-based regimens versus other regimens); number of treatment lines (one versus more than one); time elapsed from administration of last treatment (≤12 versus >12 mo); number of rituximab administrations (up to six versus more than six doses); IgG, IgA, and IgM serum levels (low versus normal); and previous radiation therapy (yes versus no). Age and previous vaccination were also evaluated among healthy controls. The variables were first assessed in univariate analysis. The multivariate model (one for each evaluated parameter) was obtained by a step-down forward procedure, starting from the initial model that contained only the constant (b0) and adding significant univariate factors. A significance level of p = 0.05 was used to retain variables in the multivariate model, and a p value = 0.10 was chosen as a threshold for the removal test of the least useful predictor. Binary logistic regression was used for the categorical outcome variable (i.e., serocon-version and seroprotection), whereas multiple linear-regression analysis was performed when the outcome was a continuous variable (i.e., corrected T1 titer). The β-coefficients of the significant results were reported. A β-coefficient <0 (i.e., odds ratio <1) is associated with a lower response.
Because responses to three Ags were available for each subject, the mean of the three corrected T1 titers was considered a robust quantitative indicator of the global humoral response. This parameter (i.e., mean corrected T1 titers), in addition to the specific corrected T1 titer, was used for subgroup analysis and for correlation analysis with B cells and serum Igs. Correlations among the aforementioned clinical parameters (χ2/Fisher exact test) and with flow cytometry data (Mann–Whitney U test) were also assessed.
The p values < 0.05 (two sided) were considered statistically significant. Analyses were performed with SPSS version 17.0 (SPSS, Chicago, IL).
Results
Subject characteristics
During October–November 2008, of 62 patients and 102 healthy subjects sequentially screened at our Institutions (University and National Cancer Institute of Genoa), 37 patients and 34 healthy controls qualified for eligibility and provided informed consent. Six of the patients did not meet the criteria for CR after restaging and were excluded. Thus, 65 vaccinated subjects were analyzed (patient group: n = 31; control group: n = 34). The study demographics are shown in Table I. The groups matched with regard to median age (66 and 62 y in patient and control group, respectively). More than one third of the subjects in each group had never received influenza vaccine, whereas the others had been vaccinated during the preceding epidemic season, as well as during several previous seasons.
Table I.
Demographic data for patients and controls
| Characteristic | Patients (n = 31) | Controls (n = 34) |
|---|---|---|
| Age (y; median [range]) | 66 (36–84) | 62 (49–81) |
| ≤60 | 36% (11/31) | 38% (13/34) |
| >60 | 64% (20/31) | 62% (21/34) |
| Men/women | 12/19 | 22/12 |
| Previous unvaccinated patients | 32% (10/31) | 29% (10/34) |
| NHL histotype at diagnosis | ||
| Aggressive | 58% (18/31) | |
| Diffuse large B cell | 42% (13/31) | |
| Others | 16% (5/31) | |
| Indolent | 42% (13/31) | |
| Follicular | 26% (8/31) | |
| Others | 16% (5/31) | |
| Applied chemotherapy regimens (in addition to rituximab) | ||
| CHOP or CHOP likea | 65% (20/31) | |
| CVP | 13% (4/31) | |
| Fludarabine-containing regimensb | 26% (8/31) | |
| Othersc | 23% (7/31) | |
| Lines of chemotherapy administered (n) | ||
| 1 | 84% (26/31) | |
| 2d | 6% (2/31) | |
| 3e | 10% (3/31) | |
| Time after treatment administration (mo; median [range]) | 29 (7–65) | |
| ≤12 | 19% (6/31) | |
| >12 | 81% (25/31) | |
| Rituximab doses (n; median [range]) | 6 (3–11) | |
| ≤6 | 81% (25/31) | |
| >6 | 19% (6/31) | |
| Radiotherapy | 32% (10/31) | |
| Abs serum level | ||
| IgG serum level (mg/dl; median, IQR [normal range]) | 842, 525–1043 (700–1600) | |
| Low serum IgG | 36% (11/31) | |
| IgA serum level (mg/dl; median; IQR [normal range]) | 148, 60–239 (70–400) | |
| Low serum IgA | 26% (8/31) | |
| IgM serum level (mg/dl; median; IQR [normal range]) | 39, 18–78 (40–230) | |
| Low serum IgM | 52% (16/31) | |
| Low IgG or IgA or IgM serum level | 55% (17/31) | |
| Low IgG, IgA, and IgM serum levels | 19% (6/31) | |
Etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (n = 3).
Fludarabine, cyclophosphamide, vincristine, and prednisone (n = 1); fludarabine, mitoxantrone, and cyclophosphamide (n = 4); fludarabine and mitoxantrone (n = 2); and fludarabine and cyclophosphamide (n = 1).
Cyclophosphamide, mesna, methotrexate, doxorubicin, vincristine, cytarabine, and leucovorin (n = 2); cisplatin, etoposide, and mitoxantrone (n = 1); cyclophosphamide (n = 1); cyclophosphamide and dexamethasone (n = 1); gemcitabine and oxaliplatin (n = 1); and cyclophosphamide, vincristine, methotrexate, and prednisone (n = 1).
First line, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; second line, rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone (n = 1). First line, fludarabine, mitoxantrone, and cyclophosphamide; second line: rituximab-cyclophosphamide, vincristine, and prednisone (n = 1).
First line, fludarabine, cyclophosphamide, vincristine and prednisone; second line, cisplatin, VP16, and mitoxantrone; third line, rituximab-cyclophosphamide (n = 1). First line, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; second line, rituximab-fludarabine, mitoxantrone, and cyclophosphamide; third line, rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (n = 1). First line, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; second line, rituximab-gemcitabine and oxaliplatin; third line, fludarabine, mitoxantrone, and cyclophosphamide (n = 1).
CVP, cyclophosphamide, vincristine, and prednisone.
Most patients (58%) had been diagnosed with aggressive lymphoma (mostly diffuse large B cell lymphoma). Median time from last treatment was 29 mo (range, 7–65 mo), and rituximab had been administered >1 y before in ~80% of patients. In all but one patient, the last administration of rituximab and chemotherapy coincided. One patient received rituximab without concomitant chemotherapy during his last cycle. The majority of patients (84%) had received one line of treatment. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens predominated (20 patients [65%]); a minority (8 patients [26%]) received fludarabine-based regimens. Low levels of serum IgM, often combined with low levels of IgG and/or IgA, were observed in 52% of patients. The low serum levels of each Ig class correlated with each other (p < 0.05). The administration of fludarabine was associated with low serum levels of IgG (p = 0.001), IgA (p = 0.001), and IgM (p = 0.037). Four of five patients who had been treated with multiple lines of chemotherapy had received fludarabine (p = 0.010); the administration of multiple chemotherapy lines was associated with low serum levels of IgG (p = 0.042) and IgA (p = 0.010), as well as with the administration of more than six cycles of rituximab (p = 0.038). In the control group, age ≤ 60 y was associated with previous administration of influenza vaccine (p = 0.005).
Reduced response to influenza vaccine by rituximab-treated NHL patients in CR
Prior to vaccination, influenza A/H3N2 GMTs in healthy controls were almost 2-fold greater than in patients (p = 0.001). Accordingly, the A/H3N2 prevaccination-seroprotection rate (Ab titers ≥ 40) was greater in controls (p = 0.005). No differences in pre-vaccination Ab titers against A/H1N1 and B strains were detected (Table II).
Table II.
Pre- and postvaccination parameters in patients and controls
| Prevaccination Parameters | Patients (n = 31) | Controls (n = 34) | p Value (Patients versus Controls)b |
|---|---|---|---|
| Strain: A/H3N2 | |||
| GMT | 22.4 | 42.5 | 0.001 |
| Seroprotection rate | 39% (12/31) | 74% (25/34) | 0.005 |
| Strain: A/H1N1 | |||
| GMT | 21.9 | 23.1 | 0.72 |
| Seroprotection rate | 39% (12/31) | 47% (16/34) | 0.50 |
| Strain: B | |||
| GMT | 11.6 | 11.5 | 0.93 |
| Seroprotection rate | 10% (3/31) | 12% (4/34) | 1.00 |
| Postvaccination Parameters | |||
| Strain: A/H3N2 | |||
| GMT | 36.6a | 135.9a | <0.00001 |
| Seroconversion rate | 23% (7/31) | 53% (18/34) | 0.012 |
| Seroprotection rate | 65% (20/31) | 94% (32/34) | 0.004 |
| MFI | 1.6 | 3.2 | |
| Strain: A/H1N1 | |||
| GMT | 41.8a | 104.3a | 0.004 |
| Seroconversion rate | 29% (9/31) | 41% (14/34) | 0.31 |
| Seroprotection rate | 74% (23/31) | 94% (32/34) | 0.039 |
| MFI | 1.9 | 4.5 | |
| Strain: B | |||
| GMT | 15.6a | 27.7a | 0.004 |
| Seroconversion rate | 3% (1/31) | 29% (10/34) | 0.005 |
| Seroprotection rate | 23% (7/31) | 44% (15/34) | 0.07 |
| MFI | 1.3 | 2.4 | |
p < 0.01 compared with prevaccination GMT.
The p values < 0.05 (significant difference) are bold.
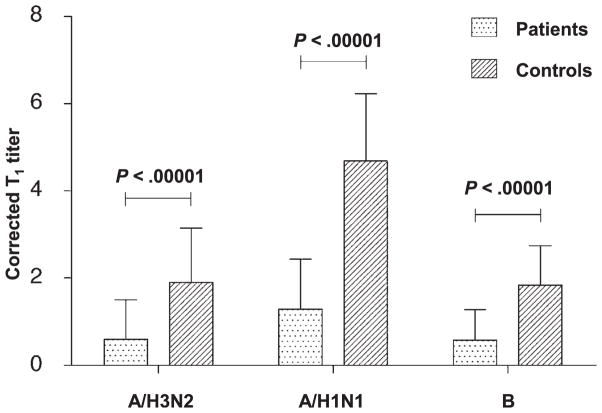
Following vaccination, GMTs increased for all three influenza strains in patients and in healthy controls (p < 0.01). However, this increase was blunted among patients and was approximately half that in controls (p < 0.05; Table II). Furthermore, even after correction for prevaccination status, according to Beyer et al. (24), the postvaccination Ab titer of each strain was much greater in controls. As shown in Fig. 1, the corrected T1 titer for each strain was more than three times lower in patients compared with healthy controls (p < 0.00001 for all comparisons). This poor increase in Ab titers among patients resulted in poor seroconversion rates. Only one patient (3%) achieved seroconversion against B strain compared with 10 controls (29%) (p = 0.005). Similarly, <25% of patients and >50% of controls seroconverted against A/H3N2 (p = 0.012). This trend was also observed for A/H1N1, without reaching significance (seroconversion rate: 29% versus 41% for patients and controls, respectively; p = 0.31). Finally, only two healthy controls (6%) were not seroprotected (i.e., lacked an Ab titer ≥40) against A/H3N2 and/or A/H1N1 viruses compared with 11 patients (35%) for A/H3N2 and 8 patients (26%) for A/H1N1 (p = 0.004 and p = 0.039, respectively). A considerable proportion of patients (77%) and controls (56%) was not sero-protected against B virus, without a significant difference between the groups (p = 0.07).
FIGURE 1.
Corrected postvaccination Ab titers are lower in patients compared with controls. Bar graphs of postvaccination Ab titer data transformed into binary logarithms and corrected for prevaccination status according to Beyer’s method. Error bars represent SD. Corrected T1 titer was significantly lower in patients compared with controls for A/H3N2 (0.60 ± 0.90 versus 1.90 ± 1.25), A/H1N1 (1.29 ± 1.15 versus 4.69 ± 1.54), and B (0.58 ± 0.70 versus 1.84 ± 0.92) strains. p < 0.00001 for all comparisons, Student t test.
Postvaccination parameters were analyzed according to the European CHMP criteria for the annual approval of influenza vaccine immunogenicity for the elderly (Fig. 2). At least one of the three reported requirements has to be met for all three viral strains to fulfill these criteria. Overall, an adequate vaccine immunogenicity was observed in controls but not in patients. All requirements for A/H3N2 and A/H1N1 and two of three requirements for influenza B were satisfied in healthy controls. In the patient group, MFI and seroconversion data did not reach the required thresholds for A/H3N2 and A/H1N1, whereas the response to influenza B was disappointing for all analyzed parameters.
FIGURE 2.
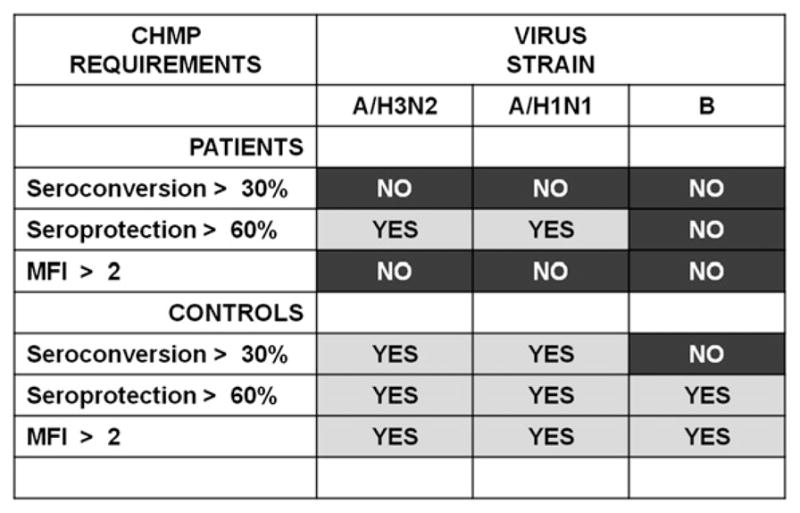
CHMP requirements against vaccine strains met after immunization in patients and controls. In the patient group, only two of nine values fulfilled CHMP requirements for subjects >60 y old, in contrast with eight of nine values in the control group. p = 0.015, Fisher exact test, two-tailed.
Thus, overall, only two of nine values fulfilled the CHMP immunogenicity requirements in the patient group compared with eight of nine values in the control group (p = 0.015, Fisher exact test).
During the epidemic season, ILI was reported by four patients and none of the controls (p = 0.047, Fisher exact test). Lower than normal serum IgM levels were detected in all four of those patients and were associated with low serum IgA levels in one case and with low serum IgA and IgG levels in another. Laboratory confirmation of influenza infection (e.g., nasopharyngeal swab and PCR) was not possible because of patient unavailability (holiday/travel, n = 3; notification 2 wk after resolution of symptoms, n = 1). Indeed, we do not have confirmed influenza strains in these patients, and we do not know whether potential influenza strains were those covered by vaccination.
Analysis of possible risk factors associated with poor response to influenza vaccination
We next evaluated postvaccination seroconversion rates, seroprotection rates, and corrected T1 titers with regard to factors that could contribute to an increased risk for nonresponse. Variables that significantly correlated with clinical response, according to univariate and multivariate analyses, are shown in Table III.
Table III.
Patient and control subgroup analysis: correlation between pretreatment factor and parameters of response to influenza vaccination
| Patients: Univariate Analysisa
|
Strain: A/H3N2
|
Strain: A/H1N1
|
Strain: B
|
All Strains
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable Analyzed | SC p Value | SP p Value | Corr.T1 Titer p Value | SC p Value | SP p Value | Corr.T1 Titer p Value | SC p Value | SP p value | Corr.T1 Titer p Value | Mean Corr.T1 Titer p Value |
| Age > 60 y | 0.66 (+) | 0.14 (−) | 0.67 (+) | 0.14 (−) | 0.12 (−) | 0.020 (−) | 0.45 (+) | 0.18 (−) | 0.68 (−) | 0.10 (−) |
| Previous vaccination | 0.81 (+) | 0.22 (−) | 0.22 (−) | 0.009 (−) | 0.61 (−) | 0.018 (−) | 0.141 (−) | 0.25 (+) | 0.29 (−) | 0.031 (−) |
| Indolent histology | 0.09 (−) | 0.77 (−) | 0.08 (−) | 0.16 (−) | 0.59 (−) | 0.28 (−) | 0.23 (+) | 0.09 (−) | 0.035 (−) | 0.043 (−) |
| Fludarabine administration | 0.43 (−) | 0.06 (−) | 0.09 (−) | 0.036 (−) | 0.006 (−) | 0.010 (−) | 0.55 (−) | 0.43 (−) | 0.025 (−) | 0.003 (−) |
| Treatment lines > 1 | 0.88 (−) | 0.023 (−) | 0.45 (−) | 0.12 (−) | 0.43 (−) | 0.31 (−) | 0.66 (−) | 0.19 (−) | 0.078 (−) | 0.14 (−) |
| Time after treatment ≤ 12 mo | 0.14 (−) | 0.90 (+) | 0.25 (−) | 0.46 (−) | 0.64 (−) | 0.42 (−) | 0.19 (+) | 0.48 (+) | 0.78 (+) | 0.41 (−) |
| Rituximab doses > 6 | 0.70 (−) | 0.08 (−) | 0.62 (−) | 0.46 (−) | 0.64 (−) | 0.46 (−) | 0.62 (−) | 0.70 (−) | 0.85 (−) | 0.50 (−) |
| Previous radiation therapy | 0.50 (+) | 0.21 (+) | 0.77 (+) | 0.94 (+) | 0.71 (−) | 0.90 (−) | 0.48 (−) | 0.25 (−) | 0.96 (+) | 0.94 (+) |
| Low IgG serum level | 0.66 (−) | 0.40 (−) | 0.43 (−) | 0.07 (−) | 0.32 (−) | 0.13 (−) | 0.45 (−) | 0.18 (−) | 0.031 (−) | 0.061 (−) |
| Low IgA serum level | 0.43 (−) | 0.06 (−) | 0.21 (−) | 0.23 (−) | 0.38 (−) | 0.29 (−) | 0.55 (−) | 0.08 (−) | 0.020 (−) | 0.063 (−) |
| Low IgM serum level | 0.60 (−) | 0.32 (−) | 0.15 (−) | 0.004 (−) | 0.47 (−) | 0.023 (−) | 0.30 (−) | 0.74 (+) | 0.026 (−) | 0.009 (−) |
| Patients: Multivariate Analysisb,c
|
Strain: A/H3N2
|
Strain: A/H1N1
|
Strain: B
|
All Strains
|
||||||
| Variables Included in the Final Models | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | Mean Corr.T1Titer p Value (β [SE]) |
|
| ||||||||||
| Previous vaccination | — | — | — | 0.030 (−) (22.67 [1.2]) | — | — | — | — | — | — |
| Fludarabine administration | — | — | — | — | 0.012 (−) (22.41 [0.96]) | 0.010 (−) (21.18 [0.42]) | — | — | — | 0.003 (−) (20.81 [0.26]) |
| Treatment line > 1 | — | 0.047 (−) (22.39 [1.2]) | — | — | — | — | — | — | — | — |
| Low IgA serum level | — | — | — | — | — | — | — | — | 0.020 (−) (20.65 [0.27]) | — |
| Low IgM serum level | — | — | — | 0.019 (−) (23.27 [1.4]) | — | — | — | — | — | — |
| Controls: Univariate Analysisa
|
Strain: A/H3N2
|
Strain: A/H1N1
|
Strain: B
|
All Strains
|
||||||
| Variables Analyzed | SC p Value | SP p Value | Corr.T1Titer p Value | SC p Value | SP p Value | Corr.T1Titer p Value | SC p Value | SP p Value | Corr.T1Titer p Value | Mean Corr.T1Titer p Value |
|
| ||||||||||
| Age > 60 y | 0.43 (−) | 0.25 (−) | 0.84 (+) | 0.016 (+) | 0.724 (+) | 0.21 (+) | 0.52 (+) | 0.85 (− | 0.74 (+) | 0.40 (+) |
| Previous vaccination | 0.20 (−) | 0.35 (−) | 0.06 (−) | 0.0002 (−) | 0.35 (−) | 0.003 (−) | 0.38 (−) | 0.76 (+) | 0.33 (−) | 0.008 (−) |
| Controls Multivariate Analysisb,c
|
Strain: A/H3N2
|
Strain: A/H1N1
|
Strain: B
|
All Strains
|
||||||
| Variables Included in the Final Models | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | SC p Value (β [SE]) | SP p Value (β [SE]) | Corr.T1Titer p Value (β [SE]) | Mean Corr.T1Titer p Value (β [SE]) |
|
| ||||||||||
| Previous vaccination | — | — | — | 0.002 (−) (23.53 [1.17]) | — | 0.003 (−) (21.63 [0.51]) | — | — | — | 0.008 (−) (20.95 [0.34]) |
− and + indicate the direction of the correlation (or trend) between the variable and the outcome (seroconversion rate, seroprotection rate, and corrected T1 titer). (−), the variable is associated with a lower response; (+), the variable is associated with a greater response. Significant correlations (p < 0.05) are shown in bold type. β coefficient < 0 (i.e., odds ratio < 1) is associated with a lower response.
The variables were first assessed in univariate analysis: seroconversion and seroprotection rates were compared with the χ2 test. Corrected T1 titers were compared with the Student t test.
Binary logistic regression and multiple linear regression analyses were performed for categorical (seroconversion and seroprotection) and continuous (corrected T1 titer) outcome variables, respectively. For each response parameter, a multivariate model was obtained by a step-down forward procedure, starting from the initial model that contained only the constant (b0) and then adding significant univariate factors. A significance level of p = 0.05 was used to retain variables in the multivariate model; a p value = 0.10 was chosen as threshold for the removal test of the least useful predictor. All statistical tests were two sided.
In multivariate analysis, — was used if the p value was >0.05.
Corr.T1Titer, corrected T1 titer; mean Corr.T1Titer, mean of the three corrected T1 titers (anti-A/H1NI, anti-A/H2N2, and anti-B hemagglutinin Abs); SC, seroconversion rate; SP, seroprotection rate.
According to the univariate analysis, younger patients (≤60 y) showed a better corrected T1 titer against A/H1N1 strain (p = 0.020). Indolent histology and low IgG serum levels were associated with a lower corrected T1 titer against B strain. The afore-mentioned results did not qualify for inclusion in the final model after multivariate analysis.
In the multivariate model, the administration of previous influenza vaccination and fludarabine-based chemotherapy and the presence of low IgA or IgM serum levels were associated with lower seroconversion rates and/or postvaccination-corrected titers against A/H1N1 (as for fludarabine and lgM) or B (as for IgA) strains. Treatment with fludarabine or more than one line of chemotherapy were the only factors associated with lack of sero-protection (for A/H1N1 and A/H3N2, respectively) included in the final model (Table III). Administration of fludarabine was the single variable that negatively correlated with the largest number of postvaccination parameters. At the univariate level, “fludarabine administration” was associated with a lack of response to A/H1N1 (seroconversion rate, seroprotection rate, and corrected T1 titer: p = 0.036, p = 0.006, and p = 0.010, respectively) and B Ags (corrected T1 titer: p = 0.025). When the humoral response was evaluated globally, the variables “fludarabine administration,” “previous vaccination,” “indolent histology,” and “low IgM serum level” were associated with a lower mean corrected T1 titer in the univariate analysis. “Fludarabine administration” was the only variable retained in the final multivariate model.
In the control group, subjects aged ≤60 y or receiving previous influenza vaccination had a lower probability of seroconverting against A/H1N1 (p = 0.016). However, according to the multivariate analysis, only the variable “previous vaccination” was included in the final model (p = 0.002) because of the strong correlation between such two variables (i.e., previous vaccination and age; p = 0.005). This reflects the characteristics of young controls, most of whom were health care professionals and, therefore, were routinely vaccinated for seasonal influenza. Even when the response was evaluated globally (mean of T1 corrected titers), “previous vaccination” still correlated negatively with the response (multivariate p = 0.008). This paradoxical association between “previous vaccination” and the worse vaccine response that we detected in controls and patients is known as “negative booster effect of previous vaccination” (27). Importantly, “previous vaccination” had no negative effect in terms of seroprotection (Table III) (27).
CD27+ memory B cells are decreased in patients and correlate with vaccine response
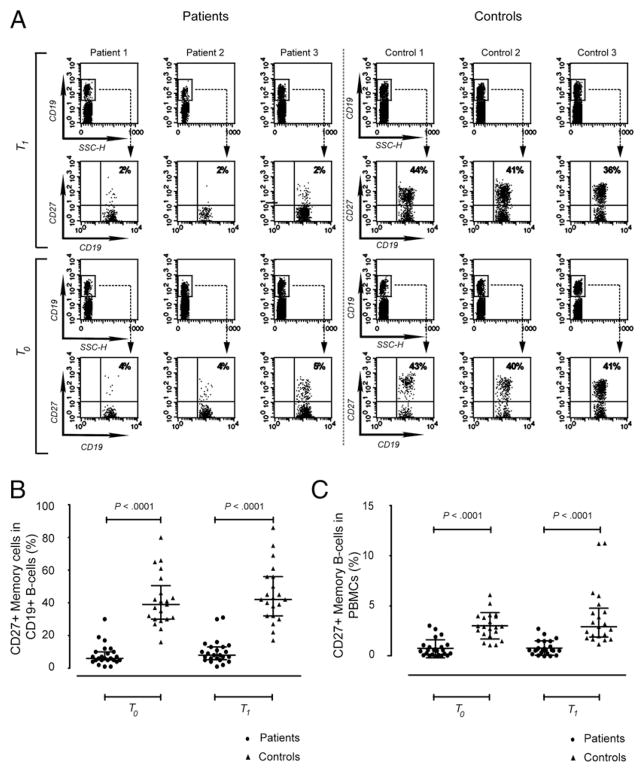
To identify possible mechanism(s) responsible for the observed defective immune responses to influenza vaccination, even long after rituximab dosing, we studied the possibility of rituximab-induced/associated persistent protracted perturbations of peripheral B cell subsets. To this end, flow cytometric analysis of B cell subsets was performed at baseline and after vaccination. Samples from 24 patients and 21 healthy controls were available for analysis. The median age of PBMC donors was 68 y (range, 36–84 y) for patients and 62 y (range, 51–71 y) for controls. At the time of analysis, patients were in CR and had been off treatment for a median of 31 mo (range, 7–65 mo). Absolute peripheral B cell counts (measured at T0) did not differ between patients and controls (163 cells/μl, interquartile range [IQR]: 40–255 cells/μl and 210 cells/μl, IQR: 150–300 cells/μl, respectively; p = 0.11). In patients, the proportion of peripheral B cells was 10.5% (IQR: 2.8–15.8%) at T0 and 7.5% (IQR: 3.2–16.5%) at T1. Similar data were observed in healthy controls at T0 (7.0%, IQR: 6.0–10.5%; p = 0.28) and at T1 (7.0%, IQR: 4.0–13.0% p = 0.93). Peripheral CD27− B cells were present in all subjects, whereas CD27+ memory B cells were substantially reduced in patient samples (Fig. 3). As shown in Fig. 3A and 3B, much lower proportions of CD27+ B cells were observed in patients (6.0%, IQR: 5.0–10.0%) compared with healthy controls (39.0%, IQR: 30.0–50.5%; p < 0.00001). This dramatic difference in the CD27+ B cell/total B cell (CD19+) ratio detected at baseline (p < 0.00001) was confirmed 4 wk later (8.0%, IQR: 5.3–13.0% versus 42.0%, IQR: 32.0–45.0% for patients and controls, respectively; p < 0.00001). CD27+ memory B cell depletion was also present when considering the proportion of CD27+ B cells among total PBMCs at T0 (0.6%, IQR: 0.1–1.0% versus 2.9%, IQR: 2.1–4.0% for patients versus controls; p < 0.00001) and at T1 (0.6%, IQR: 0.3–1.4% versus 2.9%, IQR: 1.9–4.8% for patients versus controls, respectively; p < 0.00001) (Fig. 3C) and was still evident, even in terms of absolute number, at T0 (8 cells/μl, IQR: 2–16 cells/μl versus 91 cells/μl, IQR: 45–113 cells/μl for patients versus controls, respectively; p < 0.0001). The numbers of circulating B cells or CD27+ memory B cells did not show any significant change after vaccine challenge compared with basal data in patient and control groups.
FIGURE 3.
There are fewer circulating CD27+ memory B cells in patients compared with healthy controls. A, FACS (flow cytometry)-analysis scatter plots from three representative patients and three controls showing the percentage of CD27+ B cells in the B cell population obtained from PBMCs. B cell subsets from the same subject were measured before (T0) and 4 wk after (T1) vaccination. Percentage of CD27+ memory B cells in B cells (B) and in total PBMCs (C) assessed in peripheral blood from patients and controls and obtained at the time points described in A. Longer horizontal lines represent the medians for each data group, whereas the shorter ones show the IQR. Statistical analyses were performed with the two-tailed Mann–Whitney U test.
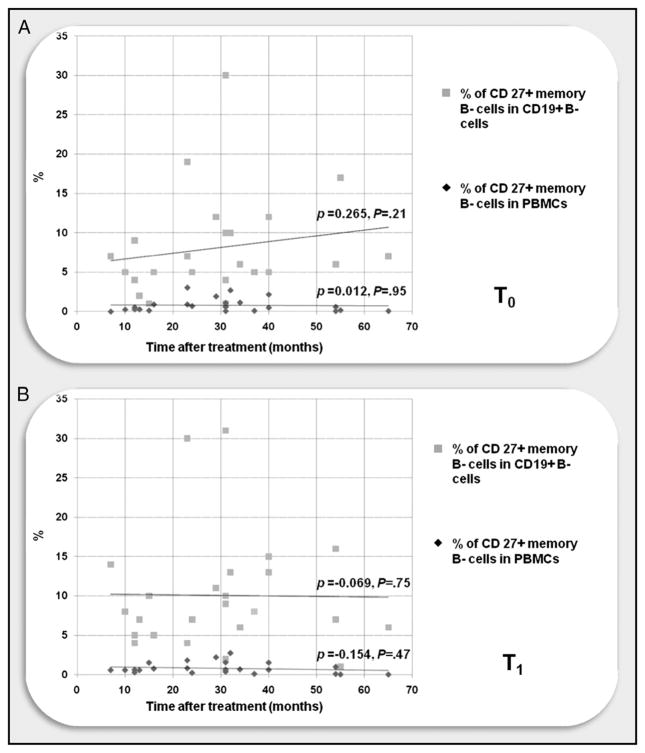
Given the possibility of an effect of the time interval since the last rituximab dosing, we controlled for memory B cell numbers and proportions as a function of time since the last rituximab administration. As shown in Fig. 4, failure to recover peripheral CD27+ memory B cell numbers over time was unrelated to time after treatment.
FIGURE 4.
CD27+ memory B cells remain depleted long after treatment. Scatter plot of FACS analysis of PBMCs from patients and controls obtained before (T0; A) and 4 wk after (T1; B) influenza vaccination. Time after treatment = months since last rituximab administration, calculated at T0. CD27+ memory B cells were still depleted long after treatment, without any significant linear correlation between time after treatment and CD27+ memory B cell levels, as shown by the continuous lines. Spearman’s rank correlation coefficient test (ρ) was used to correlate CD27+ memory B cell data and time after treatment.
Peripheral total and CD27+ memory B cells, influenza vaccine response, and IgM serum level are correlated with each other in patient group
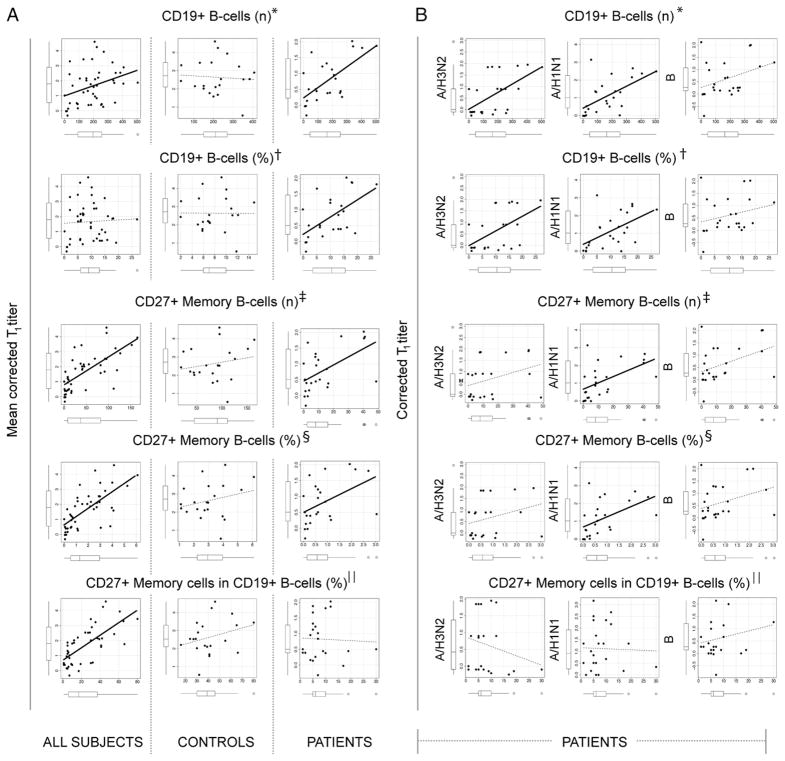
To further explore the association between peripheral B cells and the intensity of humoral response against the vaccine, we evaluated the correlation between the prevaccination data of B cell populations and the postvaccination Ab titers (Spearman’s ρ; Fig. 5). A direct correlation was observed in a first model including all patients and healthy controls. Increasing B cells (number), CD27+ memory B cells (number and percentage), or proportion of CD27+ memory cells among B cells were significantly associated with a more effective response to influenza vaccination. When the model was applied to patients or controls alone, this correlation remained significant for patients but not for healthy controls. This indicated that the distribution observed in the overall study population was the result of skewing by the patient group, as well as by the difference between the two groups. Thus, physiologic fluctuations of B cells in healthy subjects do not affect the activity of vaccination, whereas pathologic perturbations in B cell populations after rituximab-based treatment are linked to the intensity of humoral response to influenza vaccine.
FIGURE 5.
Total B cells and CD27+ memory B cells correlate with corrected T1 titers for influenza vaccination in patients but not in healthy controls. A, Scatter plots representing the correlations of number and percentage of total and CD27+ memory B cells with mean corrected T1 titers in all subjects (left panels), controls (center panels), and patients (right panels). Box-and-whisker plots on each axis show median, IQR, and 95% confidence interval for the represented variable. Lines in the plots represent the linear regression for any given pair of variables. Thick continuous lines represent significant correlations. *Number of B cells/μl; †percentage of B cells in PBMCs; ‡number of CD27+ memory B cells/μl; §percentage of CD27+ memory B cells in PBMCs; ||percentage of CD27+ memory B cells in B cells. B, Scatter plots representing the correlations between the number and percentage of total and CD27+ B cells with corrected T1 titers for each influenza strain in patients. Correlations with the H3N2 strain are in the left panels, correlations with H1N1 are in the center panels, and correlations with B are in the right panels. Graph characteristics are the same as in A.
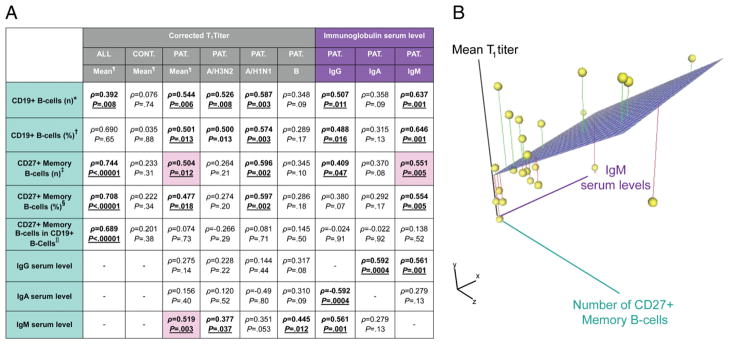
We next analyzed the possible relationship among the response to influenza vaccination, the baseline (T0) serum Igs, and peripheral B cells in NHL survivors treated with rituximab-based chemotherapy (Spearman’s ρ; Fig. 6). Flow cytometric data were also compared with other clinical parameters. Serum IgG and IgM levels significantly correlated with circulating B cells and CD27+ memory B cell data (Fig. 6A), with the stronger correlation being with IgM levels. Moreover, only IgM serum levels correlated with the response to influenza vaccination (corrected T1 titers). Therefore, IgM serum levels, CD27+ memory B cells (number and percentage), and mean corrected T1 titer seemed to be strictly correlated with each other (Fig. 6B).
FIGURE 6.
Serum IgM level and number of peripheral CD27+ memory B cells are highly correlated with mean corrected T1 titer in NHL patients treated with rituximab-based chemotherapy. A, Table showing Spearman’s coefficients (ρ) and relative p values (P) for correlations among FACS parameters, serum Ig levels, and mean corrected T1 titers for influenza vaccine in all subjects (ALL), control group (CTR), and patients (PAT) groups. The corrected T1 titers for each strain are also shown for the patient group. *Number of B cells/μl; †percentage of B cells in PBMCs; ‡number of CD27+ memory B cells/μl; §percentage of CD27+ memory B cells in PBMCs; ||percentage of CD27+ memory B cells in B cells; ¶mean corrected T1 titers for H3N2, H1N1, and B strains. B, Three-dimensional scatter plot of IgM serum level, mean corrected T1 titer, and number of peripheral CD27+ memory B cells. Intersections of the three vectors are represented in space as yellow spheres, connected to the three-dimensional regression plane (light purple surface) by lines representing least squares fitting.
Total B cells and CD27+ memory B cells were lower in those patients that had received more than one line of chemotherapy or fludarabine (Supplemental Table I). No correlation was detected between age, previous vaccination, number of rituximab doses, administration of radiation therapy and total or CD27+ memory B cell data.
Discussion
This study showed that disease-free NHL patients, treated with rituximab-containing immunochemotherapy, have a significant lack of humoral response to influenza vaccine. This was consistently detected for each of the three vaccine Ags and persisted long after treatment (50% of patients evaluated received the last chemotherapy >29 mo before the vaccination). To our knowledge, this is the first study assessing the humoral response to influenza vaccination in lymphoma patients in long-standing remission, outside the perichemotherapy period, thereby avoiding the confounding exerted by the immunosuppressive activity of the disease and/or the antiblastic-induced cytopenia.
There are few data on the activity of influenza vaccine in NHL patients treated with rituximab. Although it is generally accepted that patients with cancer undergoing treatment have a lower humoral response to influenza vaccination than do healthy controls (1), a recent large trial showed that all parameters investigated in NHL patients following vaccination fulfilled CHMP requirements (6). However, the cohort studied was more heterogeneous than ours, because some of their patients had not been treated, and only five patients had received rituximab (6). More recently, Ljungman et al. (28) described a very weak response to one or two doses of influenza vaccination in patients with various hematological malignancies who had recently discontinued treatment. These results were ascribed, in part, to the inclusion of patients treated with mAbs, because extremely insufficient responses were detected in the six patients who received rituximab. In another study, Takata et al. (29) evaluated seven NHL patients who were recently treated or were being treated with rituximab and CHOP chemotherapy; they reported virtually no Ab response against one of the three virus strains (i.e., A/H3N2). Although the small number of patients in the aforementioned studies prevents more general conclusions, additional evidence on the immunosuppressive effect of rituximab on influenza response is provided by the observation that, in patients affected by rheumatoid arthritis, it reduced the response to influenza vaccination in the peritreatment period (30–33). This is not surprising, because in the peritreatment period (e.g., first 6 mo) B cell depletion is massive, and CD19+ B cells are virtually absent in peripheral blood. The present results, obtained prospectively in a relevant and relatively homogeneous cohort of lymphoma patients, confirmed and extended previous preliminary observations from a limited number of patients and raise the concern that this immune impairment is not limited to the peritreatment period but, rather, persists long-term.
In the current study, seven of nine CHMP requirements for influenza vaccine evaluation were not fulfilled, and the European immunogenicity criteria were not met in the investigated patients (23). This was true even when adopting the CHMP criteria for the elderly, which are obviously less stringent than those for young people. Although most patients showed a postvaccination titer ≥ 40 for A strains, it is difficult to consider our cohort of patients sufficiently protected against influenza infection. Indeed, this protective threshold value has only been validated in young healthy adults (34); it may not represent a valid surrogate of clinical protection in frail patients (e.g., the elderly) (35) or in lymphoma survivors in whom the health status is often compromised by several other causes (36, 37). In this regard, the increased number of ILI cases observed during the epidemic season could be used as a clinical argument to support these considerations, although the unavailability of specimens for virological analysis prevented an etiological assessment. Notably, all four patients who developed ILI had a low serum level of IgM (+/− low serum level of IgG and/or low serum level of IgA).
A poor response to vaccination was observed against B strains in patients, as well as among the healthy controls. This finding is not surprising, because a lower response against type B influenza viruses compared with type A strains was reported in immuno-competent or immunocompromised individuals of different ages (38, 39).
After multivariate analyses, low serum IgM or IgA level, fludarabine administration, and multiple lines of treatment were associated with low serological response to influenza vaccination for at least one of the output variables considered (e.g., seroconversion rate, seroprotection rate, and corrected T1 titers). A history of fludarabine administration was the single factor with the greatest impact on the response to vaccination (in terms of number of significant correlations), and it was the sole factor confirmed by the multivariate modeling when the humoral response was evaluated globally (i.e., through the evaluation of the mean corrected T1 titer). The association among fludarabine, low serum Ig levels, and low B cell/CD27+ memory B cell values strongly suggested a possible detrimental effect of this drug on the reconstitution of the immune system. However, it is well known that fludarabine suppresses the T and B cell arm of the immune response (40, 41). The correlation between IgM (but not IgG or IgA serum levels) and the postvaccination titer (i.e., mean corrected T1 titer) and the association between low IgM serum levels and fludarabine administration are in agreement with the reported high incidence of infections associated with hypogammaglobulinemia (particularly of the IgM class) in NHL patients treated with rituximab-fludarabine regimens (14). Additionally, because cellular immune response is also correlated with vaccine protection (35), the evaluation of Ag-specific T cell responses represent another interesting subject that could be addressed in future investigations.
The coadministration of rituximab and high-dose chemotherapy associated with autologous stem cell transplantation results in a high incidence of IgG hypogammaglobulinemia with CD27+ B cell depletion (10). On the contrary, IgM hypogammaglobulinemia has not been reported frequently as one of the side effects of rituximab. However, it is noteworthy to highlight that IgM serum determinations were rarely (8, 42) included in large international rituximab trials (8, 14, 42–45).
Although the number of circulating B cells was similar in patients and healthy controls, a decrease in absolute numbers and relative percentages of CD27+ memory B cells was consistently observed in the patient group. CD27+ memory B cells (classical memory cells) represent the large majority of memory B cells (46, 47) and are considered responsible for the generation of the bulk of anti-influenza Abs (47). Although delayed recovery of CD27+ memory B cells was reported in patients followed for shorter periods (10, 11), the striking finding of the current study is that in NHL patients in CR treated with conventional rituximab-containing chemotherapy, the generalized depletion of CD27+ B cells was still detectable and profound >5 y after the last rituximab dose, and it correlated directly with low serum IgM levels and response to influenza vaccine.
The profound depletion in CD27+ B cells (<10% of CD19+ B cells in patients versus ~40% in healthy subjects) likely includes the reduction of circulating IgM+IgD+CD27+ B cells, which originate in the splenic marginal zone and in other marginal zone-equivalent areas of lymphoid tissues (48–50). IgM+IgD+CD27+ B cells, which represent ~20% of circulating B cells in healthy subjects, have been considered responsible for the secretion of low-affinity natural Abs (49, 50). Therefore, this provides a plausible explanation for the low serum IgM levels seen in our patients.
The impairment in CD27+ memory B cells may also be contributed to the low Ab HI titers observed. Because of the design of the present work, the issue cannot be addressed in further detail. Although the IgG fraction of anti-hemagglutinin Abs is the largest Ig component 1 mo after influenza vaccination, the intrinsic inability of the HI assays to differentiate the contribution of the different isotypes to Ab titers represents a limitation of the present observation.
In conclusion, disease-free NHL patients treated with rituximab-based chemotherapies, especially fludarabine, have defective responses to influenza vaccination years after treatment. Persistent perturbation of B cell compartments and Ig synthesis due to rituximab-containing regimens was identified as a mechanism contributing to this low humoral response. Although influenza vaccination remains the most relevant preventive measure in this population, it does not seem to elicit sufficient immune responses in many patients. Our results lay the foundation for larger studies to confirm whether these patients are at particular risk for infections. NHL survivors require careful postvaccination surveillance, and alternative prophylactic/therapeutic approaches represent an urgent subject.
Supplementary Material
Acknowledgments
This work was supported in part by the National Cancer Research Institute of Genoa.
We thank Dr. Gian Carlo Parodi, Rosangela Lai, Dr. Pietro Blandini, Dr. Mimmo Iannopollo, Prof. Laura Sticchi, Dr. Marisa Alberti, Dr. Guido Siffredi, and Dr. Nicoletta Provinciali for helping with donor and/or control recruitment; Dr. Paola Marroni and Dr. Michela Paganuzzi for performing blood cell counts and analyzing serological tests; and Dr. Sabrina Bacilieri for help with analyzing HI assays. D.B. and G.Z. thank Dr. Pietro Blandini for useful suggestions on multivariate analysis and good clinical practice principles, as always. D.B. thanks Dr. Claudia Berri for continuous support. We thank all study volunteers and all patients for continuous support. D.B. dedicates this article to Paola Angelica. This work is dedicated to our patients.
Abbreviations used in this article
- CHMP
Committee for Medicinal Products for Human Use
- CHOP
cyclophosphamide, doxorubicin, vincristine, and prednisone
- CR
complete remission
- GMT
geometric mean titer evaluated with hemagglutinin-inhibiting assay
- HI
hemagglutinin inhibition
- ILI
influenza-like illness
- IQR
inter-quartile range
- MFI
mean fold increase
- NHL
non-Hodgkin’s lymphoma
Footnotes
D.B. and A.D.M. conceived and designed the study and wrote the manuscript; D.B. coordinated the study; all authors analyzed and interpreted data; D.B. and G.Z. performed statistical analyses; D.B., G.Z., M.R.S., G.I., P.D., F.M.M., M.F., F.A., and A.D.M. critically reviewed the manuscript; C.M. and S.Z. analyzed FACS experiments; and D.B., G.Z., E.Z., A.B., M.R.S., E.B., O.R., M.M., G.C., F.B., and M.F. recruited patients.
The online version of this article contains supplemental material.
Disclosures
F.A. participated in speakers’ bureaus and advisory board meetings sponsored by Novartis Vaccines and Sanofi Pasteur and received research funding from Novartis Vaccines and Sanofi Pasteur. G.I. participated in speakers’ bureaus and advisory board meetings sponsored by Sanofi Pasteur and has received research funding from Crucell Berna, GlaxoSmith-Kline, and Sanofi Pasteur. P.D. received financial support for scientific research, speaker’s fees, and attendance at national and international meetings from influenza vaccine and antiviral manufacturers, not including Cruce/ISB or Roche. He has been and currently is investigator of several clinical trials on innovative vaccines produced by influenza vaccine manufacturers, not including Crucell/ISB or Roche.
References
- 1.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advisory Committee on Immunization Practices. [Accessed: August 26, 2010];Prevention and Control of Influenza Recommendation of the Advisory Committee on Immunization Practices (ACIP) 2008 Available at: http://www.ct.gov/dph/lib/dph/ACIP_recommendations_2008-2009.pdf.
- 3.World Health Organization. [Accessed: September 10, 2010];Recommendations for Influenza Vaccine Composition. Northern Hemisphere: 2008–2009. Available at: http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/
- 4.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257–2259. [PubMed] [Google Scholar]
- 5.Feery BJ, Sullivan JR, Hurley TH, Evered MG. Immunization with influenza vaccine in patients with haematological malignant disease. Med J Aust. 1977;1:292–294. doi: 10.5694/j.1326-5377.1977.tb130704.x. [DOI] [PubMed] [Google Scholar]
- 6.Centkowski P, Brydak L, Machala M, Kalinka-Warzocha E, Błasińska-Morawiec M, Federowicz I, Walewski J, Wegrzyn J, Wolowiec D, Lech-Marańda E, et al. Polish Lymphoma Research Group. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J Clin Immunol. 2007;27:339–346. doi: 10.1007/s10875-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 7.Rapezzi D, Sticchi L, Racchi O, Mangerini R, Ferraris AM, Gaetani GF. Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur J Haematol. 2003;70:225–230. doi: 10.1034/j.1600-0609.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 9.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M, Kumano K, Minauchi K, Yamaguchi K, Takeda Y, et al. Hypo-gammaglobulinemia with a selective delayed recovery in memory B cells and an impaired isotype expression after rituximab administration as an adjuvant to autologous stem cell transplantation for non-Hodgkin lymphoma. Eur J Haematol. 2006;77:226–232. doi: 10.1111/j.1600-0609.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 11.Sidner RA, Book BK, Agarwal A, Bearden CM, Vieira CA, Pescovitz MD. In vivo human B-cell subset recovery after in vivo depletion with rituximab, anti-human CD20 monoclonal antibody. Hum Antibodies. 2004;13:55–62. [PubMed] [Google Scholar]
- 12.Zoppoli G, Bruzzone B, Caligiuri P, Picciotto A, Balleari E, Bruzzone A, Icardi G, Ghio R. From a medical mistake to a clinical warning: the case of HBV mutant virus reactivation in haematological patients. Br J Haematol. 2009;144:969–970. doi: 10.1111/j.1365-2141.2008.07539.x. [Published erratum appears in 2009 Br. J. Haematol. 147: 411.] [DOI] [PubMed] [Google Scholar]
- 13.Bedognetti D, Zoppoli G, Sertoli MR, Zanardi E, Blandini P, Uccellini L, Boccardo F, Andreoli GB, Ghio R, Racchi O, et al. Relevance of HBV/HBcAb screening in lymphoma patients treated in the Rituximab era. Int J Hematol. 2010;91:342–344. doi: 10.1007/s12185-010-0522-z. author reply 345–346. [DOI] [PubMed] [Google Scholar]
- 14.Cabanillas F, Liboy I, Pavia O, Rivera E. High incidence of non-neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: a frequently unrecognized and easily treatable complication. Ann Oncol. 2006;17:1424–1427. doi: 10.1093/annonc/mdl141. [DOI] [PubMed] [Google Scholar]
- 15.Shortt J, Spencer A. Adjuvant rituximab causes prolonged hypo-gammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2006;38:433–436. doi: 10.1038/sj.bmt.1705463. [DOI] [PubMed] [Google Scholar]
- 16.Italian Ministry of Health. Circular September 15 2008. [Accessed: September 10, 2010];Prevention and control of influenza: recommendations for 2008–2009 season. Available at: http://www.salute.gov.it/influenza/newsInfluenza.jsp?id=471&menu=inevidenza&lingua=italiano.
- 17.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, et al. International Harmonization Project on Lymphoma. . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 19.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 20.de Totero D, Capaia M, Fabbi M, Croce M, Meazza R, Cutrona G, Zupo S, Loiacono F, Truini M, Ferrarini M, Ferrini S. Heterogeneous expression and function of IL-21R and susceptibility to IL-21-mediated apoptosis in follicular lymphoma cells. Exp Hematol. 2010;38:373–383. doi: 10.1016/j.exphem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Zambon M. Laboratory diagnosis of influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Blackwell Science; Oxford: 1998. pp. 291–313. [Google Scholar]
- 22.World Health Organization. [Accessed: September 10, 2010];WHO manual on animal influenza diagnosis and surveillance. 2002 Available at: http://www.wpro.who.int/internet/resources.ashx/CSR/Publications/manual+on+animal+ai+diagnosis+and+surveillance.pdf.
- 23.European Agency for the Evaluation of Medicinal Products-Committee for Proprietary Medicinal Products. [Accessed: September 10, 2010];Note for guidance on harmonization of requirements for influenza vaccines. Available at: http://www.ema.europa.eu/pdfs/human/bwp/021496en.pdf.
- 24.Beyer WE, Palache AM, Lüchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103:125–132. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, Icardi G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28:4123–4129. doi: 10.1016/j.vaccine.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. [Accessed: September 10, 2010];Influenza Like Illness case definition. Available at: http://www.acha.org/ILI_Project/ILI_case_definition_CDC.pdf.
- 27.Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, van der Water GL, Masurel N, Osterhaus AD. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine. 1996;14:1331–1339. doi: 10.1016/s0264-410x(96)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. Br J Haematol. 2005;130:96–98. doi: 10.1111/j.1365-2141.2005.05582.x. [DOI] [PubMed] [Google Scholar]
- 29.Takata T, Suzumiya J, Ishikawa T, Takamatsu Y, Ikematsu H, Tamura K. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab-CHOP. J Clin Exp Hematop. 2009;49:9–13. doi: 10.3960/jslrt.49.9. [DOI] [PubMed] [Google Scholar]
- 30.van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, Voskuyl AE, Blom M, Risselada AP, de Haan A, Westra J, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 31.Gelinck LB, Teng YK, Rimmelzwaan GF, van den Bemt BJ, Kroon FP, van Laar JM. Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis. 2007;66:1402–1403. doi: 10.1136/ard.2007.071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, Litinsky I, Comaneshter D, Levartovsky D, Mendelson E, Azar R, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67:937–941. doi: 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 33.Rehnberg M, Brisslert M, Amu S, Zendjanchi K, Håwi G, Bokarewa MI. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res Ther. 2010;12:R111. doi: 10.1186/ar3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 35.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 36.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 37.Stava CJ, Jimenez C, Vassilopoulou-Sellin R. Endocrine sequelae of cancer and cancer treatments. J Cancer Surviv. 2007;1:261–274. doi: 10.1007/s11764-007-0038-6. [DOI] [PubMed] [Google Scholar]
- 38.Evison J, Farese S, Seitz M, Uehlinger DE, Furrer H, Mühlemann K. Randomized, double-blind comparative trial of subunit and virosomal influenza vaccines for immunocompromised patients. Clin Infect Dis. 2009;48:1402–1412. doi: 10.1086/598193. [DOI] [PubMed] [Google Scholar]
- 39.Zuccotti GV, Scaramuzza A, Riboni S, Mameli C, Pariani E, Tanzi E, Zanetti A, Radaelli G. Long-lasting immunogenicity of a virosomal vaccine in older children and young adults with type I diabetes mellitus. Vaccine. 2009;27:5357–5362. doi: 10.1016/j.vaccine.2009.06.082. [DOI] [PubMed] [Google Scholar]
- 40.Goodman ER, Fiedor PS, Fein S, Athan E, Hardy MA. Fludarabine phosphate: A DNA synthesis inhibitor with potent immunosuppressive activity and minimal clinical toxicity. Am Surg. 1996;62:435–442. [PubMed] [Google Scholar]
- 41.Takada K, Danning CL, Kuroiwa T, Schlimgen R, Tassiulas IO, Davis JC, Jr, Yarboro CH, Fleisher TA, Boumpas DT, Illei GG. Lymphocyte depletion with fludarabine in patients with psoriatic arthritis: clinical and immunological effects. Ann Rheum Dis. 2003;62:1112–1115. doi: 10.1136/ard.62.11.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 43.van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, Kimby E, van t Veer M, Vranovsky A, Holte H, Hagenbeek A. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 45.Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, et al. MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 46.Ehrhardt GR, Hijikata A, Kitamura H, Ohara O, Wang JY, Cooper MD. Discriminating gene expression profiles of memory B cell subpopulations. J Exp Med. 2008;205:1807–1817. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 49.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 50.Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, Plebani A, Aiuti F, Quinti I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.