Abstract
Interleukin (IL)-27 is a heterodimeric cytokine that is known to have both stimulatory and inhibitory functions during immune responses. We investigated the effects of IL-27 on arthritis and bone erosion in the murine collagen-induced arthritis (CIA) model. We demonstrate that the inhibitory effect of IL-27 on osteoclastogenesis is associated with interferon-γ (IFN-γ) production by using an IFN-γ knockout mouse model. The IL-27-Fc was injected into both CIA and IFN-γ-deficient mice. The effects of IL-27-Fc on osteoclast differentiation were evaluated both in vitro and in vivo. The IL-27-Fc-injected mice showed significantly lower arthritis indices and fewer tartrate-resistant acid-phosphatase-positive osteoclasts in their joint tissues than untreated mice. Interleukin-27 inhibited osteoclastogenesis from bone marrow-derived mononuclear cells in vitro, which was counteracted by the addition of anti-IFN-γ antibody. The IL-27-Fc did not affect arthritis in IFN-γ knockout mice. Interleukin-27 also suppressed osteoclast differentiation in human and intriguingly, it could promote the expression of IFN-γ on priming osteoclasts. These results imply that IL-27 suppressed the generation of CIA and osteoclastogenesis, which were mediated by the induction of IFN-γ.
Keywords: collagen-induced arthritis, interferon-γ, interleukin-27, interleukin-27-Fc, osteoclastogenesis
Introduction
Interleukin (IL)-27 is a heterodimeric cytokine that consists of Epstein–Barr virus-induced protein (EBI)3 and p28 subunits and shares homology with the p40 and p35 subunits of IL-12.1 It is a member of the IL-12 family, along with IL-12, IL-23 and IL-35. It is primarily produced by activated macrophages and dendritic cells.2 Interleukin-27 is known to have pleiotropic actions during immune responses, with both activating and regulatory roles.2 Hence, its overall effects on inflammatory diseases are often debated and are dependent on the specific inflammatory disease model. Adjuvant-induced arthritis and experimental autoimmune encephalomyelitis were both suppressed by antibodies neutralizing the function of the p28 subunit of IL-27.3,4 Consistent with these findings, IL-27 induced a T helper type 1 (Th1) immune response and susceptibility to proteoglycan-induced arthritis.5 In contrast, IL-27 receptor α (IL-27Rα) -deficient mice were susceptible to experimental autoimmune encephalomyelitis and generated more IL-17-producing Th17 cells than wild-type mice, suggesting IL-27-mediated immune suppression.6 Interleukin-27 attenuated collagen-induced arthritis (CIA) when administered at the onset of disease, probably through its inhibition of Th17 differentiation.7 More recently, IL-27 was shown to abrogate receptor activator of nuclear factor-κB ligand (RANKL)-mediated osteoclastogenesis through signal transducer and activator of transcription 1 (STAT1)-dependent inhibition of c-Fos.8 It has also been reported that IL-27 inhibits human osteoclastogenesis by a direct mechanism that suppresses the responses of osteoclast precursors to RANKL.9
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by synovial inflammation and bone erosion.10 Bone erosion in RA is mediated by osteoclasts located in inflammatory pannus–bone interfaces and in subchondral locations.11,12 Osteoclasts are bone resorbing, multinucleated cells derived from the monocyte/macrophage lineage. The differentiation of osteoclasts, or osteoclastogenesis, is regulated by RANKL and macrophage colony-stimulating factor (M-CSF).13 The differentiated cells express osteoclast markers such cathepsin K, tartrate-resistant acid phosphatase (TRAP), and calcitonin receptor.11,14 Therapies that target osteoclast-mediated bone resorption are important for attenuating bone destruction in RA.
Interferon-γ (IFN-γ) has been reported to have protective effects in CIA.15 Interferon-γ receptor knockout (KO) mice show higher susceptibility to CIA than wild-type mice, which may have been a result of the loss of IFN-γ-mediated protection from bone destruction.16,17 Interferon-γ strongly suppresses osteoclastogenesis by interfering with the RANKL–RANK signalling pathway. It rapidly degrades the RANK adaptor protein, tumour necrosis factor receptor-associated factor 6 (TRAF6), which results in strong inhibition of RANKL-induced activation of nuclear factor-κB and Jun N-terminal kinase.18 It has also been reported that IL-27 augments IL-12-dependent IFN-γ production by naive T cells.19
In the present study, we investigated the effects of IL-27 on osteoclastogenesis and inflammatory arthritis in the murine CIA model. We found that IL-27 suppressed osteoclastogenesis through its induction of IFN-γ.
Materials and methods
Animals
Four- to six-week-old male DBA/1J mice were purchased from SLC, Inc. (Shizuoka, Japan). The IFN-γ-deficient C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were maintained under specific pathogen-free conditions at the Institute of Medical Science of the Catholic University of Korea and were fed standard mouse chow (Ralston Purina, St Louis, MO) and water ad libitum. All experimental procedures were examined and approved by the Animal Research Ethics Committee of the Catholic University of Korea, which conforms to all US National Institutes of Health guidelines.
Induction of CIA and assessment of arthritis
To induce CIA in DBA1/J mice, type II collagen (CII) was dissolved overnight in 0·1 M acetic acid (4 mg/ml) with gentle rotation at 4°C. Mice were injected intradermally at the base of the tail with 100 μg CII emulsified 1 : 1 (weight/volume) in complete Freund's adjuvant (Chondrex, Redmond, WA). Two weeks later, the mice were boosted intradermally with 100 μg 1 : 1 (weight/volume) CII in incomplete Freund's adjuvant (Difco, Detroit, MI). Induction of arthritis in C57BL/6 mice was performed as described previously.20 The arthritis index in these mice was scored twice per week according to the following criteria: 0 = no oedema or swelling, 1 = slight oedema and erythema limited to the foot or ankle, 2 = slight oedema and erythema from the ankle to the tarsal bone, 3 = moderate oedema and erythema from the ankle to the tarsal bone, and 4 = oedema and erythema from the ankle to the entire leg. The arthritic score of each mouse was expressed as the sum of the scores of four limbs.21
Plasmid construction
Codon-optimized mouse IL-27p28 [GenBank: 145636], IL-27EBI3 [GenBank: 015766], and the Fc-region of non-cytolytic mIgG2a22 genes were synthesized using codons for mammalian cell expression by GeneScript Co. (Piscataway, NJ) and cloned into the pUC57 plasmid. Codon-optimized mouse IL-27p28 (mp28co), the internal ribosomal entry site (IRES) of encephalomyocarditis virus, mouse IL-27EBI3 (mEBI3co), and non-cytolytic Fc (mFcm) were linked in a tandem, unidirectional arrangement. The expression cassettes of mp28co-IRES-mEBI3co and mp28co-IRES-mEBI3co-mFcm were inserted into the pGX10 vector23 using EcoRV/NcoI or EcoRV/NotI restriction enzyme sites to generate pGX10-mp28co-IRES-mEBI3co and pGX10-mp28co-IRES-EBI3co-mFcm constructs.
Administration of IL-27-Fc
For hydrodynamic injection, mice were rapidly injected intravenously with 100 μg IL-27-Fc in 2 ml saline within 5 seconds. After 8 days, the same mice were injected intramuscularly with 100 μg IL-27-Fc in the left leg by electrical stimulation (electroporation). The intramuscular injection was performed with a 31-gauge insulin syringe. After 2 days, they were injected intramuscularly with 100 μg IL-27-Fc in the right leg by electroporation.
Patients
Four RA patients who fulfilled the American College of Rheumatology 1987 revised criteria for the classification of RA24 were involved in this study. Synovial tissues were obtained from patients with RA (n = 4) or patients with osteoarthritis (OA) (n = 4). The OA patients and normal healthy volunteers were included as controls. Informed consent was obtained from all patients and healthy volunteers before the study, and this study was approved by the Ethical Committees at Seoul St Mary's Hospital.
Immunohistochemistry of mouse joints and RA synovium
Mouse joint tissue and RA and OA synovium were fixed in 4% paraformaldehyde, decalcified in EDTA-containing bone decalcifier, embedded in paraffin, and sectioned. The murine sections were stained with haematoxylin & eosin (H&E), safranin O and toluidine blue to detect proteoglycans. The sections were de-waxed using xylene and dehydrated in a gradient of alcohols. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol. Immunohistochemistry was performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Tissues were incubated with primary antibody to nitrotyrosine, nuclear factor of activated T cells c1 (NFATc1), RANK, low-density lipoprotein receptor-related protein 4 (LRP4), T-cell factor 3 (TCF3), and SRY-box containing gene 6 (SOX6) (all from Santa Cruz Biotechnology, Santa Cruz, CA) in human and IL-27, LRP4, matrix metalloproteinase 19 (MMP19) (all from Santa Cruz), and IFN-γ (R&D Systems, Minneapolis, MN) overnight at 4°C. The tissues were then incubated with a biotinylated secondary antibody and a streptavidin–peroxidase complex for 1 hr. For histological evaluation of CIA, sections were evaluated blind, and scores were determined, as previously described.25
Measurement of immunoglobulin
Blood was obtained from the mouse orbital sinus and sera were stored at −20°C until use. Anti-CII IgG1 and IgG2a antibodies were measured using the mouse IgG1/IgG2a ELISA quantification kit (Bethyl Lab Co., Montgomery, TX).
In vitro osteoclastogenesis
Bone marrow-derived monocyte/macrophage cells were isolated from the tibias and femurs of mice by flushing the bone marrow cavity with α-minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA). The cells were centrifuged, exposed to ACK buffer at room temperature for 30 seconds to remove red blood cells, and incubated with α-MEM containing penicillin/streptomycin and 10% fetal bovine serum for 12 hr to separate the floating and adherent cells. The floating cells were collected, suspended in α-MEM, counted, seeded in 48-well plates at 1 × 105 cells/well, and cultured with α-MEM in the presence of 10 ng/ml recombinant human (rh) M-CSF (R&D Systems) for 3 days to form macrophage-like osteoclast precursor cells. After 3 days in culture, non-adherent cells were washed out and these osteoclast precursor cells were then cultured in the presence of 10 ng/ml rhM-CSF and 25 ng/ml soluble rhRANKL (PeproTech, London, UK) with or without various concentrations of recombinant mouse IL-27 for 4 days to generate osteoclasts. Human peripheral blood mononuclear cells obtained from normal, healthy volunteers were isolated from buffy coats using Ficoll–Hypaque (Amersham Biosciences, Uppsala, Sweden) and density gradient centrifugation. The red blood cell-depleted cells were seeded in 24-well plates at 5 × 105 cells/well and incubated at 37°C for 2 hr to separate floating and adherent cells. Non-adherent cells were removed, and the adherent cells were washed with sterile PBS and cultured in α-MEM with 100 ng/ml rhM-CSF for 3 days to form macrophage-like osteoclast precursor cells. After 3 days in culture, these osteoclast precursor cells were further cultured in the presence of 25 ng/ml rhM-CSF, 30 ng/ml rhRANKL, and various concentrations of rhIL-27 (R&D Systems) for 9 days to generate osteoclasts. On day 3, the medium was replaced with fresh medium containing M-CSF, RANKL and IL-27.
TRAP stain
A commercial TRAP kit (Sigma, St Louis, MO) was used according to the manufacturer's instructions, but omitting the haematoxylin counterstain. TRAP-positive multinucleated cells containing three or more nuclei were scored as osteoclasts.
Reverse transcription-PCR
Messenger RNA was extracted using the Tri Reagent (Molecular Research Center, Cincinnati, OH). Reverse transcription of 2 μg total mRNA was conducted at 42°C using the Superscript Reverse Transcription system (Takara, Shiga, Japan). Polymerase chain reaction amplification of cDNA aliquots was performed by adding 2·5 mm dNTP, 2·5 U Taq DNA polymerase (Intron, Seongnam, Korea), and 0·25 μm each of sense and antisense primers. The reactions were processed in a DNA thermal cycler (PerkinElmer Cetus, Norwalk, CT). The sequences of the primers used for mouse samples were as follows: calcitonin receptor, 5′-CGG ACT TTG ACA CAG CAG AA-3′ (sense), 5′-AGC AGC AAT CGA CAA GGA GT-3′ (antisense); TRAP, 5′-TCC TGG CTC AAA AAG CAG TT-3′ (sense), 5′-ACA TAG CCC ACA CCG TTC TC-3′ (antisense); IFN-γ, 5′-GAA AAT CCT GCA GAG CCA GA-3′ (sense), 5′-TGA GCT CAT TGA ATG CTT GG-3′ (antisense); β-actin, 5′-GTA CGA CCA GAG GCA TAC AGG-3′ (sense), 5′-GAT GAC GAT ATC GCT GCG CTG-3′ (antisense). The level of mRNA expression was normalized to β-actin expression levels.
Real-time PCR
The PCR amplification and analysis were performed using a LightCycler 2.0 instrument (Roche Diagnostic, Mannheim, Germany) with software version 4.0. All reactions were performed using LightCycler Fast Start DNA master SYBR green I (Takara), according to the manufacturer's instructions. The following primers were used for human sequences: cathepsin K, 5′-TGA GGC TTC TCT TGG TGT CCA TAC-3′ (sense), 5′-AAA GGG TGT CAT TAC TGC GGG-3′ (antisense); calcitonin receptor, 5′-TGG TGC CAA CCA CTA TCC ATG C-3′ (sense), 5′-CAC AAG TGC CGC CAT GAC AG-3′ (antisense); IFN-γ, 5′-TGA CCA GAG CAT CCA AAA GA-3′ (sense), 5′-GCT GTT TCG AGG TCG AAG AG-3′ (antisense); β-actin, 5′-GGA CTT CGA GCA AGA GAT GG-3′ (sense), 5′-TGT GTT GGC GTA CAG GTC TTT G-3′ (antisense). The mRNA expression levels were normalized to β-actin expression levels.
Statistical analysis
Statistical analyses were performed with the GraphPad Prism (Version 4 for Windows, GraphPad Software, GraphPad Software Inc., San Diego, CA). P-values were calculated using a two-tailed (paired t test) and two-way analysis of variance (grouped) analysis. P < 0·05 was considered significant.
Results
The effect of IL-27 on CIA development
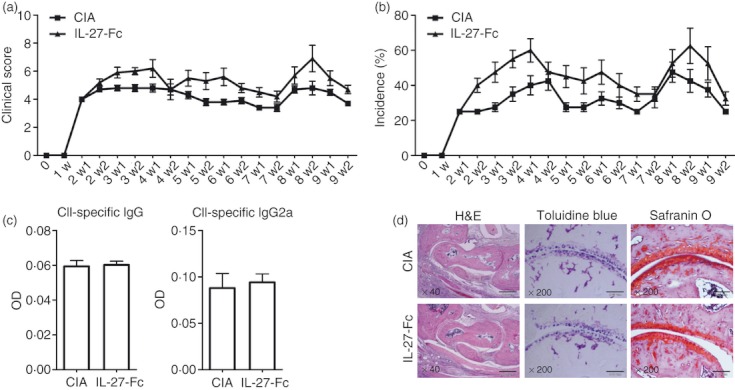
To determine whether IL-27 modulates disease severity in vivo, IL-27-Fc was administered by hydrodynamic injection into mice after the induction of arthritis. Treatment with IL-27-Fc ameliorated arthritis (Fig. 1a,b). Histological sections of knee joints stained with H&E, toluidine blue and safranin O showed that IL-27-Fc-treated mice had less severe CIA than control Fc-treated mice (Fig. 1c, inflammation, 3·67 ± 0·58 versus 1·33 ± 0·58, P < 0·05; cartilage damage, 4·00 ± 0·00 versus 1·33 ± 0·57, P < 0·05). In IL-27-Fc-treated mice, the levels of total and CII-specific IgG and IgG2a in serum were also significantly lower as compared with control-Fc-treated mice (Fig. 1d). To confirm the pathogenic engagement of RANK, NFATc1 and nitrotyrosine, which are indicators of cell damage, inflammation and the production of nitric oxide during arthritis, respectively, the joints of CIA and IL-27-Fc-treated mice were immunostained with specific antibodies directed against these molecules (Fig. 1e). Positively stained cells were strongly detected in the inflamed joints of CIA mice, whereas few positive cells were observed in the joints of IL-27-Fc-treated mice. The LRP4, an antagonist of canonical Wnt signalling,26 Tcf3, a transcription factor that interacts with β-catenin,27 and Sox6, a potent enhancer of chondroblast function,28 were also immunostained. The LRP4-positive and Tcf3-positive cells were detected in the joints of CIA mice, whereas Sox6-positive cells were more prevalent in the joints of IL-27-Fc-treated mice.
Figure 1.
The effect of interleukin-27 (IL-27) on collagen-induced arthritis (CIA) development. After primary immunization, DBA/1J mice were rapidly injected intravenously with 100 μg IL-27-Fc in 2 ml saline within 5 seconds. After 8 and 10 days, the mice were injected intramuscularly with 100 μg IL-27-Fc in the left and right legs, respectively, by electroporation. The (a) clinical score and (b) incidence rate of disease is shown for both CIA and IL-27-Fc-treated mice over time. Data are representative of three independent experiments with five per group (error bars, SD). (c) The degree of inflammation and cartilage damage is shown for representative joint sections from each group of mice. (d) The concentrations of total and type II collagen (CII)-specific IgG and IgG2a were determined by ELISA. (e) Representative images show the tibiotalar joints of CIA and IL-27-Fc-treated mice, which were subject to immunohistochemical staining for nitrotyrosine, nuclear factor of activated T cells c1 (NFATc1), receptor activator of nuclear factor-κB (RANK), low-density lipoprotein receptor-related protein 4 (LRP4), T-cell factor 3 (TCF3), and SRY-box containing gene 6 (SOX6) (magnification, × 400). *P < 0·05; **P < 0·01; ***P < 0·001, compared with CIA mice.
The effect of IL-27 on osteoclast differentiation
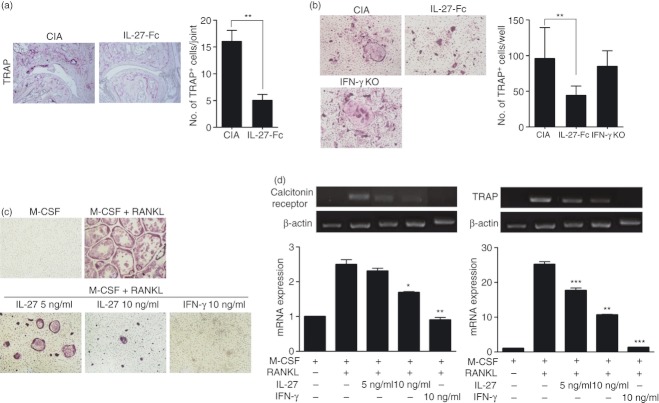
To assess the in vivo effects of IL-27-Fc on joint destruction by osteoclasts, joints from CIA and IL-27-Fc-treated mice were stained to assess TRAP expression. In CIA, TRAP-positive cells were strongly detected, whereas only a few TRAP-positive cells were detected within the joints of IL-27-Fc-treated mice (16·00 ± 3·61 versus 5·00 ± 2·00, P < 0·01, Fig. 2a). To verify the in vivo suppressive effects of IL-27 on osteoclastogenesis, bone marrow cells from CIA and IL-27-Fc-treated mice were induced to undergo osteoclast differentiation by culturing them in the presence of M-CSF and RANKL. Bone marrow cells from IFN-γ KO mice were used as a positive control for osteoclast differentiation, as IFN-γ has been shown to suppress osteoclast formation.29,30 Osteoclast differentiation of bone marrow cells from IL-27-Fc-treated mice was suppressed compared with those from CIA mice (95·67 ± 75·51 versus 44·00 ± 23·00, P < 0·01) whereas bone marrow cells from IFN-γ KO mice differentiated in a similar way to those from CIA mice (95·67 ± 75·51 versus 84·67 ± 38·28, not significant, Fig. 2b). To determine whether IL-27 inhibits osteoclast differentiation in vitro, mouse bone marrow cells were differentiated into osteoclasts in the presence of M-CSF, RANKL and various concentrations of IL-27. Interferon-γ was also tested as a positive control. The IL-27 treatment suppressed osteoclast differentiation in a similar way to treatment with IFN-γ (M-CSF plus RANKL 288·33 ± 17·50 versus M-CSF plus RANKL with IL-27 5 ng/ml 147·00 ± 3·00 or with IL-27 10 ng/ml 109·00 ± 1·00, P < 0·01, respectively, Fig. 2c). Furthermore, the relative mRNA expression of calcitonin receptor and TRAP was significantly down-regulated in osteoclasts in the presence of IL-27 (Fig. 2d).
Figure 2.
The effect of interleukin-27 (IL-27) on osteoclast differentiation. (a) Tartrate-resistant acid phosphatase (TRAP) staining of ankle joints. The number of TRAP+ cells was compared between collagen-induced arthritis (CIA) and IL-27-Fc-treated mice in Fig. 1. (b) Bone marrow cells from CIA and IL-27-Fc-treated mice in Fig. 1 were induced to undergo osteoclast differentiation by culturing them in the presence of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL). The number of multinucleated TRAP+ cells was measured by light microscopy. Data represent the average value calculated from four independent experiments. **P < 0·01, compared with the M-CSF plus RANKL culture conditions. (c) Mouse bone marrow cells from DBA/1J were cultured with or without IL-27 in osteoclast differentiation conditions for 4 days. Cells were then fixed and stained for TRAP. The number of multinucleated TRAP+ cells was measured by light microscopy. Data represent the average value calculated from four independent experiments. **P < 0·01, compared with the M-CSF plus RANKL culture conditions. (d) RNA was extracted and the expression levels of osteoclast-related genes, calcitonin receptor, and TRAP were quantified by reverse transcription-PCR. Messenger RNA levels were normalized to β-actin. Data represent the average value calculated from three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, compared with the M-CSF plus RANKL culture conditions.
Induction of IFN-γ by IL-27
To investigate the mechanism underlying inhibition of osteoclast differentiation by IL-27, mRNA expression of IFN-γ from osteoclasts differentiated in vitro with IFN-γ or IL-27 treatment was detected by reverse transcription-PCR (Fig. 3a). Interestingly, IL-27 treatment enhanced the expression of IFN-γ compared with no IL-27 stimulation. To further evaluate the IL-27-enhanced production of IFN-γ, joints from CIA and IL-27-Fc-treated mice were stained with anti-IFN-γ antibody (Fig. 3b). Over-expression of IL-27 by injection of IL-27-Fc caused an increase in the expression of IFN-γ as well as of STAT1. To better understand whether IFN-γ is an osteoclast suppressive mediator linked to IL-27, we examined the ability of IL-27 to inhibit the formation of osteoclasts in the presence of anti-IFN-γ antibody. As shown in Fig. 3(c), IL-27 could suppress the formation of osteoclasts (368·50 ± 5·50 versus 296·00 ± 8·00, P < 0·001) whereas IL-27 treatment together with anti-IFN-γ antibody could not suppress the differentiation of osteoclasts (368·50 ± 5·50 versus 393·00 ± 40·00, not significant). In contrast, IL-27 treatment with or without IFN-γ blockade had no significant suppressive effect, but rather a little enhanced effect in IFN-γ KO mice (Fig. 3d). These results suggest that IFN-γ induced by IL-27 suppresses osteoclast differentiation in vitro and in vivo.
Figure 3.
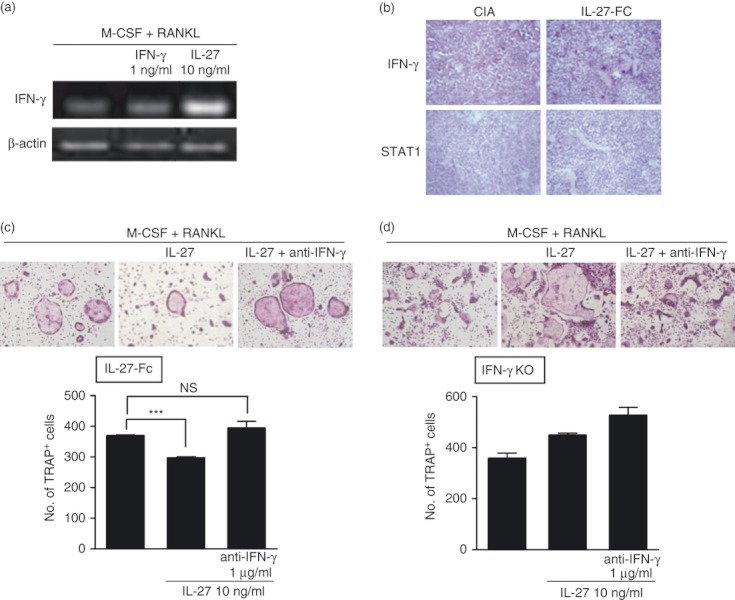
Induction of interferon-γ (IFN-γ) by interleukin-27 (IL-27). (a) Mouse bone marrow cells from DBA/1J were cultured with IFN-γ or IL-27 under osteoclast differentiation conditions for 4 days. Analysis of IFN-γ mRNA expression was performed using real-time PCR. (b) Immunohistochemical staining for IFN-γ and signal transducer and activator of transcription 1 (STAT1) was performed on ankle joint tissue from collagen-induced arthritis (CIA) and IL-27-Fc-treated mice in Fig. 1. (c, d) Mouse bone marrow cells from IL-27-Fc-treated CIA mice (c) or IFN-γ knockout (KO) (d) mice were cultured with IL-27 in the presence of anti-IFN-γ antibody. Cells were then fixed and stained for TRAP. The number of multinucleated TRAP+ cells was measured by light microscopy. Data represent the average value calculated from four independent experiments. *P < 0·05; ***P < 0·001, compared with the macrophage colony-stimulating factor (M-CSF) plus receptor activator of nuclear factor-κB ligand (RANKL) culture conditions.
The effect of IL-27 in IFN-γ-deficient mice
To confirm the mechanism of IL-27-mediated IFN-γ production and inhibition of osteoclast differentiation in vivo, we next tested the effect of IL-27 in IFN-γ-deficient mice. Interleukin-27-Fc was administered by hydrodynamic injection into IFN-γ KO mice after the induction of arthritis. In IFN-γ-deficient mice, treatment with IL-27-Fc did not ameliorate arthritis (Fig. 4a,b). Pathological analysis of joint histological sections and CII-specific IgG and IgG2a levels in serum were also not significantly different in IL-27-treated versus untreated mice (Fig. 4c,d).
Figure 4.
The effect of interleukin-27 (IL-27) in interferon-γ (IFN-γ)-deficient mice. After primary immunization, IFN-γ knockout (KO) mice were rapidly injected intravenously with 100 μg IL-27-Fc in 2 ml saline within 5 seconds. After 8 and 10 days, the mice were injected intramuscularly with 100 μg IL-27-Fc in the left and right leg, respectively, by electroporation. The (a) clinical score and (b) incidence rate of disease is shown for both collagen-induced arthritis (CIA) and IL-27-Fc-treated mice over time. Data are representative of two independent experiments with five per group (error bars, SD). (c) The concentrations of type II collagen (CII)-specific IgG and IgG2a were determined by ELISA. (d) Images show the degree of inflammation and cartilage damage based on haematoxylin & eosin, toluidine blue and safranin O staining of the tibia–talus joint in the ankle of CIA and IL-27-Fc-treated mice. Scale bars, × 40: 500 μm, × 200: 500 μm.
Suppressive effect of IL-27 on human monocyte-derived osteoclasts
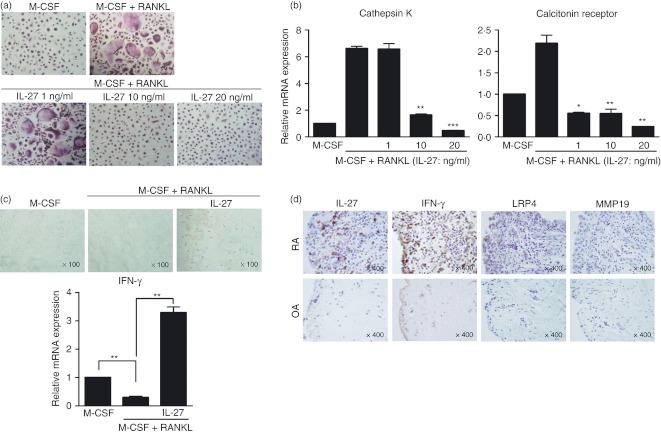
To determine whether IL-27 inhibits human osteoclast differentiation, human peripheral blood mononuclear cell-derived monocytes were cultured with various concentrations of IL-27 in the presence of M-CSF and RANKL, and TRAP-positive cells were analysed by immunohistochemistry. The IL-27 inhibited the differentiation of TRAP-positive multinuclear cells in a dose-dependent manner (M-CSF plus RANKL 231·33 ± 10·50 versus M-CSF plus RANKL with IL-27 10 ng/ml 16·67 ± 5·77 or with IL-27 20 ng/ml 1·33 ± 1·15, P < 0·01 or P < 0·001, respectively, Fig. 5a). The mRNA expression levels of cathepsin K and calcitonin receptor were decreased by IL-27 (Fig. 5b). To evaluate whether IL-27 inhibits human osteoclast differentiation via induction of IFN-γ, the expression of IFN-γ was examined by immunostaining and real-time PCR in IL-27-stimulated early-stage osteoclasts. Levels of IFN-γ protein and mRNA were increased by IL-27 (Fig. 5c). We also performed immunohistochemical staining on synovial tissues from patients with RA and OA; IL-27, IFN-γ and LRP4 were all positive in RA synovial tissues but MMP19 showed no difference between RA and OA synovial tissues (Fig. 5d).
Figure 5.
Suppressive effect of interleukin-27 (IL-27) on human monocyte-derived osteoclasts. (a) Human osteoclast precursor cells were cultured in the presence of 25 ng/ml macrophage colony-stimulating factor (M-CSF), 30 ng/ml receptor activator of nuclear factor-κB ligand (RANKL), and various concentrations of IL-27 to generate osteoclasts. After 9 days, the cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) (original magnification, × 100) and the number of TRAP+ multinucleated cells was determined by light microscopy. Data are representative of at least three independent experiments (mean and SD). **P < 0·01; ***P < 0·001, compared with the M-CSF plus RANKL culture conditions. (b) The mRNA expression levels of cathepsin K and calcitonin receptor were quantified by real-time PCR and normalized to that of β-actin. Data are representative of at least three independent experiments (mean and SD). *P < 0·05; **P < 0·01; ***P < 0·001, compared with the M-CSF plus RANKL culture conditions. (c) Monocytes from human peripheral blood mononuclear cells were cultured for 3 days with 100 ng/ml M-CSF to form macrophage-like osteoclast precursor cells. After 3 days, the cells were further cultured in the presence of 25 ng/ml M-CSF, 30 ng/ml RANKL, and 1 ng/ml IL-27. After 3 days, the cells were immunostained to detect IFN-γ (upper). The expression of IFN-γ mRNA was quantified by real-time PCR (lower). Data are representative of at least three independent experiments (mean and SD). **P < 0·01. (d) The expression of IL-27, interferon-γ (IFN-γ), low-density lipoprotein receptor-related protein 4 (LRP4), and matrix metalloproteinase 19 (MMP19) were determined in the synovium of patients with rheumatoid arthritis (RA; n = 4) or osteoarthritis (OA; n = 4) by immunohistochemical staining. Representative photographs are shown.
Discussion
In this study, we demonstrated the inhibitory effect of IL-27 on CIA generation by showing IL-27-induced inhibition of osteoclastogenesis in CIA mice. Interleukin-27 was also a strong inhibitor of human osteoclast differentiation. We found that IL-27 inhibited osteoclastogenesis via induction of IFN-γ, because this effect did not occur in IFN-γ-deficient mice or in mice treated with anti-IFN-γ antibody. Furthermore, administration of IL-27-Fc did not ameliorate joint inflammation in IFN-γ KO mice. To our knowledge, this is the first report of the inhibitory effects of IL-27 on inflammation and osteoclastogenesis via IFN-γ production.
Much has been learned of the regulatory roles of IL-27 in recent years. Initial studies found that IL-27 directed naive CD4+ T cells to differentiate into Th1 cells and augmented early Th1 responses during innate immune responses.1,2 Later reports using animal models indicated that IL-27 inhibited Th1, Th2 and Th17 responses, as well as the expansion of inducible regulatory T cells.31 There have also been numerous reports that IL-27 regulates inflammation in autoimmune diseases and RA.7,32 However, contradictory results have also been reported. Wong et al.31 detected significantly higher plasma concentrations of IL-27 in RA patients and determined that RA fibroblast-like synoviocytes (FLS) constitutively express functional IL-27 receptors cells. They also observed the activational effects of IL-27, rather than its suppressive effects, in FLS derived from RA patients. Furthermore, IL-27 induced higher levels of inflammatory cytokines and chemokines, such as IL-6, CCL2, CXCL9 and CXCL10, in RA-FLS compared with control FLS.33 The IL-27 expression was also increased in RA synovial membranes and synovial fluid.7 In our study, we found that IL-27-Fc injection ameliorated CIA, such that joint inflammation and cartilage damage were both significantly decreased. The timing of IL-27 administration may be important for its inhibitory effects on joint inflammation. When administered at the onset of the disease, IL-27 showed anti-inflammatory effects in murine models.6,7,34 We administered IL-27-Fc during the early phase of arthritis. Whether the inhibitory effects of IL-27 on arthritis will be reproduced upon administration during established arthritis needs to be investigated.
Here, we observed that the expression of IFN-γ, as well as STAT1, was enhanced in the joint tissues of IL-27-Fc-injected mice. Interferon-γ is a type II IFN produced by Th1 cells, natural killer cells, natural killer cells, and professional antigen-presenting cells. Production of IFN-γ is controlled by cytokines that are secreted by antigen-presenting cells, most notably IL-12 and IL-18.35,36 The regulatory role of IFN-γ on bone erosion or bone loss is important for maintaining homeostasis of bone metabolism in inflammatory conditions. Interferon-γ showed direct inhibition of osteoclastogenesis induced by tumour necrosis factor-α.16 The inhibitory effect of IFN-γ on osteoclastogenesis together with Toll-like receptor activation was also reported.37
The inhibitory effect of IL-27 on osteoclast generation was reported in several previous studies. In blood-derived human CD14+ cells, Kalliolias et al.9 observed that IL-27 down-regulated RANK, triggering receptor expressed on myeloid cells and abrogating RANKL-induced expression of c-Jun and NFATc1. In our study, we also observed that IL-27-Fc injection down-regulated the expression of RANK and NFATc1 in synovial tissues of CIA mice. The administration of IL-27 suppressed the development of CIA and lowered the serum levels of CII-specific IgG as well as Sox6. Treatment of mice with IL-27 also decreased serum IL-6 and collagen-specific IgG2a. Our results suggest that IL-27 is a potential therapeutic agent for RA. Spleen and lymph node cells from IL-27-treated mice produced significantly less IFN-γ and IL-17 than cells from control mice when cultured with collagen in vitro.7 In conclusion, IL-27 demonstrated inhibitory effects on joint inflammation and osteoclastogenesis through its induction of IFN-γ production. Anti-inflammatory actions and protective effects on bone erosion are both important for useful treatment of arthritis. Incorporation of the inhibitory function of IL-27 into arthritis therapies should be further investigated.
Acknowledgments
This work was supported by a grant (A092258) from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare and by the Basic Science Research Programme through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (grant numbers 2008-0059943 and 2011-0006004).
Glossary
- CIA
collagen-induced arthritis
- CII
type II collagen
- EBI
Epstein–Barr virus-induced protein
- IFN
interferon
- IL-27
interleukin-27
- IRES
internal ribosomal entry site
- KO
knockout
- LRP4
low-density lipoprotein receptor-related protein 4
- MMP19
matrix metalloproteinase 19
- mEBI3co
mouse IL-27EBI3
- mFcm
non-cytolytic Fc
- mp28co
codon-optimized mouse IL-27p28
- OA
osteoarthritis
- RA
rheumatoid arthritis
- RANKL
receptor activator of nuclear factor-κB ligand
- rh
recombinant human
- SOX6
SRY-box containing gene 6
- STAT
signal transducer and activator of transcription
- TCF3
T-cell factor 3
- Th17
interleukin-17-producing T helper
- TRAF
tumour necrosis factor receptor-associated factor
- TRAP
tartrate-resistant acid phosphatase
Disclosures
The authors declare no financial or commercial conflict of interests.
References
- 1.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–47. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–8. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–71. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 6.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 7.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67:1474–9. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa M, Takaishi H, Takito J, et al. IL-27 abrogates receptor activator of NF-κB ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009;183:2397–406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 9.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–13. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 11.Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27:968–75. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 13.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Pettit AR, Walsh NC, Manning C, Goldring SR, Gravallese EM. RANKL protein is expressed at the pannus–bone interface at sites of articular bone erosion in rheumatoid arthritis. Rheumatology (Oxford) 2006;45:1068–76. doi: 10.1093/rheumatology/kel045. [DOI] [PubMed] [Google Scholar]
- 15.Matthys P, Vermeire K, Mitera T, et al. Enhanced autoimmune arthritis in IFN-γ receptor-deficient mice is conditioned by mycobacteria in Freund's adjuvant and by increased expansion of Mac-1+ myeloid cells. J Immunol. 1999;163:3503–10. [PubMed] [Google Scholar]
- 16.Kohara H, Kitaura H, Fujimura Y, et al. IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol Lett. 2011;137:53–61. doi: 10.1016/j.imlet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 17.De Klerck B, Carpentier I, Lories RJ, et al. Enhanced osteoclast development in collagen-induced arthritis in interferon-γ receptor knock-out mice as related to increased splenic CD11b+ myelopoiesis. Arthritis Res Ther. 2004;6:R220–31. doi: 10.1186/ar1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 19.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–90. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 20.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–8. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 21.Rosloniec EF, Cremer M, Kang A, Myers LK. Collagen-induced arthritis. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1505s20. Chapter 15:Unit 15 5. [DOI] [PubMed] [Google Scholar]
- 22.Zheng XX, Steele AW, Nickerson PW, Steurer W, Steiger J, Strom TB. Administration of noncytolytic IL-10/Fc in murine models of lipopolysaccharide-induced septic shock and allogeneic islet transplantation. J Immunol. 1995;154:5590–600. [PubMed] [Google Scholar]
- 23.Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, Cho SN. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003;10:1592–9. doi: 10.1038/sj.gt.3302057. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005;14:3523–38. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Yan Y, Lim YB, et al. BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. J Cell Biochem. 2009;108:896–905. doi: 10.1002/jcb.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smits P, Li P, Mandel J, et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–90. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Mundy GR, Roodman GD. Recombinant human interferon-γ inhibits formation of human osteoclast-like cells. J Immunol. 1986;137:3544–9. [PubMed] [Google Scholar]
- 30.Fox SW, Chambers TJ. Interferon-γ directly inhibits TRANCE-induced osteoclastogenesis. Biochem Biophys Res Commun. 2000;276:868–72. doi: 10.1006/bbrc.2000.3577. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–30. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickens SR, Chamberlain ND, Volin MV, et al. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63:2289–98. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CK, Chen da P, Tam LS, Li EK, Yin YB, Lam CW. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010;12:R129. doi: 10.1186/ar3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 35.Schoenborn JR, Wilson CB. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 36.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 37.Ji JD, Park-Min KH, Shen Z, Fajardo RJ, Goldring SR, McHugh KP, Ivashkiv LB. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J Immunol. 2009;183:7223–33. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]