Abstract
Inflammation is an unstable state; it either resolves or persists. Inflammatory reactions often have a propensity for specific anatomical sites. Why inflammation persists with specific tissue tropism remains obscure. Increasing evidence suggests that stromal cells which define tissue architecture are the key cells involved, and therefore make attractive therapeutic targets. Research on stromal cells in general and fibroblasts in particular has so far been hampered by a lack of fibroblast-specific cell markers. This review highlights our increasing understanding of the role of fibroblasts in inflammation, and suggests that these cells provide the cellular basis for site specific chronic inflammation.
Keywords: chronic, fibroblast, inflammation, persistence, stromal
Introduction
Chronic inflammation occurs in a wide range of disabling diseases including rheumatoid arthritis, psoriasis and inflammatory bowel disease. One of the most important but as yet unanswered questions in inflammation research is not why inflammation occurs (episodes of self-limiting inflammation are normal and essential for the clearance of pathogens), but why it does not resolve. Historically, models of inflammation have stressed the role of antigen-specific lymphocyte responses. However, recent studies have begun to challenge the primacy of the leucocyte and are focused instead on an extended immune system in which stromal cells such as fibroblasts play a role in the persistence of the inflammatory lesion. This review aims to highlight the role that fibroblasts play in regulating the switch from acute resolving to chronic persistent inflammation.
Fibroblasts: more than just structural cells
The stroma comprises a number of cell types thought traditionally to act mainly as structural cells that produce matrix. These cell types include fibroblasts, endothelial cells, pericytes and epithelial cells. It is becoming clear that these connective tissue cells are far from inert immunologically, and play a key role in choreographing and orchestrating the immune response.
Fibroblasts are ubiquitous cells identified by their morphology, ability to adhere to plastic and lack of epithelial, vascular and leucocyte lineage markers [1]. Despite being the most abundant cells of the stroma, they have remained relatively poorly characterized in molecular terms [2]. This lack of fibroblast-specific markers has made characterization of fibroblast subsets, and hence function, difficult. Increasingly fibroblast-specific proteins have been identified, and in 2006 Kalluri and Zeisberg et al. [1] summarized the major markers. These include fibroblast activation protein 1 (FAP1), CD248 and vascular cell adhesion molecule 1 (VCAM-1), which are found on fibroblasts, as well as other markers that are better known as endothelial markers such as α-smooth muscle actin (αSMA) and vimentin. More recently, Pilling et al. [3] screened a large number of antibodies in an attempt to distinguish between monocytes, macrophages, fibrocytes (monocyte-derived) and fibroblasts. One of the striking findings from these studies is that, although many of the markers were absent from fibroblasts, none were present only on fibroblasts, highlighting the current difficulties in identifying these cells in vivo.
Fibroblasts are primarily responsible for the synthesis and remodelling of extracellular matrix components (ECM) in tissues. However, their ability to produce and respond to growth factors allows reciprocal paracrine interactions which maintain the homeostasis of adjacent cell types such as epithelial and endothelial cells [1]. These interactions regulate the morphogenesis of epithelial and endothelial structures in tissues and, as a consequence, fibroblasts play a critical role during tissue development, differentiation and repair. Therefore, understanding the biology of fibroblasts and their synthetic products is a vital area of research.
Fibroblasts are implicated in disease at a number of levels. Tissue resident fibroblasts play a crucial role in driving the inflammatory response [4]. In chronic inflammation the resolution phase is prolonged and disordered, leading to the persistent accumulation of the inflammatory infiltrate. This occurs because of the inappropriate production of survival factors such as type I interferon [5], as well as the ectopic expression and function of constitutive chemokines implicated in the retention of lymphocytes within lymphoid tissues such as the bone marrow and lymph node [6]. Fibroblasts isolated from different anatomical sites (synovium, skin, bone marrow, lymph nodes) display topographic differentiation and positional memory [7,8]. We have explored the functional consequences of these anatomical differences using models of leucocyte–fibroblast and leucocyte–endothelial–fibroblast co-culture which have allowed us to determine the effects of fibroblasts in regulating the recruitment, survival and distribution of different leucocyte subsets in vitro[9,10].
Fibroblasts are also key mediators of tissue destruction, both directly via secretion of matrix metalloproteinases (MMPs), cathepsins, inflammatory cytokines and chemokines [11] and indirectly via regulation of monocyte differentiation to osteoclasts [12]. In the rheumatoid joint, activated fibroblasts attach to and overgrow the cartilage surface, then invade and destroy cartilage and induce bone reabsorption [13]. Remarkably, fibroblasts maintain this destructive phenotype under in-vitro conditions even after multiple passages, allowing experiments to be performed in vitro with cells that are functionally representative of their in-vivo counterparts [14]. Furthermore, it has been demonstrated that the invasiveness of fibroblasts in vitro correlates with rates of bone erosion in the individual patients from whom they were isolated [15]. Strikingly, cultured rheumatoid arthritis synovial fibroblasts (but not normal or osteoarthritis synovial fibroblasts) attach to and invade co-implanted human cartilage even after multiple passages in vitro, indicating that this invasive phenotype is both stable and disease-specific [16].
Fibroblasts also play a principal role in fibrosis, tumour survival and metastasis [1]. At a cellular level the accumulation of myofibroblasts (αSMA+ fibroblasts) leads to the formation of granulation tissue and hypertrophic scars, excessive ECM production and rarefaction of the microvasculature [15]. Recently, myofibroblasts in fibrotic tissue have been shown to be resistant to apoptosis and are able to promote their own survival by killing surrounding lymphocytes [17]. A pathogeneic role for fibroblasts has also been observed in cancer [18]. Tumour stroma is comprised predominantly of cancer-associated fibroblasts (CAF). These cells, which resemble myofibroblasts, accelerate cancer cell growth by providing nutritional support, encouraging angiogenesis and facilitating tumour invasion [19]. Injection of a mixture of CAF with breast cancer cells into mice revealed that these cells help to accelerate tumour growth [20,21]. In an elegant series of experiments, a key role for fibroblasts in suppressing immune cell activity against tumours has been demonstrated recently [22].
Finally, functional similarities between fibroblasts and mesenchymal stem cells (MSC) have been highlighted recently suggesting a close relationship between these two types of stromal cells [23]. Dramatic benefits have been reported in animal models and early-phase clinical trials where MSC have proved to be capable of powerful immunosuppression [24]. However, a proinflammatory phenotype for MSC has also been described [25,26]. In fact, the administration of murine MSC in models of arthritis has led to divergent results, highlighting the need for a closer examination of the origins of MSC and fibroblasts [27,28]. MSC are defined by their ability to adhere to plastic, expression of a number of markers and ability to differentiate down osteogeneic, chondrogenic and adipogenic lineages [29]; but these properties and the markers they express are also shared by fibroblasts [30]. These observations suggest that MSC and fibroblasts share more in common than recognized previously. Such diversity in cell subsets is a well-accepted paradigm in leucocyte biology where, for example, within the same family of CD4+ T cells, regulatory as well as inflammatory subsets exist [31]. Whether similar diversity exists within the fibroblast family of cells has not yet been explored adequately.
Chronic inflammation: defined by persistence and specificity
Chronic inflammation has two major characteristics: persistence and specificity.
Persistence of inflammation requires the balance of recruitment, emigration, division and apoptosis of cells in the inflammatory lesion to be perturbed. During the initial stages of an inflammatory response, preferential recruitment of cells to the inflammatory lesion occurs in a process which is relatively well understood [32]. The mechanisms controlling the survival and retention of these leucocytes within the inflamed tissue are just as vital to the regulation of the process and yet remain largely unexplored. If increased survival or reduced egress of leucocytes occurs, the result is excessive, persistent inflammation largely irrespective of the levels of pathogen or autoantibody that triggered the initial response.
The interaction between leucocytes and stromal cells during an acute, resolving inflammatory response depends on the sequential expression of adhesion molecules, chemokines and cytokines [33–35]. Aberrant temporal and spatial expression of these proteins and their receptors leads to persistent leucocyte retention and survival in these inappropriately stable stromal cell microenvironments. For example, we have found that inappropriate production of survival factors such as interferon β[4] and stromal cell-derived factor-1 (SDF-1) [36,37] are implicated in the retention of lymphocytes within lymphoid tissues such as the bone marrow and lymph node [3,5,8,38,39]. These data suggest that while normal homeostasis and resolution of acute inflammation depends on the right cell being in the right place at the right time, it is likely that chronic inflammation involves immune cells being positioned in the wrong place at the wrong time.
During the development of an immune response, leucocytes have to be positioned appropriately within tissues. This movement of cells to appropriate niches within immune organs is driven by chemokines and their receptors. A long-standing observation has been that tissues undergoing chronic inflammatory reactions contain infiltrates of distinct subsets of leucocytes that are often organized into well-defined lymphoid-like structures (tertiary lymphoid tissue). For example, within the rheumatoid synovium, B cells organized in clusters are in close contact with macrophages, synoviocytes and CD8 T cells at the periphery of a CD4 T cell area. Stromal cells are required for this tertiary lymphoid tissue organization to occur, mediated by stromal cell production of cytokines and chemokines such as CXCL13, CCL19, CCL21, intercellular adhesion molecule 1 (ICAM-1) and VCAM-1 [40,41]. A schematic representing the role of fibroblasts in attracting and retaining lymphocytes at the site of inflammation is shown in Fig. 1. An often overlooked mesenchymal stromal cell is the fibrocyte, derived from monocyte precursors. Little detail is known about the functional abilities of this cell type, but they have the ability to produce cytokines and extracellular matrix as well as to present antigen (reviewed in [42]). These cells are found frequently at sites of chronic inflammation, including in autoimmune disease, cardiovascular disease and asthma. Mathai et al. [43] demonstrated that the presence of circulating fibrocytes correlates closely with increased lymphocyte cell numbers and these leucocytes showed an activated phenotype.
Fig. 1.
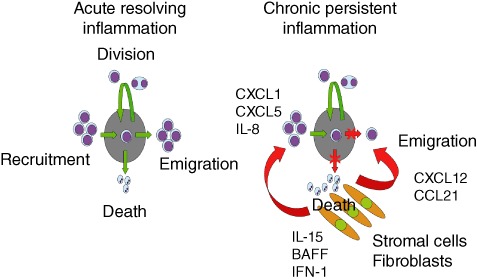
Homeostatic control of leucocyte accumulation at sites of inflammation. The left image shows the normal steady state whereby cell recruitment, emigration, cell death and division are stable and controlled to prevent the excessive build-up of leucocytes with a tissue. The right image shows how fibroblasts at an inflammatory site orchestrate the maintenance of a leucocytic infiltrate by producing survival factors such as type 1 interferon (IFN-1), interleukin (IL)-15 and B cell activating factor (BAFF) to prevent cell death. Chemokines such as CXCL12 and CCL21 prevent cell emigration and CXCL1/5 and IL-8 act to recruit leucocytes to the lesion. Unless there is active removal of survival factors and reordering of chemokine gradients, the net result is chronic accumulation, survival and retention of leucocytes at sites of disease.
There is now accumulating evidence that stromal cells define tissue topography, provide positional memory and regulate the switch from resolving to persistent inflammation [44]. Previously, Parsonage et al. [45] and Fries et al. [46] have reviewed the evidence that fibroblasts isolated from different sites exhibit distinct transcriptional profiles which define their migratory capacity, extracellular matrix production and immunomodulatory functions. Differences are also seen in fibroblasts from the same anatomical site (synovium) but different diseases (rheumatoid versus osteoarthritis arthritis), and these differences are similarly retained despite long-term culture [7,47].
The fact that these phenotypic differences are so stable in culture suggests semi-permanent epigenetic modifications (reviewed extensively in Jüngel et al. [48]). Indeed, synovial tissue from rheumatoid arthritis patients has been shown to have decreased expression of the histone deacetylases (HDAC)-1 and -2 compared to the same tissue from normal and osteoarthritis patients in one study [49], but increased activity in another, similar study [50]. Conversely, Sirt1 and 4 (also deacetylases) have been shown in a separate study to be up-regulated in synovial fibroblasts from rheumatoid compared to osteoarthritis patients [51]. In a model of inflammation driven by innate immunity (severe hypoxic pulmonary hypertension), Li et al. [52] demonstrated that pulmonary fibroblasts exhibited a profound and stable proinflammatory phenotype characterized by increased HDAC activity and high expression of cytokines and chemokines as well as VCAM and CD40. This inflammatory profile could be reduced by treating activated pulmonary fibroblasts with class I HDAC inhibitors [52]. Clearly, more work is required to understand the epigenetic changes that occur in fibroblasts during inflammation and how these might explain the stability of the phenotypes observed in culture.
Although site-specific fibroblast phenotypes are remarkably stable during in-vitro culture it is possible to alter the phenotype by changing the inflammatory stimuli given. For example, the transcriptional profile of fibroblasts isolated from the synovium can be made to resemble that of lymphoid fibroblasts, implying that fibroblast regional identity can be modified by inflammation [7,44,46,53]. This work has led to the proposal that stromal cells define an ‘area postcode’ that allows leucocytes to identify their position within the body [45]. This hypothesis predicts that components of the stromal area postcode become disordered during acute inflammatory episodes, leading to the accumulation of lymphocytes in tertiary lymphoid-like structures and consequently persistent, chronic inflammation. We would therefore predict that fibroblasts act not only as the drivers of disease but are also required to resolve inflammation by actively removing cytokine/chemokine gradients and allowing leucocytes to leave the affected area or to remove survival signals, allowing the infiltrating cells to undergo apoptosis (Fig. 2).
Fig. 2.
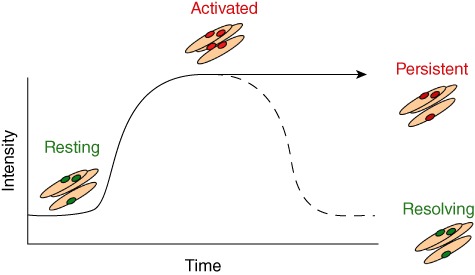
Model to demonstrate the role of fibroblasts in active resolution of inflammation. As the intensity of inflammation increases over time, fibroblasts switch from a regulatory/resting to an activated phenotype. There are two potential outcomes; either the fibroblasts can switch to a resolving phenotype and reduce their cytokine/chemokine output to allow inflammation to resolve or they may retain their activated proinflammatory phenotype and prevent the resolution of inflammation. If this occurs, it is possible that even with removal of the initial stimulus the leucocyte infiltration will remain.
Recently Lefèvre et al. [16] have shown that synovial fibroblasts from rheumatoid patients but not osteoarthritis patients have not only invasive but also systemic homing and migratory abilities. It is intriguing to speculate that in rheumatoid arthritis it is these activated migratory synovial fibroblasts that spread the disease throughout the articular joints, thereafter attracting leucocytes to the synovium.
Conclusion
The architecture of organs is adapted very closely to their function. Tissue resident stromal cells define the microanatomy and architecture of organs and provide the appropriate microenvironment in which specialized functions can occur. In addition to their landscaping properties, fibroblasts are not simply passive players in immune responses but play an active role in governing the persistence of inflammatory disease, as well as enabling immunological memory to become established in a site-specific manner [54]. The response of the immune system to tissue damage involves a carefully choreographed series of cellular interactions between immune and non-immune cells. Immune cells require stromal cells in order to home to and survive within the site of inflammation. Given that all inflammatory reactions take place within a defined background of specialized stromal cells, understanding the biology of fibroblasts in lymphoid and non-lymphoid tissues is important in understanding how immune cell infiltrates become established and persistent in chronic immune-mediated inflammatory diseases.
Therefore, populations of leucocytes recruited to sites of inflammation should not be considered in isolation but in conjunction with fibroblasts that provide survival, differentiation and positional cues upon which the formation and persistence of leucocyte infiltrates depend. In light of these data we propose that inflammation is not a generic process, but contextual, and that differences in the response of different inflammatory diseases to therapy are likely to be due to intrinsic differences in the behaviour of fibroblasts within microenvironments. Ignoring the contribution of fibroblasts to the pathogenesis of chronic inflammatory disease may account for the failure of current therapies to affect a permanent cure. We suggest that stromal cells in general and fibroblasts in particular offer a new family of organ-specific targets to treat chronic immune-mediated inflammatory diseases such as rheumatoid arthritis, psoriasis and inflammatory bowel disease.
Disclosure
The authors have no conflict of interest.
References
- 1.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 2.Filer A, Pitzalis C, Buckley CD. Targeting the stromal microenvironment in chronic inflammation. Curr Opin Pharmacol. 2006;6:393–400. doi: 10.1016/j.coph.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages and fibroblasts. PLoS ONE. 2009;10:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley CD. Why does chronic inflammatory joint disease persist? Clin Med. 2003;3:361–6. doi: 10.7861/clinmedicine.3-4-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Buckley CD, Amft N, Bradfield PF, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–9. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- 7.Filer A, Parsonage G, Smith E, et al. Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils. Arthritis Rheum. 2006;54:2096–108. doi: 10.1002/art.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsonage G, Falciani F, Burman A, et al. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003;90:688–97. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- 9.Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–82. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 10.Lally F, Smith E, Filer A, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005;52:3460–9. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller-Ladner U, Gay S. MMPs and rheumatoid synovial fibroblasts: Siamese twins in joint destruction? Ann Rheum Dis. 2002;61:957–9. doi: 10.1136/ard.61.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Pap T, Gay S. Fibroblasts and fibroblast-like synoviocytes. In: Firestein G, Budd R, Harris E, McInnes I, Ruddy S, Sergent J, editors. Kelly's Textbook of Rheumatology. 8th edn. Philadelphia: Saunders Elsevir; 2009. pp. 201–15. [Google Scholar]
- 14.Muller-Ladner U, Kriegsmann J, Franklin BN, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–15. [PMC free article] [PubMed] [Google Scholar]
- 15.Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, Breedveld FC, Toes RE, Huizinga TW. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:1999–2002. doi: 10.1002/art.21118. [DOI] [PubMed] [Google Scholar]
- 16.Lefèvre S, Knedla A, Tennie C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414–20. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallach-Dayan SB, Golan-Gerstl R, Breuer R. Evasion of myofibroblasts from immune surveillance: a mechanism for tissue fibrosis. Proc Natl Acad Sci USA. 2007;104:20460–5. doi: 10.1073/pnas.0705582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer M, Werner S. Cancer as an over-healing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 19.Barsky SH, Green WR, Grotendorst GR, Liotta LA. Desmoplastic breast carcinoma as a source of human myofibroblasts. Am J Pathol. 1984;115:329–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 21.Orimo PB, Gupta DC, Sgroi F, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science. 2010;350:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 23.Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica. 2009;94:258–63. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182:7963–73. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 26.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–19. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 28.Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyndall A, Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009;43:821–8. doi: 10.1038/bmt.2009.63. [DOI] [PubMed] [Google Scholar]
- 30.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 31.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–42. [PubMed] [Google Scholar]
- 33.Bombara MP, Webb DL, Conrad P, et al. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol. 1993;54:399–406. doi: 10.1002/jlb.54.5.399. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–74. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pablos JL, Amara A, Bouloc A, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–86. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agace WW, Amara A, Roberts AI, et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–8. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 38.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 39.Amft N, Curnow SJ, Scheel-Toellner D, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjögren's syndrome. Arthritis Rheum. 2001;44:2633–41. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Manzo A, Paoletti S, Carulli M, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35:1347–59. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- 41.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukocyte Biol. 2001;69:331–9. [PubMed] [Google Scholar]
- 42.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–35. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show and enhanced profibrotic phenotype. Lab Invest. 2010;90:812–23. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang HY, Chi J-T, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsonage G, Filer AD, Haworth O, et al. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–6. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fries KM, Blieden T, Looney RJ, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–92. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 47.Scaife S, Brown R, Kellie S, et al. Detection of differentially expressed genes in synovial fibroblasts by restriction fragment differential display. Rheumatology. 2004;43:1346–52. doi: 10.1093/rheumatology/keh347. [DOI] [PubMed] [Google Scholar]
- 48.Jüngel A, Ospelt C, Gay S. What can we learn from epigenetics in the year 2009? Curr Opin Rheumatol. 2010;22:284–92. doi: 10.1097/BOR.0b013e3283389641. [DOI] [PubMed] [Google Scholar]
- 49.49 Huber LC, Brock M, Hemmatazad H, et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007;56:1087–93. doi: 10.1002/art.22512. [DOI] [PubMed] [Google Scholar]
- 50.Kawabata T, Nishida K, Takasugi K, et al. Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R133. doi: 10.1186/ar3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niederer F, Brentano F, Ospelt C, et al. Expression of sirtuins in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60:S52. doi: 10.1002/art.24226. [DOI] [PubMed] [Google Scholar]
- 52.Li M, Riddle SR, Frid MG, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–22. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burman A, Haworth O, Hardie DL, et al. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]


