Abstract
ONO-4641 is a next-generation sphingosine 1-phosphate (S1P) receptor agonist selective for S1P receptors 1 and 5. The objective of the study was to characterize the immunomodulatory effects of ONO-4641 using preclinical data. ONO-4641 was tested in both in-vitro pharmacological studies as well as in-vivo models of transient or relapsing–remitting experimental autoimmune encephalomyelitis (EAE). In vitro, ONO-4641 showed highly potent agonistic activities versus S1P receptors 1 and 5 [half maximal effective concentration (EC50) values of 0·0273 and 0·334 nM, respectively], and had profound S1P receptor 1 down-regulating effects on the cell membrane. ONO-4641 decreased peripheral blood lymphocyte counts in rats by inhibiting lymphocyte egress from secondary lymphoid tissues. In a rat experimental autoimmune encephalomyelitis (EAE) model, ONO-4641 suppressed the onset of disease and inhibited lymphocyte infiltration into the spinal cord in a dose-dependent manner at doses of 0·03 and 0·1 mg/kg. Furthermore, ONO-4641 prevented relapse of disease in a non-obese diabetic mouse model of relapsing-remitting EAE. These observations suggest that ONO-4641 may provide therapeutic benefits in the treatment of multiple sclerosis.
Keywords: experimental autoimmune encephalomyelitis, multiple sclerosis, ONO-4641, sphingosine 1-phosphate, sphingosine 1-phosphate receptor 1
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease affecting the central nervous system (CNS) through demyelination and neurodegeneration. The aetiology of MS is notoriously heterogeneous; however, the generation of autoreactive lymphocytes directed against myelin epitopes is considered to be the initiating event. The demyelinating lesions of MS contain lymphocyte infiltrates, and these cells are involved intimately in tissue injury [1,2].
Recent studies with sphingosine 1-phosphate receptor 1 (S1P1)-deficient mice have demonstrated that S1P1 is required for lymphocyte recirculation into the blood stream [3,4]. The ligand for this receptor, sphingosine 1-phosphate (S1P), is present at high concentrations (100–400 nM) in the blood [5,6]. A concentration gradient of S1P induces the migration of lymphocytes from the lymphoid tissues into the blood [7,8]. Fingolimod (FTY720) is a first-in-class S1P receptor agonist that has shown efficacy in clinical trials of MS [9,10], and is approved as an oral drug for the prevention of relapsing forms of MS. Fingolimod is phosphorylated by sphingosine kinase 2 to produce the active metabolite fingolimod-phosphate [11], which is a non-selective S1P receptor agonist for four S1P receptors: S1P1, S1P3, S1P4 and S1P5 [12,13]. The therapeutic effects of fingolimod are thought to be mediated via S1P1. Conversely, rodent studies suggest that activity on S1P3 is associated with fingolimod's cardiovascular side effects [14,15]. The medicinal chemical strategy was thus to search for S1P receptor agonists devoid of S1P3 to reduce potential side effects.
In our efforts to identify S1P receptor agonists without S1P3 activity, we developed a next-generation S1P receptor agonist selective for S1P1 and S1P5, ONO-4641, as a potential treatment of lymphocyte-mediated diseases. MS is characterized by infiltration of lymphocytes and it is anticipated that ONO-4641 interrupts the development of the disease process. Clinical studies are under way to evaluate ONO-4641 for the treatment of MS. In this paper, we report preclinical data of ONO-4641. Effects of ONO-4641 on lymphocyte circulation and its efficacy in experimental autoimmune encephalomyelitis (EAE), animal models for MS, were investigated and our results support the therapeutic benefits of ONO-4641.
Materials and methods
Chemicals
ONO-4641 was provided by Ono Pharmaceutical Co. Ltd (Mishima, Osaka, Japan). S1P and SEW2871 were obtained from Alexis (San Diego, CA, USA) and Merck KGaA (Darmstadt, Germany), respectively. Prednisolone was obtained from Sigma-Aldrich (St Louis, MO, USA).
Cell lines
Chinese hamster ovary-K1 (CHO-K1) cells expressing human S1P1 (hS1P1), hS1P2, hS1P3, hS1P4, hS1P5 and rat S1P1 (rS1P1) were generated by transfecting CHO-K1 cells with the pIRESneo-Myc vector containing full-length cDNA for each S1P receptor using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA).
Experimental animals
Female LEW/CrlCrlj rats were obtained from Charles River Laboratories (Yokohama, Japan). Female non-obese diabetic (NOD)/ShiJcl mice were obtained from CLEA Japan Inc. (Tokyo, Japan). Animal studies were conducted in compliance with the Guidelines for Animal Studies, established by Research Headquarters, Ono Pharmaceutical Co., Ltd (Osaka, Japan).
Competitive binding assay
Competitive binding experiments of ONO-4641 for the specific binding of [33P]-S1P (American Radiolabeled Chemicals, St Louis, MO, USA) to hS1P1, hS1P2, hS1P3, hS1P4, hS1P5 and rS1P1 were performed essentially as described previously [16].
Measurement of cAMP accumulation
Cells expressing hS1P1, hS1P5 or rS1P1 and control cells were treated with forskolin (Sigma-Aldrich) and incubated with ONO-4641 or S1P at 37°C for 30 min. The cells were then lysed and the cyclic AMP (cAMP) level measured by enzyme-linked immunosorbent assay (ELISA).
Analysis of S1P1 receptor expression
Cells expressing hS1P1 were treated with ONO-4641, S1P or SEW2871 before being fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS). The cells were then incubated first with the primary antibody (clone 9E10, Sigma-Aldrich) against the Myc tag in the N-terminal domain of the receptor, and subsequently with the fluorescence-labelled secondary antibody (Invitrogen). The fluorescence intensity of antibodies bound to receptors was measured using a flow cytometer [fluorescence activated cell sorter (FACS)Calibur; BD Biosciences, San Jose, CA, USA].
Peripheral blood lymphocyte count
Female Lewis rats received a single oral dose at 0·01, 0·03, 0·1, 0·3 or 1 mg/kg of ONO-4641 or 0·5% methylcellulose (MC). At baseline and 2, 4, 8, 12, 24, 48, 72, 96 and 120 h after dosing, 200 μl blood was collected via the tail vein. The peripheral blood lymphocyte count was measured with an automated haematology analyser (SF-3000; Sysmex Corporation, Kobe, Japan). Female NOD mice received a single oral dose at 0·01, 0·03 and 0·1 mg/kg of ONO-4641 or 0·5% MC. Twenty-four h after administration, blood was collected from abdominal vena cava of the mice.
Measurement of lymphocyte accumulation in secondary lymphoid tissues
Cells derived from secondary lymphoid tissues removed from Lewis rats were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Dojindo Laboratories, Kamimashiki-gun, Japan) and suspended at a cell density of 5 × 107 cells/ml. ONO-4641 (0·1 mg/kg) or 0·5% MC was administered orally to Lewis rats; 2·5 h after administration, 1 ml of the adoptive cell suspension was transferred intravenously to the rats. Inguinal, axillary and mesenteric lymph nodes were removed and blood was collected 1·5 h after adoptive cell transfer. The numbers of transferred lymphocytes were calculated from the counted number of labelled cells and Flow-Count standard beads (Beckman Coulter, Brea, CA, USA) in each sample, which were measured with a flow cytometer.
Rat EAE model
Myelin basic protein (MBP; Sigma-Aldrich) was dissolved in dead tubercle bacilli (Difco Laboratories, Detroit, MI, USA) suspension to prepare 20 μg/ml suspension at dead tubercle bacilli concentration of 1000 μg/ml. This suspension was mixed with an equal volume of Freund's complete adjuvant (CFA) (Chemicon International, Temecula, CA, USA) to prepare as an inducer. Female Lewis rats were immunized by subcutaneous administration of the inducer at a volume of 0·1 ml into the footpad. ONO-4641 (0·01, 0·03 and 0·1 mg/kg), prednisolone (3 mg/kg) or 0·5% MC was administered orally once daily from immunization days 4–21. Neurological symptoms were scored in accordance with a method described by Rausch et al. [17]. More specifically, the severity of paralysis was graded on a scoring scale to determine the clinical score (normal: 0; flaccid tail: 1; hindlimb weakness or ataxia: 2; hindlimb complete paralysis: 3). Rats were also immunized by the inducer into the footpad and the lumbosacral spinal cord was removed on immunization day 16 and fixed in formalin. Haematoxylin and eosin (H&E)-stained specimens of the lumbosacral spinal cord were prepared and the number of perivascular cellular infiltrates was measured to assess cellular infiltration [18].
Mouse relapsing–remitting EAE model
Myelin oligodendrocyte glycoprotein 35–55 (MOG; AnaSpec, Fremont, CA, USA) was dissolved in PBS to prepare 2 mg/ml suspension. This suspension was mixed with an equal volume of CFA (Difco Laboratories) containing 4 mg/ml of dead tubercle bacilli to prepare as an inducer. NOD mice were immunized into the left footpad with the inducer at a volume of 0·05 ml. This was followed by an intravenous injection of 300 ng of pertussis toxin (PTX; Sigma-Aldrich, Inc.). A second PTX injection was administered 48 h later. Animals that achieved remission after the initial onset were selected and grouped, and ONO-4641 (0·01, 0·03 or 0·1 mg/kg) or 0·5% MC was administered orally to each group once daily for 8 weeks. Neurological symptoms were graded according to the degree of paralysis from 0 to 5, with 0 indicating normal, 1 flaccid tail, 2 partial paralysis of right hind leg, 3 paralysis of right hind leg, 4 paralysis of fore legs, and 5 moribundity or death [19]. A clinical score of 5 was assigned to dead animals until the end of observation.
Results
ONO-4641 has an agonistic action for S1P1 and S1P5
In order to investigate the binding affinity of ONO-4641 for S1P receptors, competitive binding experiments of this substance were performed for the specific binding of [33P]-S1P to S1P receptors (Table 1). ONO-4641 inhibited the specific binding of [33P]-S1P to hS1P1 and hS1P5 in a concentration-dependent manner, with Ki values of 0·626 and 0·574 nM, respectively. ONO-4641 also inhibited the specific binding to hS1P4, but the Ki value of ONO-4641 was 28·8 nM, approximately 50 times higher than those for hS1P1 and hS1P5. Conversely, the inhibitory rate of ONO-4641 on the specific binding of [33P]-S1P to hS1P2 and hS1P3 was less than 50% even at a concentration of 10 μM. To investigate the species difference between human and rat, the binding affinity of ONO-4641 for rS1P1 was examined. The Ki value of ONO-4641 for rS1P1 was 0·772 nM, indicating that ONO-4641 has a high binding affinity for rS1P1 comparable to that for hS1P1 (Table 1).
Table 1.
Binding affinities of ONO-4641 and sphingosine 1-phosphate (S1P) for S1P receptors
| Ki (nM) (95% CI) | ||
|---|---|---|
| Receptor | ONO-4641 | S1P |
| hS1P1 | 0·626 (0·567–0·694) | 0·221 (0·195–0·249) |
| hS1P2 | >5450 | 0·543 (0·453–0·649) |
| hS1P3 | >5630 | 0·0507 (0·0459–0·0560) |
| hS1P4 | 28·8 (25·8–32·2) | 5·57 (5·11–6·07) |
| hS1P5 | 0·574 (0·513–0·644) | 0·225 (0·207–0·243) |
| rS1P1 | 0·772 (0·656–0·906) | 0·298 (0·253–0·350) |
The half maximal inhibitory concentration (IC50) values of ONO-4641 and S1P for the specific binding of [33P]-S1P to S1P receptors were determined using Prism version 4·02 (GraphPad Software). The Ki values were calculated from the obtained IC50 values and are expressed with the respective 95% confidence intervals (CI) based on the results from experiments performed in triplicate.
Next, an agonistic action of ONO-4641 on S1P1 and S1P5 was investigated based on the inhibitory effect of forskolin-stimulated cAMP accumulation, because it was reported that S1P inhibited adenylate cyclase via S1P1 and S1P5 coupled with Gi protein [20]. The half maximal effective concentration (EC50) values of ONO-4641 for hS1P1 and hS1P5 were 0·0273 and 0·334 nM, respectively. ONO-4641 also had an agonistic action on rS1P1 with an EC50 value of 0·0286 nM, suggesting that there was no difference between human and rat in the agonistic action of ONO-4641 for S1P1 (Table 2).
Table 2.
Agonistic action of ONO-4641 on sphingosine 1-phosphate (S1P1) and S1P5 receptors
| EC50 (nM) (95% CI) | |||
|---|---|---|---|
| hS1P1 | hS1P5 | rS1P1 | |
| ONO-4641 | 0·0273 (0·0121–0·0617) | 0·334 (0·244–0·456) | 0·0286 (0·00972–0·0843) |
| S1P | 0·627 (0·280–1·40) | 0·681 (0·461–1·01) | 0·518 (0·360–0·746) |
The half maximal effective concentration (EC50) values of ONO-4641 and S1P for the inhibitory effects on cAMP accumulation in cells expressing hS1P1, hS1P5, and rS1P1 were determined using Prism version 4·02 (GraphPad Software). The EC50 values are expressed with their respective 95% confidence intervals (CI) based on the results from experiments performed in triplicate.
ONO-4641 induces S1P1 down-regulation
It was reported that S1P receptor agonists such as fingolimod and SEW2871 exert their effects by down-regulating S1P1 on the cell membrane [21–23]. We examined whether ONO-4641 also induces S1P1 down-regulation on the cell membrane. Both the natural ligand S1P and S1P1-selective agonist SEW2871 were used as control. In cells expressing S1P1, ONO-4641 induced S1P1 down-regulation in a concentration-dependent manner and by approximately 90% at concentration of 25 nM (Fig. 1a). S1P and SEW2871 also decreased the expression of S1P1 receptors on the cell membrane. The EC50 values of ONO-4641, S1P, and SEW2871 were 0·778, 132 and 118 nM, respectively (Fig. 1b).
Fig. 1.
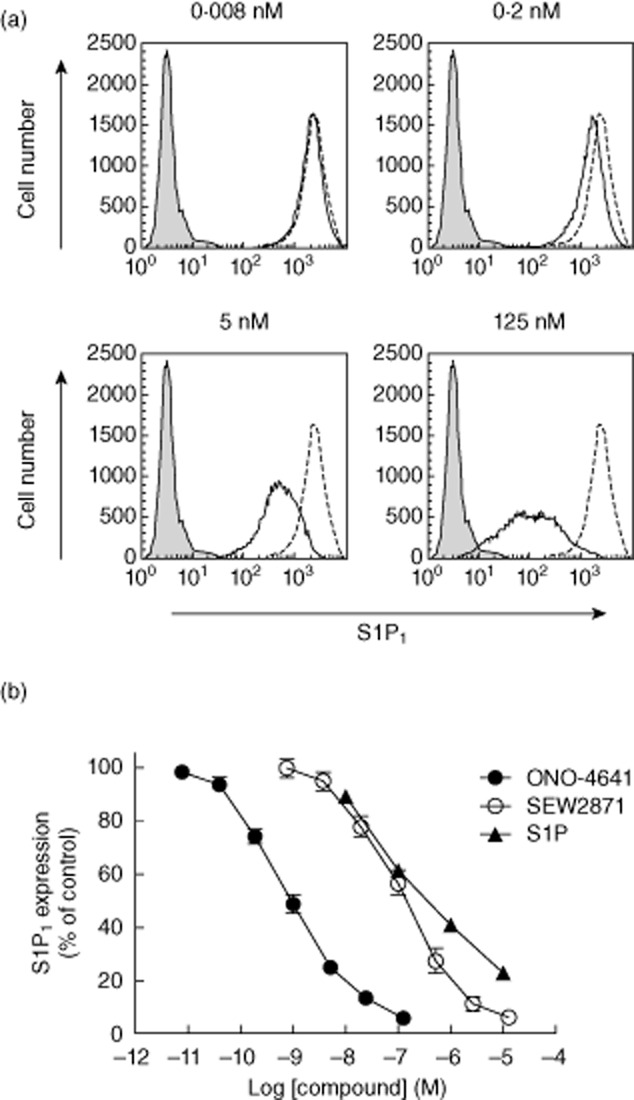
(a) Chinese hamster ovary (CHO)-K1 cells stably expressing human sphingosine 1-phosphate (hS1P1) were incubated at 37°C for 60 min with ONO-4641 (0·008–125 nM, open black solid line) or control (open black dotted line). CHO-K1 cells that do not express hS1P1, as negative control group, are also shown (closed grey line). Data are representative of three independent experiments. (b) S1P1 down-regulating effects of ONO-4641 (0·008–125 nM), S1P (10–10 000 nM) and SEW2871 (0·8–12 500 nM) were evaluated in hS1P1-expressing cells. The expression (%) of S1P1 receptors was relative to a mean S1P1 receptor-specific fluorescence intensity taken as 100% in the control group. The S1P1 expressions in the ONO-4641 (•), S1P (▴), and SEW2871 (○) are expressed as mean ± standard error based on the results from experiments performed in triplicate.
ONO-4641 decreases peripheral blood lymphocytes and induces accumulation of lymphocytes in lymphoid tissues
We examined whether ONO-4641 decreases peripheral blood lymphocyte count similar to other S1P receptor agonists [24–26]. In normal Lewis rats, the peripheral blood lymphocyte count was decreased over time, and the lowest count was observed 12 h after administration in the ONO-4641-treated groups. ONO-4641 decreased peripheral blood lymphocytes dose-dependently and had the maximum effect at doses of 0·1 mg/kg or more (Fig. 2).
Fig. 2.
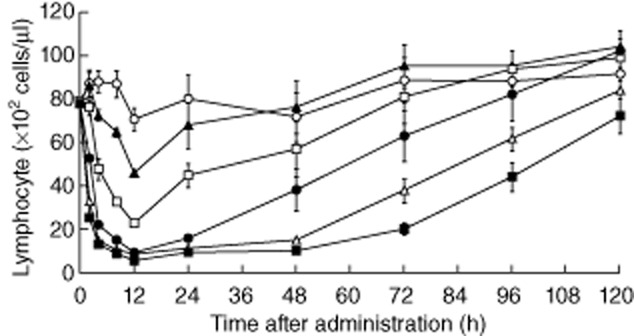
Female Lewis rats received a single oral dose of 0·01, 0·03, 0·1, 0·3 or 1 mg/kg of ONO-4641 or 0·5% methylcellulose (MC) (control). Blood was collected via the tail vein before (0) and 2, 4, 8, 12, 24, 48, 72, 96 and 120 h after administration to measure the peripheral blood lymphocyte count at each time-point. The peripheral blood lymphocyte count in the ONO-4641 0·01 mg/kg (▴), 0·03 mg/kg (□), 0·1 mg/kg (•), 0·3 mg/kg (▵) and 1 mg/kg (▪) groups and control group (○) is expressed as mean ± standard error (n = 6).
To address potential mechanisms of pharmacological effects of ONO-4641, we next examined whether ONO-4641 has an effect on circulating lymphocytes in secondary lymphoid tissues (Fig. 3), because other S1P receptor agonists have been reported to decrease peripheral blood lymphocytes by preventing lymphocyte egress from secondary lymphoid tissues [3,27,28]. The study was conducted using an adoptive cell transfer approach to verify ONO-4641-induced accumulation of circulating lymphocytes in lymphoid tissues of rats at a dose of 0·1 mg/kg, the dose associated with the maximum decrease in peripheral blood lymphocytes. The numbers of transferred lymphocytes in inguinal, axillary and mesenteric lymph nodes were significantly higher in the ONO-4641 0·1 mg/kg group compared with that in the control group. In contrast, the number of the transferred lymphocytes in peripheral blood was significantly lower in the ONO-4641 0·1 mg/kg group (Fig. 3).
Fig. 3.
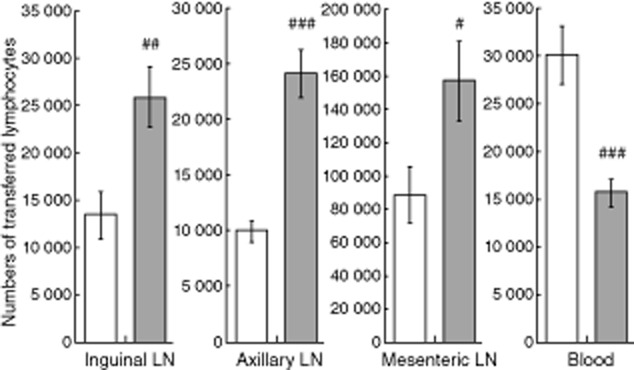
Female Lewis rats received a single oral dose of 0·5% methylcellulose (MC) (control) or ONO-4641 (0·1 mg/kg). The adoptive cell suspension was injected intravenously to transfer adoptive cells 2·5 h after administration of 0·5% MC or ONO-4641. Inguinal, axillary and mesenteric lymph nodes were removed and blood was collected 4 h after administration of 0·5% MC or ONO-4641. The number of transferred lymphocytes in inguinal, axillary and mesenteric lymph nodes, and in peripheral blood, was calculated from the flow cytometry result. The data in the control group (□) and the ONO-4641 0·1 mg/kg group (▪) are expressed as mean ± standard error from 10 animals in each group. Student's t-test was performed for comparison of the data of each tissue between the control and the ONO-4641 0·1 mg/kg groups, with a P-value of less than 5%; #P < 0·05; ##P < 0·01; ###P < 0·001.
ONO-4641 suppresses onset of disease and inhibits cellular infiltration into spinal cord in an EAE model
To assess the therapeutic impact of ONO-4641, we investigated the disease-preventive effect of ONO-4641 in a rat EAE model. Lewis rats were immunized with MBP in adjuvant. In the control group, typical monophasic EAE developed with transient paralysis. EAE was not observed in rats injected with adjuvant alone (non-induction group). The clinical scores of the ONO-4641 0·03 and 0·1 mg/kg groups remained lower than that in the control group. The maximum clinical scores decreased dose-dependently in the ONO-4641 groups and those in the ONO-4641 0·03 and 0·1 mg/kg groups were significantly lower than that in the control group (Fig. 4a). Specifically, paralysis was inhibited completely in seven of eight animals in the ONO-4641 0·1 mg/kg group (Table 3).
Fig. 4.
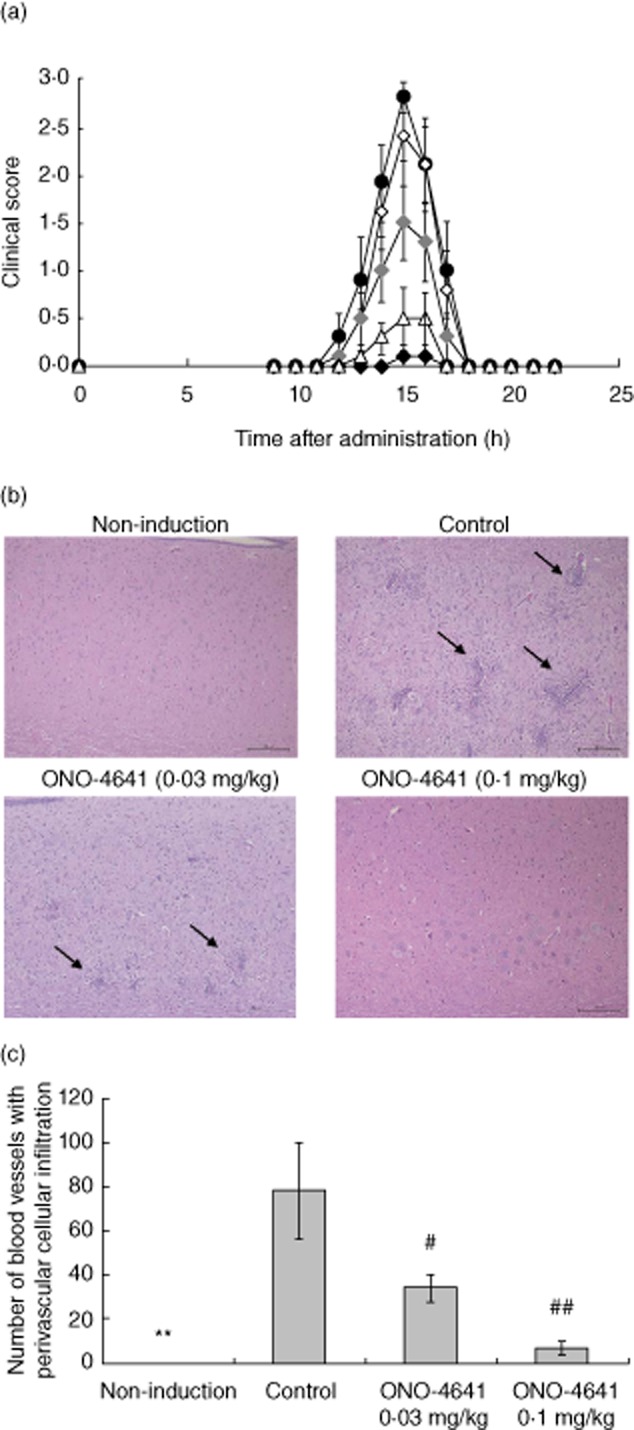
(a) Time profile of clinical score in control and ONO-4641 groups. In female Lewis rats, the inducer was administered subcutaneously into the footpad; 0·5% methylcellulose (MC), ONO-4641 (0·01, 0·03 or 0·1 mg/kg) or prednisolone 3 mg/kg was administered orally once daily from immunization days 4–21. Neurological symptoms were assessed on the day of immunization and from immunization days 9–22. The clinical score in the control (•), ONO-4641 0·01 mg/kg (◊), 0·03 mg/kg (♦), 0·1 mg/kg (♦) and prednisolone (▵) are expressed as mean ± standard error (s.e.) (n = 8). (b) Representative photographs of perivascular cellular infiltrates in the lumbosacral spinal cord. Cellular infiltration was assessed based on the number of perivascular cellular infiltrates. The inducer was administered into the footpad in ONO-4641 or control groups. Adjuvant alone was administered in the non-induction group. ONO-4641 (0·03 and 0·1 mg/kg) or 0·5% MC was administered orally once daily from immunization days 4–15. On immunization day 16, the spinal cord was removed to prepare haematoxylin and eosin (H&E)-stained specimens. The number of perivascular cellular infiltrates was measured to assess cellular infiltration (scale bars, 100 μm). Arrows indicate some of the perivascular cellular infiltrates. (c) For the H&E-stained specimens, the number of perivascular cellular infiltrates was measured under 40-fold magnification. The measurement range was within 1 cm from the proximal end of the long axis of the spinal cord. The number of perivascular cellular infiltrates is expressed as mean ± s.e. (n = 8). Dunnett's test was performed for comparison between the control and ONO-4641 groups, with a P-value of less than 5%; #P < 0·05; ##P < 0·01. A t-test was performed for comparison between the control and non-induction groups, with a P-value of less than 5%. **P < 0·01.
Table 3.
Maximum clinical score in the rat experimental autoimmune encephalomyelitis (EAE) model
| Group | Maximum clinical score |
|---|---|
| Control group | 2·9 ± 0·13 |
| ONO-4641 0·01 mg/kg group | 2·6 ± 0·26 |
| ONO-4641 0·03 mg/kg group | 1·9 ± 0·35# |
| ONO-4641 0·1 mg/kg group | 0·1 ± 0·13### |
| Prednisolone 3 mg/kg group | 0·6 ± 0·32*** |
For each animal, the highest clinical score recorded during the observation period was used as the maximum clinical score. The maximum clinical score is expressed as mean ± standard error from eight animals in each group. The Steel test was performed for comparison between the control and ONO-4641 groups, with a P-value of less than 5%; #P < 0·05; ###P < 0·001. Wilcoxon's rank sum test was performed for comparison between the control and prednisolone groups, with a P-value of less than 5%; ***P < 0·001.
We also investigated the effect of ONO-4641 on cellular infiltration into the lumbosacral spinal cord of the EAE model (Fig. 4b,c). On immunization day 16, the number of perivascular cellular infiltrates in the lumbosacral spinal cord, which was measured to assess cellular infiltration, was 78·3 ± 21·31 in the control group. Among these specimens, those in the control group were observed for CD3-positive T cells in cell-infiltrated lesions (data not shown). In contrast, in animals of the non-induction group, no perivascular cellular infiltrates were found in the lumbosacral spinal cord. In the ONO-4641 0·03 and 0·1 mg/kg groups, the numbers were 34·4 ± 6·53 and 7·3 ± 2·86, respectively, decreasing dose-dependently and differing significantly from that in the control group (Fig. 4b,c).
ONO-4641 prevents relapse in relapsing–remitting EAE model
We next investigated the effect of ONO-4641 in a NOD mouse model of relapsing–remitting EAE. In normal NOD mice, the number of peripheral blood lymphocytes was decreased by approximately 20, 60 and 80% at 24 h after a single oral dose of 0·01, 0·03 and 0·1 mg/kg of ONO-4641, respectively (Fig. 5a). In the control group of the NOD mouse model of relapsing–remitting EAE, the relapse rate was 90·0% (nine of 10 animals), and two of the nine animals died. The cumulative clinical score in the control group was 65·4 ± 18·50. In contrast, none of animals in the ONO-4641 0·1 mg/kg group had a relapse; that is, ONO-4641 completely prevented relapse at a dose of 0·1 mg/kg (Fig. 5b). In the ONO-4641 groups, two of the nine animals in the 0·01 mg/kg died. In the ONO-4641 0·01, 0·03 and 0·1 mg/kg groups, the cumulative clinical scores were 65·4 ± 22·93, 9·8 ± 6·60 and 0·0 ± 0·00, respectively, indicating that ONO-4641 was effective in preventing relapse in a dose-dependent manner ( Fig. 5, Table 4).
Fig. 5.
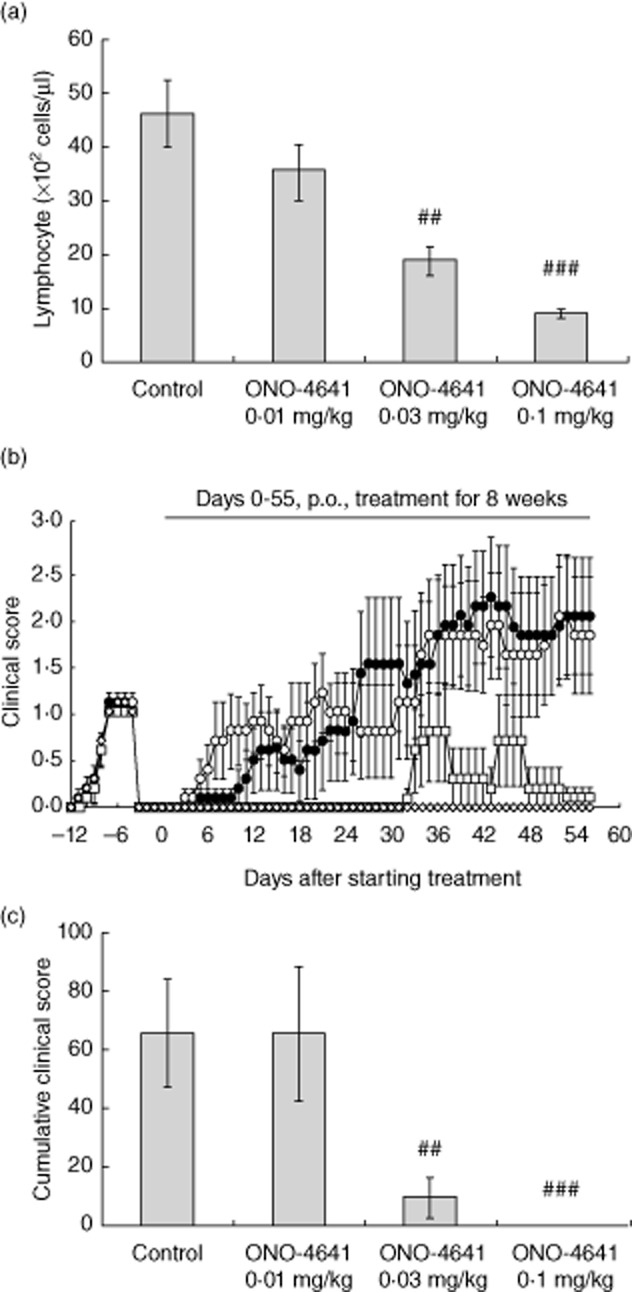
(a) Effect of ONO-4641 (0·01, 0·03 or 0·1 mg/kg) on number of peripheral blood lymphocytes in normal non-obese diabetic (NOD) mice 24 h after a single oral administration. Data are represented as mean ± standard error (s.e.) (n = 5). The P-values were determined by Dunnett's test; ###P < 0·001. (b) From the day of grouping (day 0 of treatment) to day 55 of treatment, 0·5% methylcellulose (MC) or ONO-4641 (0·01, 0·03 or 0·1 mg/kg) was administered orally to each group once daily for 8 weeks. Animals were observed for neurological symptoms on the day of immunization and from day 7 of immunization to day 56 of treatment. Clinical scores were expressed as the mean value ± s.e. for the control group (○, n = 10), ONO-4641 0·01 mg/kg group (•, n = 10), ONO-4641 0·03 mg/kg group (□, n = 9), ONO-4641 0·1 mg/kg group (◊, n = 10). (c) The cumulative value of the clinical scores for each animal obtained during the period from the day of grouping to the end of the experiment was evaluated as the cumulative clinical score and expressed as the mean value ± s.e. The Steel test was used to compare the scores between the control and ONO-4641 groups, in which a P < 0·05 was considered statistically significant (##P < 0·01; ###P < 0·001).
Table 4.
Relapse rates in the mouse model of relapsing-remitting experimental autoimmune encephalomyelitis (EAE)
| Group (no. of animals) | Relapse rates |
|---|---|
| Control group (n = 10) | 90·0% |
| ONO-4641 0·01 mg/kg group (n = 10) | 80·0% |
| ONO-4641 0·03 mg/kg group (n = 9) | 22·2% |
| ONO-4641 0·1 mg/kg group (n = 10) | 0·0% |
During the period from the day of grouping to the end of experiment, clinical scores of 1 or greater were regarded as relapse, and the percentage of animals that had relapsed is presented as the relapse rate.
Discussion
In this study, we investigated in-vitro and in-vivo pharmacological properties of ONO-4641, which is currently under clinical investigation in patients with MS. ONO-4641 selectively activates S1P1 and S1P5, and showed a more potent S1P1 down-regulating effect than the natural ligand, S1P. There was a difference between the binding affinity (Ki value of 0·626 nM) and the agonistic action (EC50 value of 0·0273 nM) of ONO-4641 on S1P1, while those values of S1P were similar. The reason for the difference is unclear; however, the potent agonistic action of ONO-4641 may account for the more potent S1P1 down-regulating effect than S1P. Fingolimod, a first-in-class S1P receptor agonist, has been approved recently for the treatment of relapsing MS. Fingolimod is a prodrug and phosphorylated reversibly to fingolimod-phosphate, the active moiety [11,12]. Fingolimod-phosphate activates S1P1 on lymphocytes via high-affinity receptor binding and subsequently induces S1P1 down-regulation on the cell membrane [21,22]. It is believed that down-regulation of S1P1 in lymphocytes by fingolimod-phosphate renders them unresponsive to the S1P gradient and prevents the egress from secondary lymphoid tissues [3,27,28]. It is presumed that the therapeutic actions of ONO-4641, like fingolimod, involve the functional antagonism of S1P1. In fact, ONO-4641 caused the S1P1 down-regulation and decreased peripheral blood lymphocyte count by inhibiting egress of lymphocytes from secondary lymphoid tissues. Furthermore, ONO-4641 suppressed the onset of disease and cellular infiltration into the spinal cord in a dose-dependent manner in the rat EAE model. As CD3-positive T cells were observed mainly in the infiltrated lesions and ONO-4641 inhibited the cellular infiltration into the lumbosacral spinal cord of the EAE model, the therapeutic effect of ONO-4641 involves the inhibition of T cell infiltration into the CNS. In this model, ONO-4641 had the maximum disease-preventive effect at 0·1 mg/kg, the dose required for the maximum decrease in peripheral blood lymphocytes. These findings suggest that ONO-4641 prevents the development of EAE by reducing recirculation of lymphocytes and prevention of their infiltration into the CNS.
We also investigated the effect of ONO-4641 in an animal model of relapsing–remitting MS that reflects the clinical course of the disease more accurately. A NOD mouse model of relapsing–remitting EAE, which features clear separation of remission and episodes [19], unlike other relapsing–remitting EAE models 29, was considered to be a more appropriate model to evaluate effect of test substance to induce and hold remission. In the relapsing–remitting EAE model, ONO-4641 was shown to prevent relapse at doses of 0·03 mg/kg or more and completely prevent relapse at a dose of 0·1 mg/kg. There is a correlation between the reduction in peripheral blood lymphocytes by ONO-4641 and prevention of the relapse of EAE in the NOD mouse model, indicating that ONO-4641 prevents relapse through the reduction of lymphocyte recirculation as with the rat EAE model.
It is noteworthy that the reduction in peripheral blood lymphocytes by ONO-4641 was reversible and the lymphocyte count recovered over time. Peripheral blood lymphocytes are composed of an array of lymphocyte subsets, out of which T and B cells play a key role in the adaptive immune system. These two subsets express S1P1 receptor highly [30,31], and are home to secondary lymphoid organs and tissues to monitor antigens. We confirmed that ONO-4641 decreased the number of these two subsets in the peripheral blood of rats (data not shown). We also investigated if ONO-4641 affects cellular functions of human T and B cells. Effects of ONO-4641 on T cell proliferation and interferon (IFN)-γ production in response to anti-CD3 plus anti-CD28 antibodies and on B cell proliferation and immunoglobulin (Ig)G production in response to anti-IgM plus interleukin (IL)-4 treatment were measured. ONO-4641 showed no obvious effects on T and B cell functions even at 1000 nM, which corresponds to more than 10 times the concentration needed for therapeutic effects (data not shown). Taken together, these observations suggest that ONO-4641 inhibits lymphocyte infiltration into the disease lesions by regulating lymphocyte recirculation without affecting their functions, and exerts immunomodulatory actions.
Recent studies with an EAE model using CNS-specific conditional S1P1 knock-out mice found that S1P1 was involved in the pathogenesis of EAE by regulating cells residing in the CNS, such as astrocytes [32,33], suggesting the possibility that S1P1 receptor agonists inhibit onset of EAE through not only immunological effects but neurological modulation. These studies demonstrated that functional antagonism of S1P1 on astrocytes could contribute to the efficacy of fingolimod in EAE. ONO-4641 acts on not only S1P1 but S1P5, which is expressed abundantly in the CNS, predominantly on oligodendrocytes [34,35]. CNS penetration of ONO-4641 after a single oral administration in rats was observed, supporting its access to S1P1/S1P5 in the CNS. Whether or not ONO-4641 has direct effects on the cells of the CNS remains to be investigated.
Data from rodent studies suggest that possible side effects in the cardiovascular and respiratory systems are caused mainly by S1P3 [14,15], although the functions of S1P receptors may differ in humans [36]. ONO-4641 does not have any affinity for S1P3, and is therefore expected to avoid potential side effects corresponding to S1P3. Additionally, it is anticipated that ONO-4641, which activates S1P1 and S1P5 as a direct agonist, allows more accurate control of its pharmacological activities in vivo compared to a prodrug such as fingolimod.
In conclusion, ONO-4641 is a potent and selective S1P1/S1P5 agonist that demonstrates clear therapeutic benefits in EAE models. Considering these preclinical results, it is expected that ONO-4641 shows therapeutic effects in patients with MS. ONO-4641 may also represent a new therapeutic option for the treatment of lymphocyte-mediated tissue inflammation such as in autoimmune diseases.
Acknowledgments
The authors would like to thank Dr Jerold Chun for critical review of the manuscript and helpful advice.
Disclosure
The authors are employees of Ono Pharmaceutical Co. Ltd.
References
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- 3.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 4.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 5.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 6.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. J Biochem. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 11.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 13.Yanagawa Y, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer's patches by FTY720-induced lymphocyte homing. Immunology. 1998;95:591–594. doi: 10.1046/j.1365-2567.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 15.Forrest M, Sun SY, Hajdu R, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 16.Sanada Y, Mizushima T, Kai Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS ONE. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rausch M, Hiestand P, Foster CA, Baumann DR, Cannet C, Rudin M. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- 18.Koh CS, Kwaan HC, Paterson PY. Neurovascular fibrinolytic activity in normal Lewis rats and rats with cell-transferred experimental allergic encephalomyelitis. J Neuroimmunol. 1990;28:189–200. doi: 10.1016/0165-5728(90)90012-c. [DOI] [PubMed] [Google Scholar]
- 19.Onuki M, Ayers MM, Bernard CC, Orian JM. Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc Res Tech. 2001;52:731–739. doi: 10.1002/jemt.1057. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto H, Takuwa N, Gonda K, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 21.Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 22.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 23.Jo E, Sanna MG, Gonzalez-Cabrera PJ, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu H, Takahashi M, Kaneko T, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111:222–229. doi: 10.1161/01.CIR.0000152101.41037.AB. [DOI] [PubMed] [Google Scholar]
- 26.Piali L, Froidevaux S, Hess P, et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337:547–556. doi: 10.1124/jpet.110.176487. [DOI] [PubMed] [Google Scholar]
- 27.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 28.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka H, Sugahara K, Shimano K, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–448. [PubMed] [Google Scholar]
- 30.Cinamon G, Matloubian M, Lesneski MJ, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 31.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 32.Choi JW, Gardell SE, Herr DR, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 34.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21:1503–1514. doi: 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- 35.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 36.Gergely P, Wallstrom E, Nuesslein-Hildesheim B, et al. Phase I study with the selective S1P1/S1P5 receptor modulator BAF312 indicates that S1P1 rather than S1P3 mediates transient heart rate reduction in humans. Mult Scler. 2009;15:S125–126. [Google Scholar]


