Abstract
Regulatory T cells (Tregs) are crucial in mediating immune homeostasis and promoting the establishment and maintenance of peripheral tolerance. However, in the context of cancer their role is more complex, and they are thought to contribute to the progress of many tumours. As cancer cells express both self- and tumour-associated antigens, Tregs are key to dampening effector cell responses, and therefore represent one of the main obstacles to effective anti-tumour responses. Suppression mechanisms employed by Tregs are thought to contribute significantly to the failure of current therapies that rely on induction or potentiation of anti-tumour responses. This review will focus on the current evidence supporting the central role of Tregs in establishing tumour-specific tolerance and promoting cancer escape. We outline the mechanisms underlying their suppressive function and discuss the potential routes of Tregs accumulation within the tumour, including enhanced recruitment, in-situ or local proliferation, and de-novo differentiation. In addition, we review some of the cancer treatment strategies that act, at least in part, to eliminate or interfere with the function of Tregs. The role of Tregs is being recognized increasingly in cancer, and controlling the function of these suppressive cells in the tumour microenvironment without compromising peripheral tolerance represents a significant challenge for cancer therapies.
Keywords: cancer, regulatory, suppression, therapy, Treg
Introduction
Since Burnet and Thomas proposed the cancer immunosurveillance hypothesis in the late 1950s, the involvement of the immune system in the recognition and eradication of nascent transformed cells and, therefore, in the control of malignancies has been a matter of intensive debate [1,2]. An improved molecular understanding of tumour-associated antigens (TAAs), and the use of genetically defined animal models to dissect out the role of different components of the immune system in tumour control has led to a general acceptance of the immunosurveillance hypothesis, despite the lack of consensus in results from some experimental mouse models (reviewed in [3]). It is also clear that the immune system has a role in shaping the immunogenicity of tumours [1,2,4], a concept supported increasingly by both epidemiological studies and direct evidence [5,6]. Known as immuno-editing, and first proposed by Dunn and colleagues, this broader model involves three stages: elimination, equilibrium and escape [1,2]. The immune system is thus recognized to have dual roles in the control of malignancy; to protect the host from emerging tumours as well as to sculpt them, resulting in variants with reduced immunogenicity or that are better equipped to evade or suppress immunological attack [1,2,7]. During the equilibrium phase, the precise nature of the interaction between the immune system and the tumour will determine the eventual outcome, be it elimination or escape [2,7]. Recently, Hanahan and Weinberg proposed the addition of immune evasion to the original six hallmarks of cancer they outlined in 2000 and tumour-associated inflammation as an enabling characteristic for the acquisition of these hallmarks [5]. Understanding how the balance between the immune system and the tumour is established during the equilibrium phase, and how it can be reorientated towards elimination, will aid in the design and development of improved therapeutic strategies aimed at cancer eradication.
Regulatory T cells
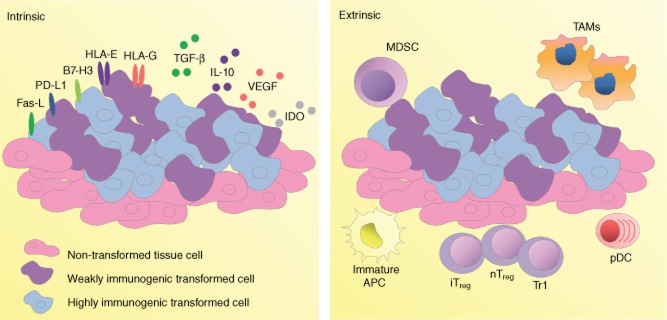
Identification of tumour-specific tolerance without generalized immunodeficiency or immunosuppression has been demonstrated in a number of models, thus defining tumours as sites of immune privilege [8–11]. This is driven by both tumour-intrinsic and -extrinsic mechanisms (summarized in Fig. 1 [2]). Because most TAAs are modified or aberrantly expressed self-antigens, it is conceivable that regulatory T cells (Tregs) play a critical role in this process [12]. Indeed, accumulating evidence implicates Tregs as one of the principal cell types suppressing TAA-specific lymphocyte activity and tumour eradication, and thus one of the major obstacles to effective anti-tumour immunotherapy [2,12–17].
Fig. 1.
Down-modulation of anti-tumour immune responses. Mechanisms for down-regulating anti-tumour immune responses can be categorized into tumour-intrinsic (left panel) and tumour-extrinsic (right panel). The first includes secretion of immunosuppressive cytokines or other soluble factors, alteration of immunogenicity and deletion or manipulation of tumour-associated antigen-specific effector cell function by the tumour cells or surrounding parenchyma. Tumour-intrinsic immunosuppressive mechanisms include secretion of immunosuppressive cytokines [interleukin (IL)-10 and transforming growth factor (TGF)-β], growth factors [vascular endothelial growth factor (VEGF)], or enzymes [indoleamine 2,3-dioxygenase (IDO)] and expression of inhibitory cell-surface molecules such as programmed cell death ligand (PD-L), Fas ligand (Fas-L), the co-inhibitory receptor B7 homologue 3 (B7-H3) and non-classical human leucocyte antigen (HLA) molecules (HLA-E/G). Tumour-intrinsic mechanisms promote a microenvironment that enhances regulatory T cell (Treg) function. Extrinsic mechanisms involve a range of suppressive cells including suppressive T cells (inducible and natural Tregs and Tr1 T cells) and a heterogeneous population of myeloid-derived suppressor cells (MDSCs), alternatively activated M2-like tumour-associated macrophages (TAMs), immature antigen-presenting cells (APCs) and plasmacytoid dendritic cells (pDCs). The cells exert their effects via both interaction with infiltrating anti-tumour inflammatory cells and contribution to the suppressive tumour microenvironment.
Tregs, first identified by Sakaguchi et al. [18], are the mediators of peripheral tolerance to self- and innocuous environmental antigens [12,19]. Originally characterized as CD4+CD25+ lymphocytes, they were also found to express the forkhead/winged helix family transcription factor FoxP3. This is critical for both the development of Tregs and for their suppressive capacity, and to an extent has also enabled improved characterization of these cells [12,20]. Two major subsets of Tregs exist: natural Treg (nT) cells, generated during T cell development in the thymus upon high-avidity interactions with peptide/class II major histocompatibility complex, and adaptive or inducible Treg (iTreg) cells that arise in the periphery upon naive CD4+ T cell interaction with tolerogenic antigen-presenting cells (APCs) [2]. While the nTreg population is considered to have relatively stable FoxP3 expression [21], plasticity within the iTreg compartment has been identified. In some models, loss of FoxP3 expression and acquisition of effector lineage-defining cytokines occurs [22], although in other experimental settings such instability has not been observed [23]. As FoxP3 can be expressed transiently in both human activated CD4+ cells [24,25] and murine T cells [26] with no demonstrable suppressive capacity, plasticity of FoxP3 expression may be a function of uncommitted cells that express this transcription factor transiently rather than a property of committed Tregs [26]. Therefore, while it appears that Tregs represent a stable subset, there may still be potential for Treg induction from effector T cells (Teff) upon exposure to certain stimuli present such as in the tumour microenvironment. Both the stability of Tregs and potential Teff conversion within the tumour context may have important implications on therapeutic strategies.
It is likely that tumour-specific tolerance relies on both nTreg and iTreg populations, and independent contributions of these two populations have been shown in a murine tumour-induced tolerance model [14]. However, others [27] have shown that the Treg population in renal cell carcinoma patients co-expresses FoxP3 and Helios, an Ikaros family transcription factor expressed by nTregs, but not iTregs [28,29]. Furthermore, it was the FoxP3+Helios+ population that expanded after interleukin (IL)-2 administration [27]. Therefore, it may be that the nTreg population is more important in tumour progression, at least in some tumours. This suggests that previous studies correlating increased Treg presence with disease progression require re-evaluation to determine whether the expanded population represents nTregs. While the implications for expanded nTreg over iTreg populations remain to be elucidated, inhibition of Helios expression has been shown to attenuate the suppressive capacity of nTregs [28], and as such could be employed therapeutically.
Classical CD4+CD25+FoxP3+ T cells can down-modulate anti-tumour responses by both release of soluble factors and direct cell–cell contact [12]. The suppressive mechanisms employed by these cells, reviewed extensively elsewhere, are outlined in Fig. 2 [12,19]. The relative contributions of each of these mechanisms remain to be elucidated, particularly in the tumour context [19]. In addition, there are several other subsets of T cells with regulatory capacity, but these are beyond the scope of this review.
Fig. 2.
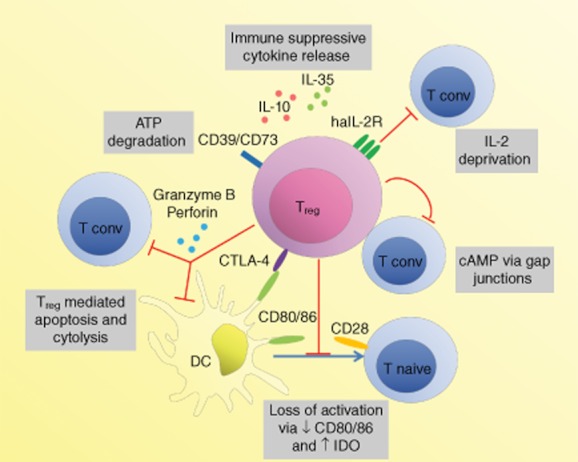
Mechanisms of regulatory T cell-mediated suppression. Regulatory T cells (Tregs) can mediate suppression through their effects on dendritic cells (DCs), such as physical inhibition of interaction between DCs and conventional T cells (Tconv) or deprivation of co-stimulation or soluble factors. Cytotoxic T lymphocyte-associated antigen (CTLA)-4 on Tregs interacts with CD80 and CD86 on DC with high affinity. In addition to inhibiting co-stimulation via CD28 on Tconv, this can lead to down-regulation of CD80 and CD86 expression on the DC (either through transendocytosis or transcriptional regulation), thereby further reducing their co-stimulatory capacity and Tconv activation and proliferation. It can also lead to up-regulation of indolamine 2,3-dioxygenase (IDO) in DC, which can mediate suppression of Tconv by tryptophan depletion and apoptosis through tryptophan catabolites. Perforin and granzyme B from Tregs induces apoptosis and cytolysis of both Tconv and DC. Deprivation of interleukin (IL)-2 via the high-affinity IL-2 receptor (haIL-2R, consisting of a CD25, CD122, CD132 complex) and adenosine triphosphate (ATP) through CD39 and CD73 can lead to the inhibition of Tconv activation or T cell apoptosis. The cytokines IL-10 and IL-35 can mediate suppression, and IL-35 can also act as an autocrine Treg growth factor. While secreted TGF-β induces IDO in DCs, how much the cytokine contributes to Treg-mediated suppression is under debate. Cyclic adenosine monophosphate (cAMP) transferred to Tconv via gap junctions inhibits their IL-2 production and subsequent proliferation. A number of other suppression mechanisms have been proposed. The relative contributions of these processes remain to be determined. Generally, spatiotemporal context may determine which of these mechanisms dominate. CD39: nucleoside triphosphate diphosphohydrolase; CD73: ecto-5′-nucleotidase.
Mechanisms driving regulatory T cell accumulation within tumours
It is critical that the processes that contribute to Treg accumulation in tumours are understood, as this underpins successful treatment strategies. Three different, but not mutually exclusive, modes of Treg accumulation within the tumour mass can be envisaged: (i) increased trafficking, (ii) preferential Treg expansion and (iii) de-novo differentiation, where the latter two can occur either locally within the tumour microenvironment or distally in tumour-draining lymph nodes (TDLNs).
It has been suggested that Tregs display an enhanced capacity for infiltration of, and accumulation within, the tumour in comparison to Teffs [2,12,13]. In support of this, preferential recruitment has been observed in ovarian [30] and breast carcinoma [31], and also Hodgkin's lymphoma [32]. It is reliant on chemokine-driven mechanisms, and several chemokines and their cognate receptors have been implicated. The chemokine receptors CCR4 and CCR8 are expressed by Tregs [33] and the CCR4 ligand CCL22 has been shown to be produced by both tumour cells and tumour-infiltrating macrophages [13,17,34]. Blockade of CCL22 reduced Treg infiltration into ovarian tumours and induced tumour rejection in a murine xenograft model [30]. CCR4 also appears to facilitate Treg tumour infiltration in gastric cancer [35] and oesophageal squamous cell carcinoma [36]. Another chemokine, CCL28, can be expressed by tumour cells during hypoxia, and it is reported to recruit preferentially Tregs expressing CCR10 [37], while in a murine model of pancreatic cancer Tregs have been shown to be recruited to the tumour site via a CCL5/CCR5 axis [38]. Targeting these chemokine receptors may inhibit Treg accumulation within tumours, although receptor and ligand redundancy and promiscuity within the chemokine–chemokine receptor system provides a significant hurdle to overcome for therapy.
A second mechanism could be through expansion of Tregs within the tumour mass (in situ) or in the TDLNs. IL-2 is essential for Treg development and homeostasis [13], and it has been proposed that Teff-derived IL-2 within the tumour mass could promote Treg proliferation, and the presence of Ki-67 (a nuclear protein expressed by proliferating cells) has been observed in tumour-infiltrating Tregs [2]. Furthermore, expansion of Tregs with potent suppressive capacity has been shown upon IL-2 administration in peripheral blood of metastatic melanoma or renal cell carcinoma patients [39], as well as within the tumour mass of patients with renal cell carcinoma [40]. Dendritic cell-derived transforming growth factor (TGF)-β-dependent proliferation of Tregs within the TDLNs in rodent tumour models has also been reported [41].
A third mechanism involving de-novo conversion of FoxP3– T cells into Tregs may play an important role in Treg accumulation in tumours [14]. The role of TGF-β in the induction of Tregs is well established [42], and tumour cell-derived TGF-β can contribute to the induction of Tregs [43]. Similar observations have been made for the effects of indoleamine 2,3-dioxygenase [44], in addition to its capacity to directly activate Tregs in TDLNs [45]. In support of local differentiation, Kurt and colleagues carried out a comparison of CD3+ T cells in progressing and rejected tumours, and found that tumour-infiltrating lymphocytes (TILs) with suppressive capacity have the same T cell receptor β chain variable region (TCR-Vβ) usage as those that promote tumour rejection [10]. However, more recently it has been reported that local conversion does not contribute significantly to the Treg pool in carcinogen-induced tumours, as the T cell receptor repertoires between Teff and Treg cells are largely distinct within the tumour microenvironment [46]. A high degree of overlap was observed between Tregs in the tumour and TDLNs, suggesting that they are induced in the TDLNs [46]. Indeed, other work has shown that CD4+CD25+ T cells within the tumour microenvironment can be derived from the existing peripheral Treg pool [47], while Valzasina and colleagues have postulated that Treg de-novo conversion in TDLNs is the principal mechanism of Treg accumulation in tumour-bearing thymectomized and CD25-depleted mice [48]. They also suggested that the expansion of Tregs in the TDLN occurs at the expense of CD4+ Teffs, and thus results in a concomitant reduction in the Teff pool [48]. However, de-novo conversion, even in the TDLNs, may not be the major mechanism in lymphosufficient animals or, indeed, at later time-points during disease. In light of the recently published data on enriched nTreg over iTreg in renal cell carcinoma patients, de-novo induction of Tregs in the periphery does not appear to contribute significantly to the Treg pool, at least in this malignancy [27]. Moreover, data from Curiel and colleagues show decreasing populations of Tregs in TDLNs with cancer progression [30], and therefore there may be a temporal component that governs which of these modes dominates.
Regulatory T cells and cancer prognosis
Since the first studies suggesting the involvement of suppressive T cells in inhibiting effective anti-tumour responses [49], Treg participation in anti-tumour responses has been studied extensively in animal models, and many malignancies have now been shown to contain infiltrating Treg populations [12]. Typically constituting 5–10% of murine and human peripheral CD4+ T cell pool, Tregs are enriched significantly within the tumour mass, peripheral blood or ascites, where they may constitute up to 20–30% of total CD4+ cells [12,17,48]. This apparent enrichment is of particular importance because Tregs appear to suppress in a dose-dependent manner [2,12,30]. While an abundance of TILs was proposed initially to correlate with favourable prognosis [50], it is now recognized that in many instances it is rather the balance between Teff and Treg populations that determines disease outcome [2,51,52]. Furthermore, there exists a strong correlation between tumour progression and Tregs, with increased numbers or frequency of Tregs associated with poor prognosis in a variety of cancers, including ovarian [30], breast [53], non-small cell lung [54], hepatocellular [55], renal cell [40,56], pancreatic [57], gastric [58] and cervical [59] carcinomas. This can be demonstrated experimentally, where adoptive transfer of Tregs results in reduced anti-tumour immune responses in mouse models [60,61]. In contrast, however, increased levels of Tregs in patients with colorectal carcinoma [16,62,63], follicular lymphoma, germinal centre-like diffuse large B cell lymphoma and Hodgkin's lymphoma [64] have been shown to be associated with increased survival, and in head and neck squamous cell carcinoma with improved local tumour control [65] and survival [66]. This correlation between Treg abundance and favourable prognosis perhaps seems counterintuitive, but might relate to the ability of these cells to suppress inflammation. Inflammation is one of the critical processes promoting tumour growth in many tissues by fostering proliferation, angiogenesis and metastasis [4,5,67]. Indeed, in patients with colorectal carcinoma, it has been proposed that Tregs suppress pro-tumourigenic T helper type 17 (Th17) cell-driven responses against the gut microbiota [16], and a similar down-regulation of pro-tumourigenic inflammatory responses by Tregs has been proposed in head and neck squamous cell carcinoma [16,65]. Conversely, there is also clear evidence that Tregs in colorectal cancer suppress tumour-specific T cell responses, and increase the chance of recurrence after surgical resection [68]. Thus, the type and location of the tumour, and the inflammatory milieu within the tumour mass, may determine precisely how Tregs impact on tumour progression.
Therapeutic targeting of regulatory Tregs
Strategies for tumour eradication have focused mainly on either enhancing tumour immunogenicity or promoting anti-tumour effector responses, although both have been largely unsuccessful [12,13]. This is thought be due primarily to the highly immunosuppressive environment found within the tumour. As Tregs are involved critically in shaping the tumour microenvironment to prevent optimal function of effector cells, Treg depletion or manipulation of their suppressive functions have been explored increasingly as therapeutic strategies.
Augmenting anti-tumour immune responses by anti-CD25 antibody-mediated Treg depletion has been explored extensively, but has generated variable outcomes. Not surprisingly, depletion of Tregs has been shown to increase anti-tumour immunity and vaccine efficacy, and to result in overall improved responses to immunotherapy in murine studies [14,34,61,69,70]. Two studies using depletion of CD4+CD25+ cells with an anti-CD25 antibody (PC61) in mice showed the efficient eradication of syngeneic tumours in vivo [69,71]. Depletion of CD4+CD25+ alone or in combination with vaccination has since been shown to contribute to improved anti-tumour responses and/or tumour regression in various tumour models [70,72,73], although some studies have failed to show any effect on tumour growth.
Clinically, non-specific depletion of CD25+ cells using daclizumab, a monoclonal antibody (mAb) against CD25, has been effective in the induction of anti-tumour responses when administered together with peptide vaccines in metastatic breast cancer patients [74]. Denileukin diftitox [DAB389IL-2 (ONTAK)], a recombinant protein composed of IL-2 and the active domain of diphtheria toxin, has been shown useful in the treatment of cutaneous T cell lymphoma [75]. It has also been shown to enhance anti-tumour effects of dendritic cell vaccines in renal cell carcinoma patients [76]. However, it had no effect on survival in patients treated previously for non-small cell lung carcinoma [77], and had no impact on Treg numbers, Treg function or disease progression in patients with metastatic melanoma or renal cell carcinoma [78]. Furthermore, Powell and colleagues reported that, while administration of LMB-2 (a CD25-directed immunotoxin) to metastatic melanoma patients resulted in preferential reduction of circulating Treg numbers, it did not have any significant effects on the anti-tumour immune responses induced by vaccination [79].
Interestingly, the efficacy of several non-specific chemotherapeutic agents may be due in part to the depleting effects on Treg populations [12]. Among the most studied is cyclophosphamide, an alkylating agent which has been shown to induce protective anti-tumour responses in both animal models [61,80,81] and in combination with vaccination in patients with metastatic melanoma [82] or breast cancer [83]. Peripheral blood samples from patients with metastatic cancers treated with metronomic doses of cyclophosphamide had a selective reduction in circulating Tregs and improved anti-tumour immunity [84]. Cyclophosphamide also potentiated anti-tumour responses in hormone-refractory prostate cancer patients [85], and there is some evidence that cyclophosphamide inhibits the optimal function of Tregs [86]. However, no effect on the total Treg pool, or Treg suppressive capacity, was observed with a single administration of cyclophosphamide in combination with bacilli Calmette–Guérin (BCG) immunotherapy in a Phase I trial of metastatic carcinoma [87]. In tumour-bearing mice, paclitaxel (an inhibitor of mitosis) given in metronomic doses with antigen-specific immunotherapy was able to reduce the number of Tregs independent of its known role in angiogenesis inhibition [88]. Previous studies in a murine mammary carcinoma model showed a small but significant immunomodulatory effect of doxorubicin or paclitaxel administration with anti-tumour vaccination [89]. Reduction in Treg numbers and suppressive capacity upon paclitaxel administration has also been confirmed in patients with non-small cell lung carcinoma [90].
An important consideration for therapies relying on Treg depletion is the rapid recovery. It has been described previously that the Treg compartment is repopulated in 48 days following depletion in mice [91], and similar effects have been reported in humans [76,92]. Furthermore, depletion of Tregs should be carried out with caution because the agents described may target both Teff and Treg populations, as CD25 may be expressed on more than 25% of human T cells [17]. Clinically, treatment of renal cell carcinoma with autologous T cells depleted of Tregs produced a transient reduction in peripheral Treg numbers, which coincided with increased antigen-independent and tumour-specific T cell responses. This suggests that although Treg depletion is generally transient, it can result in a potential therapeutic window for generating effective anti-tumour responses [93].
Interfering with some of the principal functions of Tregs is also being investigated. Cytotoxic T lymphocyte-associated antigen (CTLA)-4, expressed constitutively by Tregs, appears to be implicated in tumour control [2,12]. Studies targeting this molecule have been studied extensively in murine tumour models [94–96]. Clinically, ipilimumab (human anti-CTLA-4 mAb; MDX-010) has been effective in Phase III trials, increasing overall survival in patients with advanced metastatic melanoma [97]. However, patients with stage IV melanoma or renal cell carcinoma showed no reduction in Treg numbers or inhibited suppressive capacity, and it has been postulated that the effect of anti-CTLA-4 treatment is due to increased activation of Teffs [98]. Others have suggested that there is a Treg-depleting effect early after initial administration which permits the optimal induction of effector cell responses [99]. There is additional evidence that the suppressive mechanisms of CTLA-4 and Tregs are, at least in part, independent because even in the absence of CD25+ cells CTLA-4 blockade has been shown to induce anti-tumour responses [34]. Targeting glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR), a receptor expressed on Tregs, CD4+CD25– T cells and APCs, has also been explored [12]. With murine cells in vitro it was observed that administration of the cognate ligand (GITRL), as well as an agonistic antibody (DTA-1), resulted in the suppression of regulatory T cells [100,101]. Anti-GITR antibody treatment has been effective in promoting anti-tumour responses in the murine B16 tumour model [61] as well as in carcinogen-induced sarcoma in mice [102], but its effect in humans remains to be investigated.
Certain therapies have been shown to modulate the Teff/Treg ratio. A combination of Gvax [granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting B16 tumour cell-based vaccine] or Fl3vax (Flt3 ligand-secreting B16 tumour cell-based vaccine) with CTLA-4 blockade has been shown to be effective in increasing the ratio of Teff to Treg, resulting in rapid tumour rejection in murine tumour models [103,104]. In combination with anti-OX40, cyclophosphamide has been shown to have similar effects [105]. Interfering with the programmed cell death (PD)-1/PDL-1 axis has also been explored and has been shown to be effective in tumour rejection through a similar increase in the Teff/Treg ratio [106]. This suggests that approaches aimed at both interference with Treg function and enhancement of Teff activation and function may be equally important, and through synergy yield effective anti-tumour responses.
An important implication to therapeutic targeting of Tregs is their involvement in maintaining peripheral tolerance. A number of studies have shown that depleting Tregs or interfering with their function may lead to the development of mild to severe autoimmune reactions in humans. Similarly, anti-CD25 antibody treatment in mice resulted in increased anti-tumour immunity but also a concomitant increase in autoimmunity [69]. These potential side effects need to considered and monitored in future clinical studies.
Conclusions
The immune system shapes established tumours, resulting in an increased ability to withstand immunological attack. When a dynamic state of equilibrium is established upon interaction of the immune system with the developing tumour, strong T cell responses are key to acquiring long-lasting tumour-specific immunity. The mechanisms of establishing tumour-specific tolerance, with a principal contribution from Tregs, are becoming increasingly a focus of research, yet the field is replete with unanswered questions. Development of new therapeutic strategies that interfere with the generation and function of Tregs, or result in their specific depletion, will require improved targeting of Tregs, a better understanding of the role these cells play in different tumour types and clearer insight into the molecular mechanisms that are responsible for their accumulation and survival within tumours. Based on the data currently available, it is clear that the timing and dose of certain therapeutics is of key importance. One of the major caveats for cancer immunotherapy is the incidence of adverse reactions, such as hypersensitivity and autoimmunity, which arise as a consequence of targeting Tregs. Development of effective therapeutic strategies to target cancer will rely on combining control of Treg function and Teff/Treg ratios, while minimizing the loss of peripheral tolerance. This remains a significant challenge for the future of cancer treatment.
Acknowledgments
ARF is supported by a Medical Research Council Program grant held by RJN and GJG.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber TH, Podack ER. A critical analysis of the tumour immunosurveillance controversy for 3-MCA- induced sarcomas. Br J Cancer. 2009;101:381–386. doi: 10.1038/sj.bjc.6605198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Betts G, Jones E, Junaid S, et al. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61:1163–1171. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–924. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 8.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 9.Radoja S, Rao TD, Hillman D, Frey AB. Mice bearing late-stage tumors have normal functional systemic T cell responses in vitro and in vivo. J Immunol. 2000;164:2619–2628. doi: 10.4049/jimmunol.164.5.2619. [DOI] [PubMed] [Google Scholar]
- 10.Kurt RA, Park JA, Panelli MC, et al. T-lymphocytes infiltrating sites of tumor rejection and progression display identical V-beta usage but different cytotoxic activities. J Immunol. 1995;154:3969–3974. [PubMed] [Google Scholar]
- 11.Kurt RA, Park JA, Schluter SF, Marchalonis JJ, Akporiaye ET. TCR V-beta usage and clonality of T cells isolated from progressing and rejected tumor sites before and after in vitro culture. Int Immunol. 2000;12:639–646. doi: 10.1093/intimm/12.5.639. [DOI] [PubMed] [Google Scholar]
- 12.Zou WP. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 13.Menetrier-Caux C, Curiel T, Faget J, Manuel M, Caux C, Zou W. Targeting regulatory T cells. Target Oncol. 2012;7:15–28. doi: 10.1007/s11523-012-0208-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 15.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 16.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nizar S, Copier J, Meyer B, et al. T-regulatory cell modulation: the future of cancer immunotherapy? Br J Cancer. 2009;100:1697–1703. doi: 10.1038/sj.bjc.6605040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunological self-tolerance maintained by activated T-cells expressing Il-2 receptor alpha-chains (Cd25) – breakdown of a single mechanism of self-tolerance causes various autoimmune-diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 19.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3(+) T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou XY, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1104. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsov YP, Niec RE, Josefowicz S, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4(+) T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Miyao T, Floess S, Setoguchi R, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Elkord E, Sharma S, Burt DJ, Hawkins RE. Expanded subpopulation of FoxP3(+) T regulatory cells in renal cell carcinoma co-express Helios, indicating they could be derived from natural but not induced Tregs. Clin Immunol. 2011;140:218–222. doi: 10.1016/j.clim.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47:1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton AM, Korty PE, Tran DQ, et al. Expression of helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3(+) T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel TJ, Coukos G, Zou LH, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 31.Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 32.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 33.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutmuller RPM, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizukami Y, Kono K, Kawaguchi Y, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3(+) regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama T, Kono K, Izawa S, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 37.Facciabene A, Peng XH, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–U141. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 38.Tan MCB, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadzadeh M, Rosenberg ST. IL-2 administration increases CD4(+)CD25(hi) Foxp3(+) regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen HK, Donskov F, Nordsmark M, Marcussen N, von der Maase H. Increased Intratumoral FOXP3-positive regulatory immune cells during interleukin-2 treatment in metastatic renal cell carcinoma. Clin Cancer Res. 2009;15:1052–1058. doi: 10.1158/1078-0432.CCR-08-1296. [DOI] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4(+)CD25(+) regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen WJ, Jin WW, Hardegen N, et al. Conversion of peripheral CD4(+)CD25(–) naive T cells to CD4(+)CD25(+) regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu VC, Wong LY, Jang T, et al. Tumor evasion of the immune system by converting CD4(+) CD25(–) T cells into CD4(+) CD25(+) T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 44.Curti A, Pandolfi S, Valzasina B, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25(–) into CD25(+) T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 45.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hindley JP, Ferreira C, Jones E, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–746. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 48.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4(+)CD25(–) lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 49.Greene MI, Fujimoto S, Sehon AH. Nature of immunosuppressor T cells and their factors in tumor-bearing host (Tbh) J Immunol. 1976;116:791–806. [PubMed] [Google Scholar]
- 50.Clemente CG, Mihm MG, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8(+) tumor-infiltrating lymphocytes and a high CD8(+)/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 53.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 54.Petersen RP, Carnpa MJ, Sperlazza J, et al. Tumor infiltrating FOXP3(+) regulatory T-cells are associated with recurrence in pathologic stage INSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 56.Griffiths RW, Elkord E, Gilham DE, et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3(+) regulatory T cells increases during the progression of pancreatic ductal adenocarcinorna and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 58.Perrone G, Ruffini PA, Catalano V, et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer. 2008;44:1875–1882. doi: 10.1016/j.ejca.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Jordanova ES, Gorter A, Ayachi O, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8(+)/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 60.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+T cell immunity against a tumor/self-antigen is augmented by CD4(+) T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3(+) T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 63.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3(+) regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 65.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4(+) T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 66.Zhang YL, Li J, Mo HY, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4–15. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 68.Pellegatta S, Cuppini L, Finocchiaro G. Brain cancer immunoediting: novel examples provided by immunotherapy of malignant gliomas. Exp Rev Anticancer Ther. 2011;11:1759–1774. doi: 10.1586/era.11.102. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25(+)CD4(+) T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 70.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25(+) regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 71.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 72.Comes A, Rosso O, Orengo AM, et al. CD25(+) regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 73.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4(+)CD25(+) regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 74.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Cancer Vaccines. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 75.Talpur R, Jones DM, Alencar AJ, et al. CD25 expression is correlated with histological grade and response to denileukin diftitox in cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:575–583. doi: 10.1038/sj.jid.5700122. [DOI] [PubMed] [Google Scholar]
- 76.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerena-Lewis M, Crawford J, Bonomi P, et al. A phase II trial of denileukin diftitox in patients with previously treated advanced non-small cell lung cancer. Am J Clin Oncol Cancer Clin Trials. 2009;32:269–273. doi: 10.1097/COC.0b013e318187dd40. [DOI] [PubMed] [Google Scholar]
- 78.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB(389)IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Powell DJ, Felipe-Silva A, Merino MJ, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida S, Nomoto K, Himeno K, Takeya K. Immune-response to syngeneic or autologous testicular cells in mice. 1. Augmented delayed footpad reaction in cyclophosphamide-treated mice. Clin Exp Immunol. 1979;38:211–217. [PMC free article] [PubMed] [Google Scholar]
- 81.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4(+)CD25(+) regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 82.Berd D, Mastrangelo MJ. Effect of low-dose cyclophosphamide on the immune system of cancer patients – depletion of Cd4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 83.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4(+) CD25(+) regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lord R, Nair S, Schache A, et al. Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study. J Urol. 2007;177:2136–2140. doi: 10.1016/j.juro.2007.01.143. [DOI] [PubMed] [Google Scholar]
- 86.Lutsiak MEC, Semnani RT, De Pascalis R, Kashmiri SVS, Schlom J, Sabzevari H. Inhibition of CD4(+)25(+) T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 87.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4(+)CD25(+) regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4(+)CD25(+) T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen CA, Ho CM, Chang MC, et al. Metronomic chemotherapy enhances antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis. Mol Ther. 2010;18:1233–1243. doi: 10.1038/mt.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eralp Y, Wang X, Wang JP, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER-2/neu in a murine mammary carcinoma model. Breast Cancer Res Treat. 2004;88:S246–S247. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Dermawan K, Jin ML, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219–229. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Laurie KL, Van Driel IR, Gleeson PA. Role of CD4(+)CD25(+) immunoregulatory T cells in the induction of autoimmune gastritis. Immunol Cell Biol. 2002;80:567–573. doi: 10.1046/j.1440-1711.2002.01127.x. [DOI] [PubMed] [Google Scholar]
- 92.Powell DJ, de Vries CR, Allen T, Ahmadzadeh M, Rosenberg SA. Inability to mediate prolonged reduction of regulatory T cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy. J Immunother. 2007;30:438–447. doi: 10.1097/CJI.0b013e3180600ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thistlethwaite FC, Elkord E, Griffiths RW, et al. Adoptive transfer of T(reg) depleted autologous T cells in advanced renal cell carcinoma. Cancer Immunol Immunother. 2008;57:623–634. doi: 10.1007/s00262-007-0400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 95.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 97.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Mahony D, Janik JE. Commenton ‘analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade’. J Immunol. 2006;176:5136. doi: 10.4049/jimmunol.176.9.5136. [DOI] [PubMed] [Google Scholar]
- 100.Kim JD, Choi BK, Bae JS, et al. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–569. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- 101.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 102.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3(+)CD25(+)CD4(+) regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Curran MA, Allison JP. Tumor vaccines expressing Flt3 ligand synergize with CTLA-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmadzadeh M, Johnson L, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]