Abstract
Objectives. To determine pharmacy students’ attitude toward and knowledge of reporting serious adverse drug events (ADEs) to the Food and Drug Administration (FDA).
Method. A 58-item survey questionnaire constructed to measure respondents’ intention to report ADEs (3 items), attitude toward reporting ADEs (20 items), knowledge of ADE reporting (9 items), and demographic data was administered to all third-year (final-year) pharmacy students at the Appalachian College of Pharmacy.
Results. The majority of the 58 students who responded (91% response rate) intended (84%) and planned (85.3%) to report serious ADEs when they encounter them. Most respondents had favorable attitudes toward reporting serious ADEs to the FDA; respondents believed that reporting serious ADEs was valuable (5.6 ± 1.5, mean ± SD), good (3.0 ± 1.7), and beneficial (5.7 ± 1.5). Many students also believed that ADE reporting resulted in increased risk of malpractice, compromised relationships with physicians, broken trust with patients, disruption of the normal workflow, and was time consuming. Many students had inadequate knowledge on reporting ADEs.
Conclusion. Although pharmacy students had strong intentions and favorable attitudes toward ADE reporting, they had inadequate knowledge of how to report serious ADEs.
Keywords: adverse drug events, adverse drug event reporting, pharmacovigilance, theory of planned behavior, drug safety
INTRODUCTION
In 2010, over 770,000 ADEs occurred in the United States, costing up to $5.6 billion annually.1 An ADE is injury or harm resulting from the use of a drug including from adverse drug reactions and overdoses. ADEs cause many hospital admissions and deaths and result in a considerable use of hospital resources.2 ADEs are associated with unnecessary patient and societal costs.3 Most ADEs are preventable through careful monitoring and reporting. Healthcare professionals in the United States are encouraged to report ADEs that they encounter to the FDA through MedWatch. ADE reporting by healthcare providers is a professional responsibility. The voluntary reporting of ADEs helps to improve future medication use and safety.4 By reporting ADEs, healthcare professionals share the clinical experience of medication therapy and build a better safety profile for the medication(s) involved.
Despite the need for and importance of ADE reporting and the existence of an elaborate system to collect ADE reports, few ADEs are reported (<1%).1 Underreporting of ADEs, including serious or severe ADEs to spontaneous reporting systems by healthcare professionals is common.5-7 Failure to report ADEs is influenced by many factors, such as lack of time, indifference, malpractice litigation concerns, lack of motivation, lack of economic incentive, complacency, lack of knowledge on ADE reporting requirements, and negative attitudes.5,6,8,9 In addition, reluctance to send reports based on mere suspicion of ADEs, lack of confidence in recognizing adverse drug reactions, misconceptions about ADE reporting, and difficulty in accessing means of reporting (eg, forms)10-16 also affect the reporting of ADEs. ADE reporting is also hampered by healthcare professionals’ attitudes toward reporting17 and possibly by personal characteristics.
Pharmacy educators should adequately prepare students regarding patient and drug safety.18 Pharmacy students are expected to have adequate knowledge concerning the process, procedure, and importance of ADE reporting. US pharmacy students have misconceptions relating to the way adverse event reports are handled and the influence of Health Insurance Portability and Accountability Act regulations on reporting.19 However, no known study has investigated the intent and attitudes of pharmacy students toward ADE reporting in the United States, as well as the demographic factors that are related to ADE reporting.
A pilot study was conducted to determine pharmacy students’ attitude toward and knowledge of reporting serious ADEs to the Food and Drug Administration (FDA). The specific objectives of the study were to determine: (1) pharmacy students’ intention to report serious ADEs to the FDA; (2) pharmacy students’ attitude toward reporting serious ADEs to the FDA; (3) pharmacy students’ knowledge of reporting serious ADEs; and (4) the relationship between demographic factors (eg, age and gender) and attitude toward reporting serious ADEs. The study hypotheses were: (1) pharmacy students have strong intentions to report serious ADEs to the FDA; (2) pharmacy students have positive attitudes toward reporting serious ADEs to the FDA; (3) pharmacy students have adequate knowledge of reporting serious ADEs to the FDA; (4) there is no relationship between pharmacy students’ attitudes toward reporting serious ADEs and age; and (5) there is no relationship between pharmacy students’ attitudes toward reporting serious ADEs and gender.
The study’s theoretical framework was based on the theory of planned behavior, which postulates that behavior is determined by behavioral intention and perceived behavioral control.20 Intention is in turn shaped by attitudes toward the behavior, social norms, and perceived control over the behavior. The theory of planned behavior has been used successfully to predict behaviors of pharmacists7,21,22 and other healthcare professionals.23-25 Thus, the theory of planned behavior provides a promising framework for predicting pharmacy students’ intention and behaviors and was used as the guiding theoretical framework in the current study.
METHODS
This cross-sectional study was approved by the Edward Via College of Osteopathic Medicine Institutional Review Board. The study was undertaken at the Appalachian College of Pharmacy (ACP) in Oakwood, Virginia, which offers an accelerated 3-year doctor of pharmacy (PharmD) program. Students complete classroom-based studies during the first 2 years, and then proceed to practice experiences during the third and final year. Education regarding the role of pharmacists in minimizing risks associated with medication products occurs mainly in the Drug Information, Clinical Research and Biostatistics course. In addition, drug safety issues are discussed in other courses throughout the curriculum to promote a culture of safety in the minds of students. However, the college does not offer a standalone required or elective course on medication safety. The study only included third-year pharmacy students who had completed the classroom-based curriculum and had been exposed to clinical practice through practice experiences. First- and second-year students were excluded from the study.
Data were collected using a survey instrument adapted from previous research.6,7,26 The survey instrument had 58 items designed to measure the respondents’ intention, attitude, knowledge of ADE reporting, and demographic data.
All students attending a third-year seminar during the 2011 spring semester were invited to complete the survey instrument. Students’ participation was voluntary; consenting students completed and returned the survey instrument to the faculty investigator.
Intention was measured by 3 items, each of which used a Likert-type response scale ranging from extremely unlikely to extremely likely (2 items) or a response scale ranging from strongly disagree to strongly agree. Attitude was measured by 12 items. The responses to the first 8 items were on a Likert-type scale (eg, extremely unlikely to extremely likely). Attitude was also measured using 4 items, with response scales ranging from worthless to valuable; pleasant to unpleasant; good to bad; and harmful to beneficial. Student knowledge of ADE reporting was measured using 9 true or false items. Data on the students’ gender and year of birth were also collected.
Data analysis was conducted using PASW Statistics 18 (SPSS, Chicago, Ill). Means, standard deviations, and frequency distributions were computed for all study variables. An alpha level of 5% was used for significance (p ≤ 0.05). An independent t test was used to compare students’ attitudes and intentions by gender. One way analysis of variance was used to compare mean attitude differences by age category. Pearson correlation was used to compute the correlation between attitude, and intention and age. Each student’s knowledge level was computed by adding up all the correct items and then dividing by the total number of items. The knowledge scores were then converted to a percentage. Power analysis was conducted using G*Power, version 3.0.10 (Erdfelder, Faul, and Buchner, Institute for Experimental Psychology, Heinrich-Heine University-Dusseldorf, Germany).
RESULTS
Of the 64 eligible third-year students, 58 completed the survey instruments for a 91% response rate. Twenty-eight respondents were male and 29 were female. Most respondents were 20 to 30 years of age, with a mean age of 28.8 ± 6.3 years.
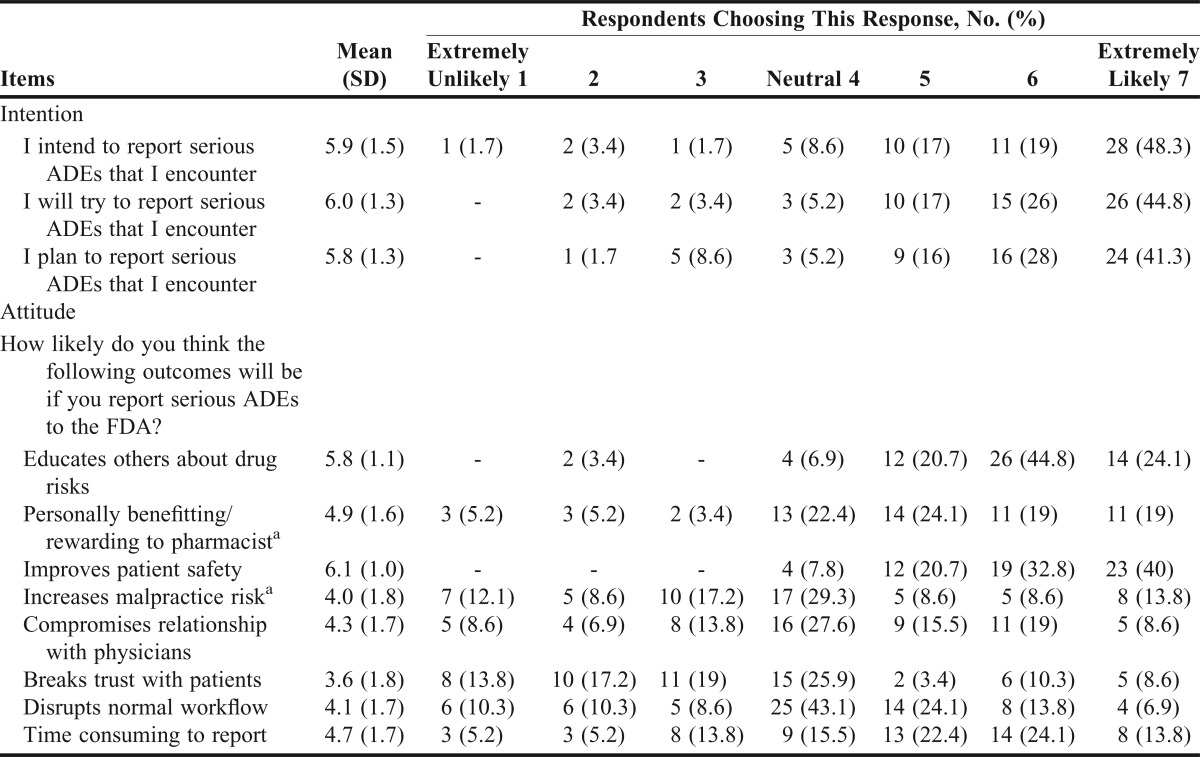
A majority of students indicated they intend to (84.0%), will try to (87.8%), and plan to (85.3%) report serious ADEs that they encounter to the FDA (Table 1). Most students (77.6%) believed that pharmacists have a moral obligation to report serious ADEs that they encounter to the FDA.
Table 1.
Attitude and Intention to Report Serious Adverse Drug Events (ADEs) to the Food and Drug Administration (FDA) (n=58)
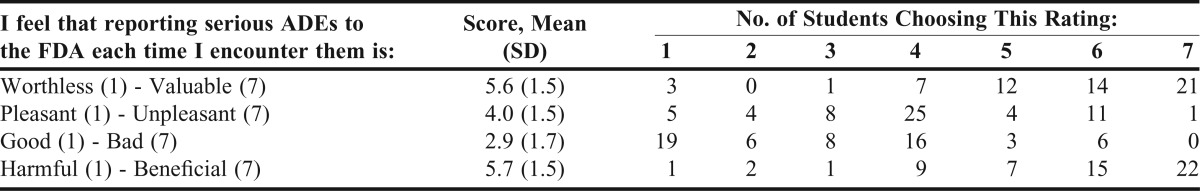
A majority of respondents believed that reporting serious ADEs was valuable (5.6 ± 1.5), good (2.9 ± 1.7), and beneficial (5.7 ± 1.5) (Table 2). However, students believed that reporting serious ADEs to the FDA was neither pleasant nor unpleasant (4.0 ± 1.5) (Table 2).
Table 2.
Pharmacy Students’ Attitudes Toward Reporting Serious Adverse Drug Events (ADEs) Each Time Encountered (N = 58)
Most respondents had favorable attitudes toward reporting serious ADEs to the FDA, and believed that reporting ADEs educates others about drug risks (5.8 ± 1.1), is personally rewarding (4.9 ± 1.6), and improves patient safety (6.1 ± 0.9) (Table 1). However, many respondents believed that reporting serious ADEs could result in negative outcomes including increased risk of malpractice, compromised relationships with physicians, broken trust with patients, and disruption of the normal workflow, and was time consuming (Table 1).
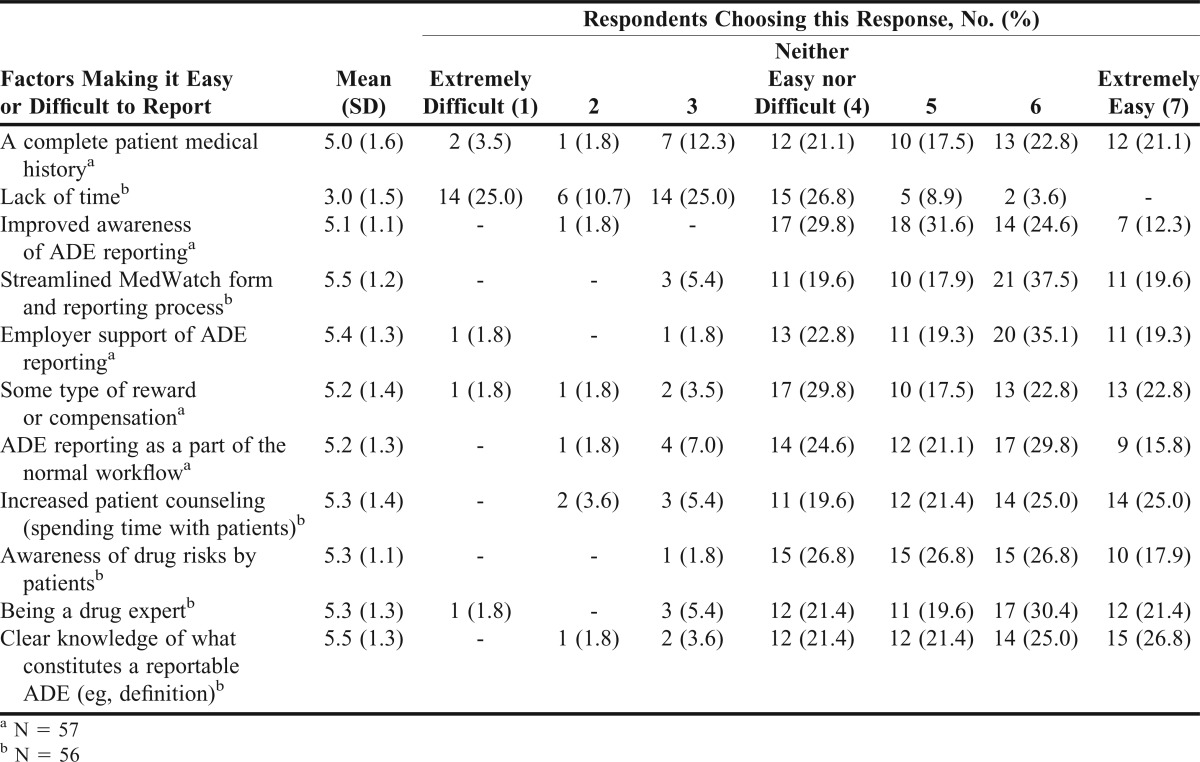
Students believed 10 of the 11 items would make it easier for them to report serious ADEs that they would encounter to the FDA. For example, students believed that having a clear knowledge of what constitutes a reportable ADE (eg, definition) (5.5 ± 1.3); having a complete patient medical history (5.0 ± 1.6); and having a streamlined MedWatch form and reporting process (5.5 ± 1.2) would make it easier for them to report serious ADEs to the FDA (Table 3).
Table 3.
Factors Making It Easy or Difficult to Report Serious Adverse Drug Events (ADEs) to the Food and Drug Administration (N = 58)
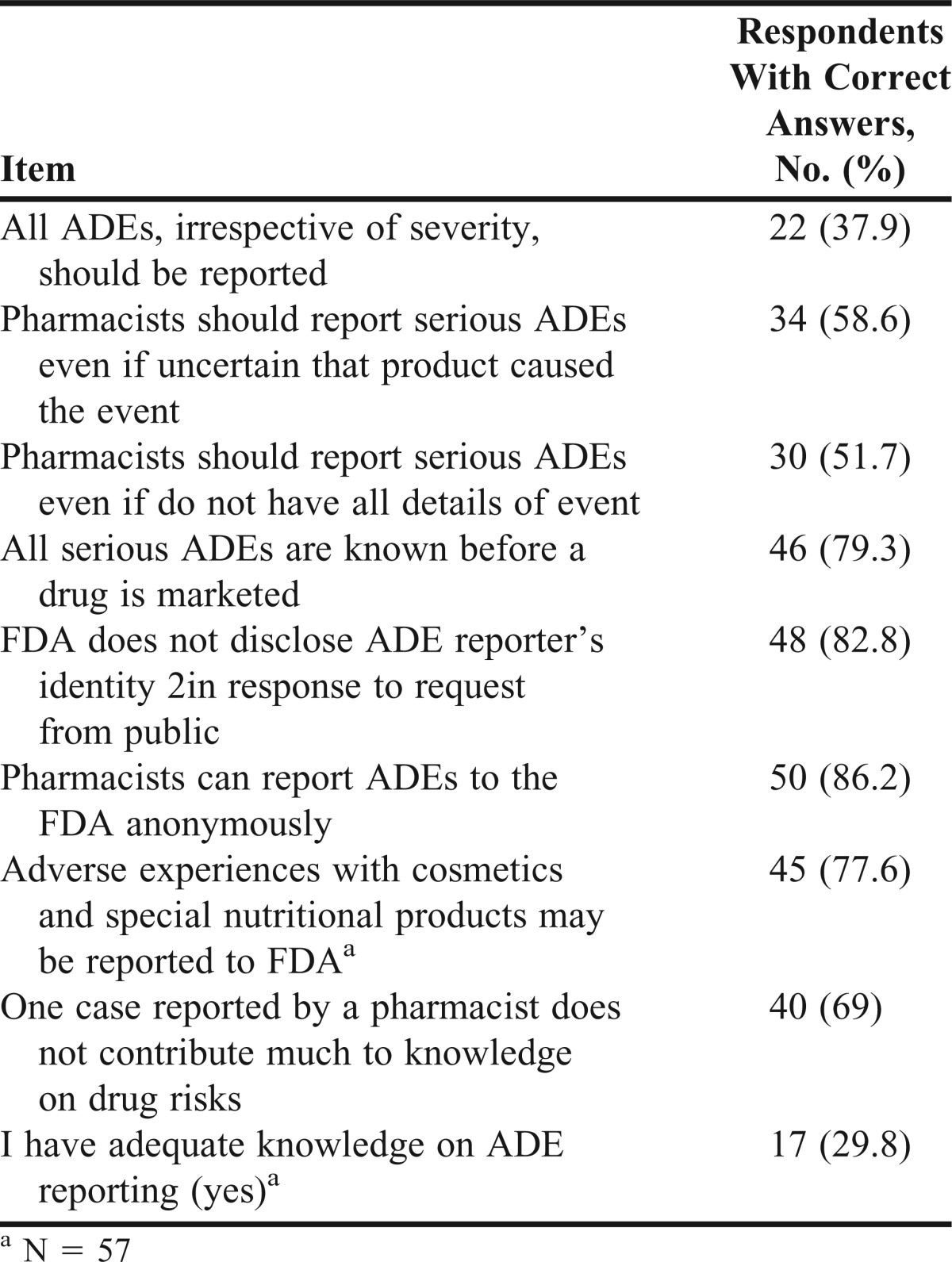
Only about 30% of students believed that they had adequate knowledge on ADE reporting (Table 4). Less than 70% of the students obtained correct answers on 4 of the 8 knowledge items (Table 4). Most students (62.1%) did not know the correct answer to the item: “All ADEs, irrespective of severity, should be reported” (correct answer = false).
Table 4.
Knowledge Concerning Reporting Adverse Drug Events (ADEs) to the Food and Drug Administration (N = 58)
There was no significant difference in mean attitude scores of students by gender for all but 1 attitude item (p > 0.05). Female students ( 6.4 ± 0.7) more strongly agreed that reporting serious ADEs to the FDA improves patient safety than did male students (5.7 ± 1.0; p = 0.004). In addition, there was no significant difference in mean attitude scores of students by age category.
DISCUSSION
Pharmacy students have strong intentions to report serious ADEs to the FDA. A majority of students intend to (84.0%), will try to (87.8%), and plan to (85.3%) report serious ADEs to the FDA. This finding concurs with Gavaza and colleagues who found that Texas pharmacists had a strong positive intent to report serious ADEs to the FDA.7 This finding is encouraging and may result in more reports from students if these intentions are translated into behavior. Behavioral intention and its antecedents are important predictors of ADE reporting behavior among pharmacists, other factors being equal.7
Pharmacy students have mostly positive attitude toward reporting serious ADEs to the FDA. Students believed that ADE reporting was valuable, good, and beneficial. Students also believed that reporting serious ADEs educates others about drugs risks, and improves patient safety. Previous studies found that pharmacists have positive and favorable attitude toward ADE reporting.6,17
However, in line with findings of previous studies involving pharmacists, students also believed that ADE reporting resulted in several negative outcomes including disrupting the normal workflow, breaking trust with patients, and compromising relationship with physicians.6 It is not clear why students held these negative beliefs concerning ADE reporting. These beliefs, if they persist, may reduce reporting of ADEs by these students when they begin professional practice.
Similar to Gavaza and colleagues, a majority of students perceived themselves to have inadequate knowledge about reporting serious ADEs.26 In addition, less than 70% of students correctly answered 4 of the 8 knowledge items. These findings show that pharmacy students may not have a complete understanding of when and how to report serious ADEs that they may encounter. Few pharmacy students knew that only serious ADEs were to be reported to the FDA (37.9%). The study indicates that students are not adequately prepared to report serious ADEs and thus may be less likely to report serious ADEs to the FDA as professionals in the future.
The inadequate knowledge found in this study may explain the perennial problem of low reporting of ADEs by practicing pharmacists. More should be done to enhance pharmacy students’ knowledge. These findings concur with Holdford and colleagues,27 who found existence of content and competency gaps with respect to drug safety among pharmacy students. Colleges and schools of pharmacy have been reported not to cover some important safety topics in their curricula. It is not clear whether the students’ inadequate knowledge was caused by gaps in the coverage of some drug safety topics in the curriculum or to other factors. As observed previously, there is room for improvement in educating pharmacy students about drug safety.
This study has several limitations. A sample of 58 pharmacy students at only 1 college is small. The results may not be generalizable to all pharmacy students nationwide. Studies with large samples and including students from multiple schools should be conducted to verify these findings. This pilot study was conducted with students enrolled in an accelerated PharmD program and they may be different from those enrolled in traditional (4-year) PharmD programs. Also, causal inferences cannot be made from this nonexperimental cross-sectional study as we did not control for all possible confounding variables such as students’ grade point average, general interest in drug safety, and type of practice experiences the students had completed. Finally, social desirability response bias cannot be completely ruled out. Students may have reported strong intentions and positive attitudes because they knew that reporting ADEs is an expected behavior. However, there was no incentive for students to be deceptive given that the survey was anonymous.
CONCLUSION
This study demonstrated that pharmacy students have strong intentions and favorable attitudes toward reporting serious ADEs, but inadequate knowledge concerning ADE reporting. Further studies with larger sample sizes and involving students at traditional PharmD programs are needed to further understand pharmacy students’ attitudes toward reporting ADEs to the FDA.
REFERENCES
- 1. Agency for Healthcare Research and Quality. Reducing and Preventing Adverse Drug Events To Decrease Hospital Costs. In: Research in Action, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2001.
- 2.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. Br Med J (Clin Res Educ). 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VA Center for Medication Safety and VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medical Advisory Panel. Adverse Drug Events, Adverse Drug Reactions and Medication Errors: Frequently Asked Questions. VA Center for Medication Safety and VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medical Advisory Panel.; 2006. http://www.pbm.va.gov/vamedsafe/Adverse%20Drug%20Reaction.pdf. Accessed November 10, 2011. [Google Scholar]
- 4.Faich GA. National adverse drug reaction reporting. 1984-1989. Arch Intern Med. 1991;151(8):1645–1647. [PubMed] [Google Scholar]
- 5.Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gavaza P, Brown CM, Lawson KA, Rascati KL, Wilson JP, Steinhardt M. Influence of attitudes on pharmacists' intention to report serious adverse drug events to the Food and Drug Administration. Br J Clin Pharmacol. 2011;72(1):143–152. doi: 10.1111/j.1365-2125.2011.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavaza P, Brown CM, Lawson KA, Rascati KL, Wilson JP, Steinhardt M. Examination of pharmacists' intention to report serious adverse drug events (ADEs) to the FDA using the theory of planned behavior. Res Soc Adm Pharm. 2011;7(4):369–382. doi: 10.1016/j.sapharm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Inman WHW. Detection and investigation of adverse drug reactions. In: Davies DM, editor. Textbook of Adverse Drug Reactions. 3rd ed. Oxford: Oxford University Press; 1985. pp. 49–62. [Google Scholar]
- 9.Gavaza P, Brown CM, Khoza S. Texas pharmacists' opinions on reporting serious adverse drug events to the Food and Drug Administration: a qualitative study. Pharm World Sci. 2010;32(5):651–657. doi: 10.1007/s11096-010-9420-y. [DOI] [PubMed] [Google Scholar]
- 10.Sweis D, Wong IC. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23(2):165–172. doi: 10.2165/00002018-200023020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoedpidemiol Drug Saf. 2007;16(2):223–228. doi: 10.1002/pds.1313. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino M, Leoni O, Banfi F, Lecchini S, Frigo G. Attitudes to adverse drug reaction reporting by medical practitioners in a Northern Italian district. Pharmacol Res. 1997;35(2):85–88. doi: 10.1006/phrs.1996.0138. [DOI] [PubMed] [Google Scholar]
- 13.Bäckström M, Mjörndal T, Dahlqvist R, Nordkvist-Olsson T. Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol. 2000;56(9):729–732. doi: 10.1007/s002280000202. [DOI] [PubMed] [Google Scholar]
- 14.Biriell C, Edwards RI. Reasons for reporting adverse drug reactions-Some thoughts based on an International review. Pharmacoepidemiol Drug Saf. 1997;6(1):21–26. doi: 10.1002/(SICI)1099-1557(199701)6:1<21::AID-PDS259>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Vallano A, Cereza G, Pedros C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653–658. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateman DN, Sanders GL, Rawlins MD. Attitudes to adverse drug reaction reporting in the Northern Region. Br J Clin Pharmacol. 1992;34(5):421–426. [PMC free article] [PubMed] [Google Scholar]
- 17.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51(1):81–86. doi: 10.1046/j.1365-2125.2001.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combined report of the 2005-06 Argus Commission, Academic Affairs, Professional Affairs, and Research and Graduate Affairs Committees. Preparing competent pharmacists and pharmacy faculty for the future. Am J Pharm Educ. 2006;70(Suppl):Article S5. [Google Scholar]
- 19.Kalari S, Dormarunno M, Zvenigorodsky O, Mohan A. Pharmacy student perceptions of adverse event reporting. Am J Pharm Educ. Sep 10. 2011;75(7):Article 131. doi: 10.5688/ajpe757131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211. [Google Scholar]
- 21.Coleman CL. Examining influences of pharmacists' communication with consumers about antibiotics. Health Commun. 2003;15(1):79–99. doi: 10.1207/S15327027HC1501_4. [DOI] [PubMed] [Google Scholar]
- 22.Herbert KE, Urmie JM, Newland BA, Farris KB. Prediction of pharmacist intention to provide Medicare medication therapy management services using the theory of planned behavior. Rese Soc Adm Pharm. 2006;2(3):299–314. doi: 10.1016/j.sapharm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Feng JY, Wu YWB. Nurses' intention to report child abuse in Taiwan: a test of the theory of planned behavior. Res Nurs Health. 2005;28(4):337–347. doi: 10.1002/nur.20087. [DOI] [PubMed] [Google Scholar]
- 24.Godin G, Belanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals' intentions and behaviours: A systematic review of studies based on social cognitive theories. Implement Sci. 2008;3(36):1–12. doi: 10.1186/1748-5908-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millstein SG. Utility of the theories of reasoned action and planned behavior for predicting physician behavior: a prospective analysis. Health Psychol. Sep 1996;15(5):398–402. doi: 10.1037//0278-6133.15.5.398. [DOI] [PubMed] [Google Scholar]
- 26.Gavaza P, Brown CM, Lawson KA, Rascati KL, Wilson JP, Steinhardt M. Texas pharmacists' knowledge of reporting serious adverse drug events to the Food and Drug Administration. J Am Pharm Assoc. 2011;51(3):397–403. doi: 10.1331/JAPhA.2011.10079. [DOI] [PubMed] [Google Scholar]
- 27.Holdford DA, Warholak TL, West-Strum D, Bentley JP, Malone DC, Murphy JE. Teaching the science of safety in US colleges and schools of pharmacy. Am J Pharm Educ. 2011;75(4):Article 77. doi: 10.5688/ajpe75477. [DOI] [PMC free article] [PubMed] [Google Scholar]