Abstract
Objective. To assess pharmacy faculty trainers’ perceptions of a Web-based train-the-trainer program for PharmGenEd, a shared pharmacogenomics curriculum for health professional students and licensed clinicians.
Methods. Pharmacy faculty trainers (n=58, representing 39 colleges and schools of pharmacy in the United States and 1 school from Canada) participated in a train-the-trainer program consisting of up to 9 pharmacogenomics topics. Posttraining survey instruments assessed faculty trainers’ perceptions toward the training program and the likelihood of their adopting the educational materials as part of their institution’s curriculum.
Results. Fifty-five percent of faculty trainers reported no prior formal training in pharmacogenomics. There was a significant increase (p<0.001) in self-reported ability to teach pharmacogenomics to pharmacy students after participants viewed the webinar and obtained educational materials. Nearly two-thirds (64%) indicated at least a “good” likelihood of adopting PharmGenEd materials at their institution during the upcoming academic year. More than two-thirds of respondents indicated interest in using PharmGenEd materials to train licensed health professionals, and 95% indicated that they would recommend the program to other pharmacy faculty members.
Conclusion. As a result of participating in the train-the-trainer program in pharmacogenomics, faculty member participants gained confidence in teaching pharmacogenomics to their students, and the majority of participants indicated a high likelihood of adopting the program at their institution. A Web-based train-the-trainer model appears to be a feasible strategy for training pharmacy faculty in pharmacogenomics.
Keywords: pharmacogenomics, curriculum, pharmacy colleges and schools, faculty development, train-the-trainer
INTRODUCTION
Pharmacogenomics is defined as “the genome-wide analysis of genetic determinants of drug efficacy and toxicity.”1 Over the last decade, there has been an explosion of scientific data and information surrounding pharmacogenomics. Dissemination of pharmacogenomic information to students is an important component of contemporary curricula for colleges and schools of pharmacy and medicine throughout the United States.2-4 In 2002, the American Association of Colleges of Pharmacy (AACP) Academic Affairs Committee drafted core competencies in pharmacogenetics and pharmacogenomics that included: (1) genetic basis of disease, (2) drug discovery and disposition/drug targets, and (3) ethical applications and social and economic implications.5 Further, as a basis of curricular evaluation, the Accreditation Council for Pharmacy Education Accreditation Standards and Guidelines recommend that a foundation in pharmacogenomics/genetics include topics related to the genetic basis for disease, drug action, drug metabolism, and individualizing drug doses.6 However, the breadth and depth of pharmacogenomics instruction and the expertise at most colleges and schools of pharmacy are inadequate to align with current accreditation standards and guidelines.3 In 2009, 55% of pharmacy colleges and schools reported no current plans for faculty development programs dedicated to pharmacogenomics instruction. However, there was strong interest (88% of 66 colleges and schools) in obtaining access to a shared curriculum on pharmacogenomics.4
Pharmacogenomics is a rapidly evolving field for which shared educational materials on the topic are not readily available. Further, there appears to be a gap between healthcare providers’ knowledge and the increasing amount of evidence-based information relevant to pharmacogenomics.7 In 2009, the University of California, San Diego (UCSD) Skaggs School of Pharmacy and Pharmaceutical Sciences, with funding from the Centers for Disease Control and Prevention, began developing evidence-based educational materials through an educational program entitled Pharmacogenomics Education Program (PharmGenEd): Bridging the Gap between Science and Practice.8 The program was designed for licensed clinicians and health professional students to increase awareness and knowledge of the validity, utility, and potential benefits and harms of pharmacogenomic testing. A driving force behind this program was to create a shared curriculum that could be integrated with ease into the curriculum of health degree programs. The curriculum addresses therapeutic areas in which pharmacogenomic testing can be applied. Each educational module includes evidence-based recommendations for pharmacogenomic testing and patient case scenarios that help students develop skills to synthesize clinical data and formulate recommendations for pharmacogenomic testing.
In accordance with effective dissemination strategies applied in other content areas,9-13 a train-the-trainer approach was used to equip faculty with the necessary teaching materials to integrate pharmacogenomics content into their institutions’ classroom course(s). To maximize use of existing technologies, training sessions were conducted by means of online webinars. The purpose of this report is to assess pharmacy faculty trainers’ perceptions of the Web-based train-the-trainer program for PharmGenEd.
METHODS
Participating pharmacy faculty trainers were recruited at professional meetings, from professional organizations’ listservs, and through personal contacts, Web sites, and e-mail announcements to all faculty members and deans registered on the AACP listserv. At the time of recruitment in the spring of 2010, our target population for dissemination was all 120 colleges and schools of pharmacy in the United States. Faculty members were invited to participate if they were teaching or were planning to teach a pharmacogenomics course at their respective institutions in the upcoming academic year. Participants signed a Faculty Trainer Agreement form, which outlined the scope of the program, a list of shared curriculum topics, available training sessions, and administration of 4 survey instruments: faculty posttraining, student pretest, student posttest, and program implementation. The results of only the faculty posttraining survey instrument are presented here.
The faculty posttraining survey instrument was adapted from an instrument previously used to evaluate a tobacco-cessation train-the-trainer program (Rx for Change) for pharmacy faculty.11 Prior to use, the survey instrument was beta-tested by 5 individuals and revised accordingly. Faculty trainers agreed to incorporate at least 1 module into their courses and complete and return all survey instruments. Electronic access to PharmGenEd (http://pharmacogenomics.ucsd.edu) educational materials was granted to faculty participants who signed the agreement and completed training requirements. Faculty members did not receive monetary compensation for participating in the program. All evaluation procedures and survey instruments were approved by the UCSD Human Research Protections Program.
The PharmGenEd team identified and invited expert authors to develop educational materials for targeted content areas, including PowerPoint (Microsoft, Redmond, WA) presentations, detailed speaker notes, 10 self-assessment multiple-choice items, and a list of recommended readings. The PharmGenEd staff developed a coordinator’s guide to highlight implementation logistics, including: (1) the PharmGenEd program, (2) how to download instructional materials, (3) how to implement the pharmacogenomics curriculum, and (4) program evaluation materials that are requested by PharmGenEd staff. The shared curriculum modules were designed to be implemented during the latter years of the pharmacy curriculum; faculty members could use each module in its entirety or partially to integrate with their existing curricula. The modules, each of which was designed to be presented within 60 minutes, were developed using an iterative process among authors, peer reviewers, and the PharmGenEd staff. The content expert authors developed the modules using the PharmGenEd PowerPoint template. They also received guidelines for how the module should be structured. With the exception of the module on economic issues, each module was organized into the following sections: (1) objectives; (2) outline; (3) case study; (4) overview of drug/therapeutic class; (5) gene/allele of interest; (6) population prevalence; (7) clinical relevance in terms of efficacy, toxicity, and dosing; (8) available pharmacogenomic test; (9) testing recommendation from prescribing information or guidelines (if any); (10) summary; (11) case summary; and (12) references. At least 3 reviewers per module provided comments and suggestions using a reviewer form that evaluated scientific merit, clinical application, and editorial and/or stylistic issues.
Between August and October 2010, content expert authors and the PharmGenEd staff conducted 8 live webinars (1 for each module) and 1 prerecorded webinar for the Concepts and Clinical Applications module. The PharmGenEd staff posted all webinar links on the PharmGenEd Web site, which was accessible to participants throughout the academic year. During the first hour of each module, the content expert authors taught the course content. During the second hour, the content expert conducted a question-and-answer session, encouraging audience participants to ask questions verbally or by using the chat function. Each webinar was recorded and made accessible to faculty trainers for future viewing through the PharmGenEd Web site. Potential faculty trainers could view these webinar recordings before determining whether to use the PharmGenEd modules and integrate them into their curricula.
After all of the live webinar training sessions had been conducted, a 29-item posttraining survey instrument was mailed to faculty trainers who had participated in at least 1 webinar. Each survey instrument was assigned a unique code for each institution and mailed through Fed-Ex. Prepaid return labels were included with each mailing for faculty trainers to use in returning their survey instruments, and outgoing and incoming mailings were recorded and tracked. The survey instrument, which was designed to parallel prior survey instruments assessing the impact of train-the-trainer programs for pharmacy faculty, assessed key factors hypothesized to be associated with adoption of the PharmGenEd program.9,11
The posttraining survey instrument included sociodemographic data such as sex, age, race, ethnicity, academic rank, degrees, number of years in current position, and specialty or discipline. Faculty trainers reported the topic (specific modules) and format (live and/or recorded) of the train-the-trainer webinar session(s) they attended. They also reported whether they had (1) received any formal training in pharmacogenomics or teaching students about pharmacogenomics; (2) previous experience in teaching pharmacogenomics – including class lectures, laboratories or workshops, continuing education programs, or classes to patients; and (3) worked as a clinician in a setting that uses pharmacogenomic testing.
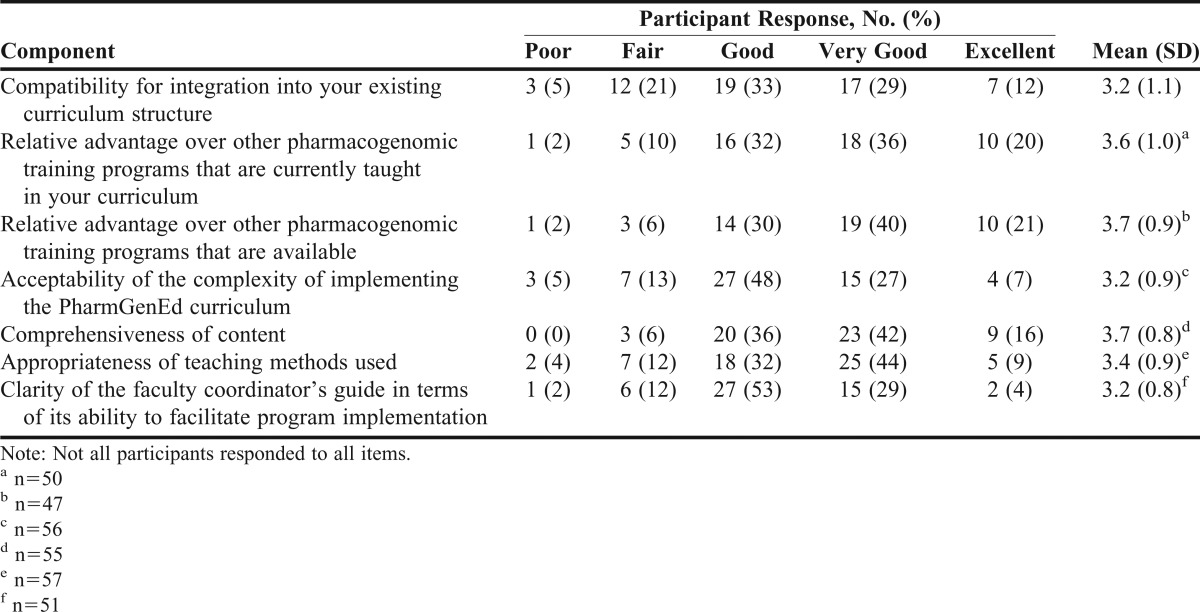
Faculty trainers rated their overall ability to teach pharmacogenomics to their students before and after participating in the PharmGenEd training. They estimated the percentage of program content that (1) was completely new, (2) was previously learned but needed review, and (3) was an unnecessary review (the sum of the percentages totaled 100%). For the PowerPoint lectures slides, instructor notes, coordinator’s guide, and PharmGenEd Web site, faculty trainers rated the overall quality (1=poor, 2=fair, 3=good, 4=very good, 5=excellent), overall usefulness (1=not at all useful, 2=a little useful, 3=moderately useful, 4=very useful, 5=extremely useful), and overall likelihood of use at their institution (1=not at all likely, 2=a little likely, 3=moderately likely, 4=very likely, 5=extremely likely). In a manner consistent with prior train-the-trainer studies9,11 that applied Rogers’ Diffusion of Innovations as a theoretical framework,9,11,14 we evaluated faculty trainers’ perceptions toward adoptability of the PharmGenEd program. Using a 5-point response scale (1=poor, 2=fair, 3=good, 4=very good, and 5=excellent), faculty trainers rated the PharmGenEd program with respect to: (1) compatibility for integration into existing curriculum structure; (2) relative advantage of other pharmacogenomics training programs that were available; (3) relative advantage over other pharmacogenomic training programs that were taught in the curriculum; (4) acceptability of the complexity of implementing PharmGenEd curriculum; (5) appropriateness of teaching methods used; and (6) clarity of the coordinator’s guide in its ability to facilitate program implementation. Additionally, we assessed confidence in skills for teaching PharmGenEd materials and likelihood of adoption of PharmGenEd materials during the upcoming academic year (2010–2011).
Faculty trainers’ perceptions were assessed regarding how important it is for each of the therapeutic modules to be covered in their college’s or school’s required pharmacogenomics coursework (1=not at all important, 2=a little important, 3=moderately important, 4=very important, 5=extremely important). Faculty trainers were asked to indicate whether they personally had the ability to determine whether PharmGenEd materials would be integrated into their school’s curricula (yes, no, not sure) and to estimate the total number of minutes of pharmacogenomics content they anticipated teaching at their institution during the upcoming academic year. For colleges and schools that had more than 1 survey respondent, the estimated total number of minutes was computed as an average of responses.
Finally, faculty trainers indicated whether they were interested in using the PharmGenEd materials to train licensed health professionals and whether they would recommend the PharmGenEd train-the-trainer program to other pharmacy faculty members or faculty members from other health professions schools (eg, medical, nursing, dental).
Summary statistics were computed to characterize survey responses. Group comparisons were made using chi-squared tests of independence or comparisons of group means, as appropriate. In all cases, comparisons of means were made using both parametric and nonparametric tests, and the conclusions were the same. As such, parametric test results were chosen for presentation to aid interpretability. Analyses were conducted using SPSS, version 19 (IBM, Armonk, NY).
RESULTS
Of 81 faculty trainers who expressed interest in using the PharmGenEd educational materials and signed a faculty agreement, 61 (75%) returned posttraining survey instruments (including 58 complete survey instruments, 2 blank forms, and 1 incomplete survey instrument). Of 20 faculty trainers who did not return the survey instrument, 14 could not be reached by telephone or e-mail, 4 were unable to implement in the upcoming year because of curricular issues, 1 had left the university, and 1 could not continue for health reasons. The final sample of respondents (n=58) represented 39 colleges and schools of pharmacy in the United States (33% of 120 schools; 22 private and 17 public) and 1 school in Canada.
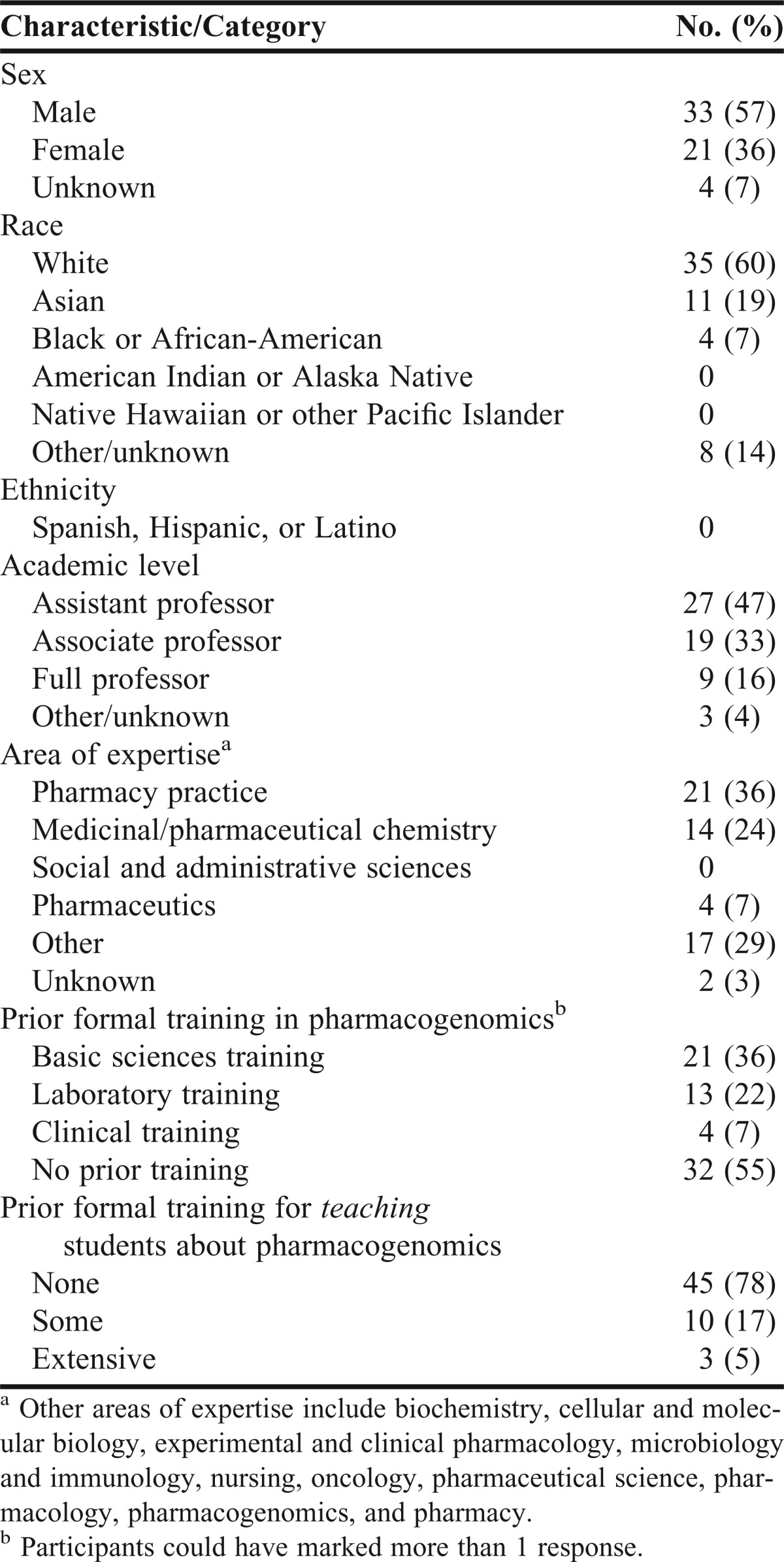
Most respondents were male, white, at the assistant professor rank, an average age of 46 ± 10.1 years, and had worked a median of 4.0 ± 8.2 years in their current position (Table 1). Approximately 36% of the respondents self-identified pharmacy practice as their discipline. Prior to participating in the PharmGenEd program, 36% of respondents had received formal pharmacogenomics training in the basic sciences, 22% had laboratory training, and 7% had clinical training. Over half (55%) reported no prior formal pharmacogenomics training. Twenty-two percent had some or extensive prior training for teaching this topic. In the past, 69% had taught classroom lectures on pharmacogenomics, 17% had taught laboratories or workshops, 19% had taught continuing education programs, and 5% had taught classes about pharmacogenomics to patients. Four participants (7%) had worked as a clinician in a setting that used pharmacogenomic testing.
Table 1.
Characteristics of Faculty Trainers Who Participated in a Train-the-Trainer Webinar in a Pharmacogenomics Shared Curriculum (n=58)
Each faculty trainer participated in a median of 2.5 of 9 available modules, either as live or recorded sessions (range = 0-9; interquartile range [IQR] = 1-6). The Concepts and Clinical Applications module, which was offered as a prerecorded webinar only, was viewed by 53% of faculty trainers. Of the webinars that were offered as both live and prerecorded, the most commonly attended and/or viewed modules were Oncology II (45%), Oncology I (43%), Cardiology I (40%) and Psychiatry II (36%). For logistical reasons, only 4 of 9 modules (Oncology II, Concepts and Clinical Applications, Psychiatry II, and Oncology I) were available prior to September, when most colleges and schools begin their semesters/quarters.
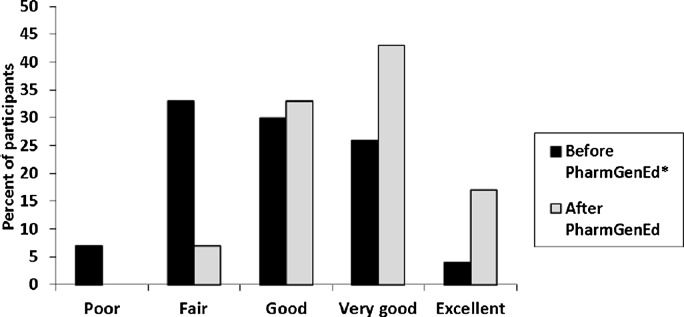
On average, faculty trainers reported that 36% of the content was completely new, 36% of the content was a necessary review, and 28% was an unnecessary review. After the training, the proportion of faculty trainers rating their confidence for teaching pharmacogenomics as “very good” or “excellent” was 43% and 17%, respectively. We observed a significant increase (2.9 ± 1.0 vs 3.7 ± 0.8; p<0.001) in self-reported ability to teach pharmacogenomics to pharmacy students (Figure 1). For faculty trainers without prior training in teaching pharmacogenomics (n=44), the mean self-reported abilities to teach was 2.6 ± 0.9 before and 3.6 ± 0.8 after PharmGenEd training (p<0.001). For faculty trainers with some or extensive prior training (n=13), the mean self-reported abilities to teach pharmacogenomics to pharmacy students was 3.6 ± 0.9 before and 4.2 ± 0.8 after the PharmGenEd training (p<0.001). Faculty trainers rated their posttraining confidence for teaching the pharmacogenomic materials as poor, 0%; fair, 11%; good, 23%; very good, 46%; and excellent, 20%.
Figure 1.
Faculty Trainers’ Self-Ratings of Overall Ability to Teach Pharmacogenomics to Pharmacy Students (n=58; p<0.001). *Assessed at posttraining: “Before your PharmGenEd webinar(s) participation, how would you have rated your overall ability to teach pharmacogenomics to your students?”
Mean ratings for perceived quality and usefulness of various PharmGenEd educational materials were at least 3.6 for perceived quality and at least 3.4 for perceived usefulness (Table 2). Table 3 presents faculty trainers’ perceived attributes of the PharmGenEd program. Of the 9 teaching modules, the Concepts and Applications module was perceived to be the most important topic to cover in required coursework for pharmacogenomics (mean, 4.4), followed by Cardiology I (mean, 4.2), Oncology II (mean, 4.1), and Cardiology II (mean, 4.1).
Table 2.
Perceived Quality, Usefulness, and Likelihood of Using the Various PharmGenEd Program Components (N=58)
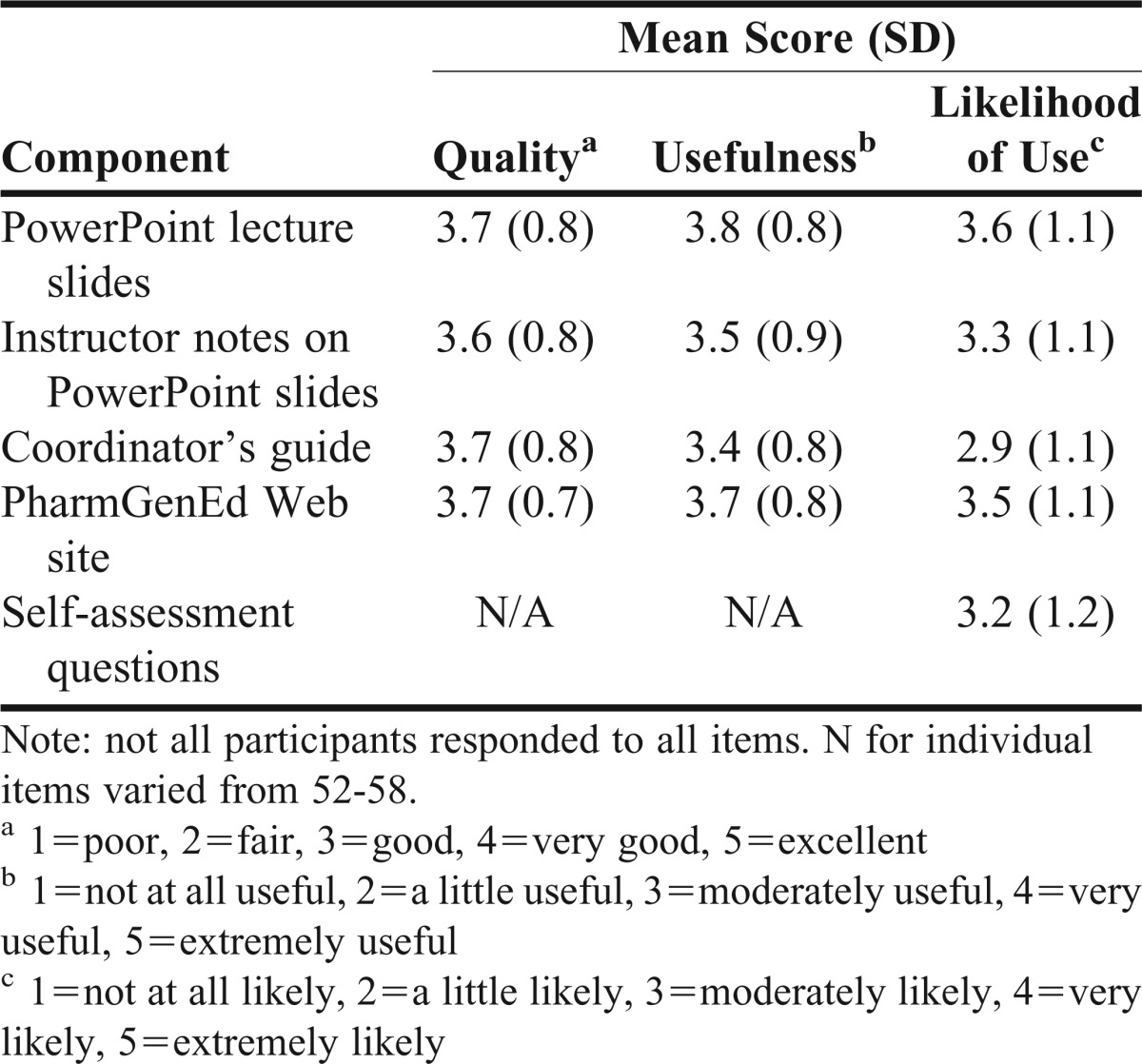
Table 3.
Perceptions of the PharmGenEd Program (N=58)
More than half (58%) of respondents indicated that they personally had the ability to determine whether the PharmGenEd materials would be integrated into their institutions’ curricula; 14% were unsure and 28% did not have the ability. Nearly two-thirds (64%) indicated at least a “good” likelihood of adopting the PharmGenEd materials at their school during the upcoming academic year. Of 37 schools for which 1 or more respondents estimated a nonzero total number of minutes of pharmacogenomics content they anticipated would be taught at their college or school in the upcoming academic year, the median number of minutes was 255 (range = 40-3300; IQR = 120-628). Three schools estimated zero minutes.
Over two-thirds (68%) of respondents indicated interest in using the PharmGenEd educational materials to train licensed health professionals. Ninety-five percent of faculty trainers indicated that they would recommend the program to other pharmacy faculty members who might be interested in teaching pharmacogenomics to their students, and 75% would recommend the program to faculty members from other health professional schools.
DISCUSSION
In this initial launch of the pharmacogenomics education program, PharmGenEd, we observed a significant increase in instructors’ self-rated confidence to teach pharmacogenomics to pharmacy students following participation in the train-the-trainer program. Nearly all participants (93%) rated their overall confidence to teach pharmacogenomics as good, very good, or excellent following completion of the training. This finding is notable because 55% of participants had not received prior formal training in pharmacogenomics, and 78% indicated they never received formal training for teaching pharmacogenomics. In concordance with prior research,4 our results confirmed a high level of interest for obtaining access to a pharmacogenomics shared curriculum.
Findings from this study demonstrate that participation in the training significantly increased perceived confidence for teaching pharmacogenomics. In a manner similar to prior train-the-trainer studies,9,11 pre- and post-training confidence for teaching were both assessed posttraining. This measurement approach is a more sensitive method of assessing the impact of a training program13,15-17 because it controls for response-shift bias (ie, the change in the study participants’ knowledge or understanding of the material as a result of the educational intervention).18 For example, in this study, participants may have provided elevated pretraining ratings of their abilities because they did not yet fully appreciate their lack of knowledge in a given content area until after participation in an educational program.
As with prior shared curricula for pharmacy educators,9,11 the PharmGenEd shared curriculum, its national dissemination plan, and the evaluation of the dissemination process were developed and grounded in Rogers’ Diffusion of Innovations Theory. This theory characterizes the process by which an innovation is communicated through various channels to networks of people over time, in various stages of obtaining knowledge, persuasion, decision, implementation, and confirmation.14 The goals of the PharmGenEd shared curriculum are to provide educational materials to bridge the knowledge gap and provide a repository of materials for curricular implementation. As such, the curriculum was specifically designed to possess characteristics that would enhance its likelihood of adoption by colleges and schools of pharmacy. Through national meeting presentations, publications, and listserv announcements from AACP, the observability of the PharmGenEd program was enhanced on a national level prior to and during the dissemination phase. Faculty trainers consistently rated the quality, usefulness, and likelihood of using the various PharmGenEd educational materials as good to excellent.
Approximately three-fourths of faculty members indicated that compatibility for integrating the curriculum into their existing curriculum was good, very good, or excellent. Compared with a tobacco cessation train-the-trainer program that also attempted to recruit all pharmacy colleges and schools in the United States (98% participation; 83 of 85 schools), our participation rate was lower. Attendance might have been impacted by the production timing of these modules, as they became available in August and September, coinciding with the beginning of the academic term for several colleges and schools of pharmacy. Lower attendance also was possible because (1) tobacco cessation is the leading cause of disease and death, and it influences virtually all organ systems in the body; (2) for nearly all pharmacy colleges and schools, some level of tobacco content was already present in the curriculum;19 and (3) trainings were conducted as live programs and included all-expense paid travel to San Francisco for up to 2 faculty members from each college and school. A follow-up assessment, currently underway, will estimate the extent to which implementation actually occurs.
The PharmGenEd shared curriculum is available (free of charge, online registration required) for colleges and schools of pharmacy and faculty members who desire evidence-based materials in clinical applications of pharmacogenomics for instructional purposes in their classrooms. The primary advantage of shared curricula is that having access to turnkey, evidence-based educational materials and training provided at no cost to faculty members reduces the amount of time and effort that individual instructors would otherwise put forth in order to develop similar content. Because pharmacogenomics is a recent addition to the accreditation requirements for doctor of pharmacy degree programs, a shared curriculum will foster the development of faculty expertise for teaching pharmacogenomics and will also facilitate the rate of uptake of the topic.20 The program’s flexibility is conducive to adoption in that either partial or full PowerPoint slide sets can be incorporated into each lecture, and either 1 or all lectures can be incorporated within 1 course or spread across different courses. In addition, all curricular materials are posted electronically, thus allowing easy access to the materials for faculty trainers and the ability to update materials as often as necessary to reflect new knowledge in the rapidly evolving field of pharmacogenomics.
Limitations of the study include the self-reported nature of data without validation, such as actual ability (vs perceived ability) to teach pharmacogenomics. Our results are inherently susceptible to social desirability bias because our measurements relied on self-reported ratings. The study is also limited by the small number of faculty trainers, representing only 39 colleges and schools of pharmacy, which raises questions about the generalizability to all colleges and schools in the United States. In addition, there was variability in the attendance at webinars and views of the educational modules, which also may influence the findings. While our data suggest the train-the-trainer model appears to be a viable approach for effective broad-scale dissemination of a pharmacogenomics curriculum, future research with long-term follow-up data will be needed to assess program adoption and sustainability.
CONCLUSION
PharmGenEd is the first shared curriculum in pharmacogenomics that was specifically designed with clinical applications using case studies. Faculty responses from our first-year dissemination efforts were largely positive, with increased confidence in participants’ perceived ability to teach pharmacogenomics content and high likelihood of adopting the shared curriculum into their respective institutions’ curricula. A Web-based approach to building a shared curriculum appears to be effective in disseminating educational materials to pharmacy faculty.
ACKNOWLEDGMENTS
This study was funded by the Centers for Disease Control and Prevention (CDC grant No. 1U38GD000070 to Kuo). Contents presented in this article are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. We appreciate the CDC staff (Michele Reyes, PhD, Daurice Grossniklaus, PhD, Jeanette St. Pierre, MPH, MA, and H. Mack Anders, MPA) who provided suggestions, comments, and critiques about the PharmGenEd program development and evaluation.
The authors acknowledge research assistants Ashley To, BA; Sandra MacGowan; Tiffany Sie; Eunice Hong; and Omar Felix for their contributions with survey mailings, data entry, and data management tasks. Dr. Lucinda Maine, Dr. Robert Kerr, and staff from AACP were instrumental to the success of the programs. Study measures and elements of the PharmGenEd train-the-trainer Web site were contributed by the authors of Rx for Change, a shared tobacco-cessation curriculum for which dissemination and evaluation were funded through a grant from the National Cancer Institute.
Finally, we thank PharmGenEd authors, peer-reviewers, and faculty members for their enthusiasm, thoughtful and constructive feedback, and assistance in promoting pharmacogenomics education for pharmacy students nationwide.
REFERENCES
- 1.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 2.Green JS, O'Brien TJ, Chiappinelli VA, Harralson AF. Pharmacogenomics instruction in US and Canadian medical schools: implications for personalized medicine. Pharmacogenomics. 2010;11(9):1331–1340. doi: 10.2217/pgs.10.122. [DOI] [PubMed] [Google Scholar]
- 3.Latif DA, McKay AB. Pharmacogenetics and pharmacogenomics instruction in colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2005;69(2):Article 23. [Google Scholar]
- 4.Murphy JE, Green JS, Adams LA, Squire RB, Kuo GM, McKay A. Pharmacogenomics in the curricula of colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2010;74(1):Article 7. doi: 10.5688/aj740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JA, Bootman JL, Evans WE, et al. Pharmacogenomics: a scientific revolution in pharmaceutical sciences and pharmacy practice: report of the 2001-2002 academic affairs committee. Am J Pharm Educ. 2002;66(Winter Suppl):12S-16S. [Google Scholar]
- 6.American Council on Pharmaceutical Education. Chicago, Ill: ACPE; 2011; Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree. Available at: http://www.acpe-accredit.org/standards/default.asp. Accessed November 29, 2012. [Google Scholar]
- 7.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am J Pharm Educ. 2011;75(3):Article 51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo GM, Ma JD, Lee KC, et al. Institutional Profile: University of California San Diego Pharmacogenomics Education Program (PharmGenEd TM): bridging the gap between science and practice. Pharmacogenomics. 2011;12(2):149–153. doi: 10.2217/pgs.10.213. [DOI] [PubMed] [Google Scholar]
- 9.Assemi M, Mutha S, Hudmon KS. Evaluation of a train-the-trainer program for cultural competence. Am J Pharm Educ. 2007;71(6):Article 110. doi: 10.5688/aj7106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brimmer DJ, McCleary KK, Lupton TA, Faryna KM, Hynes K, Reeves WC. A train-the-trainer education and promotion program: chronic fatigue syndrome–a diagnostic and management challenge. BMC Med Educ. 2008;8:49. doi: 10.1186/1472-6920-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corelli RL, Fenlon CM, Kroon LA, Prokhorov AV, Hudmon KS. Evaluation of a train-the-trainer program for tobacco cessation. Am J Pharm Educ. 2007;71(6):Article 109. doi: 10.5688/aj7106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albright CL, Farquhar JW, Fortmann SP, et al. Impact of a clinical preventive medicine curriculum for primary care faculty: results of a dissemination model. Prev Med. 1992;21(4):419–435. doi: 10.1016/0091-7435(92)90051-i. [DOI] [PubMed] [Google Scholar]
- 13.Stratos GA, Katz S, Bergen MR, Hallenbeck J. Faculty development in end-of-life care: evaluation of a national train-the-trainer program. Acad Med. 2006;81(11):1000–1007. doi: 10.1097/01.ACM.0000242475.41549.66. [DOI] [PubMed] [Google Scholar]
- 14.Rogers EM. Diffusion of Innovations. 5th ed. New York: The Free Press; 2003. [Google Scholar]
- 15.Bray JH, Howard GS. Methodological considerations in the evaluation of a teacher-training program. J Educ Psychol. 1980;72(1):62–70. [Google Scholar]
- 16.Green ML. A train-the-trainer model for integrating evidence-based medicine training into podiatric medical education. J Am Podiatr Med Assoc. 2005;95(5):497–504. doi: 10.7547/0950497. [DOI] [PubMed] [Google Scholar]
- 17.Skeff KM, Stratos GA, Bergen MR. Evaluation of a medical faculty development program. A comparison of traditional pre/post and retrospective pre/post self-assessment ratings. Eval Health Professions. 1992;15(3):350–366. [Google Scholar]
- 18.Howard GS, Daily PR. Response-shift bias: a source of contamination of self-report measures. J Appl Psychol. 1979;64(2):144–150. [Google Scholar]
- 19.Hudmon KS, Bardel K, Kroon LA, Fenlon CM, Corelli RL. Tobacco education in U.S. schools of pharmacy. Nicotine Tob Res. 2005;7(2):225–232. doi: 10.1080/14622200500055392. [DOI] [PubMed] [Google Scholar]
- 20.Maine LL. Sharing our wealth. Am J Pharm Educ. 2007;71(6):Article 114. doi: 10.5688/aj7106114. [DOI] [PMC free article] [PubMed] [Google Scholar]