Abstract
Leptin modulates T cell function and plays an important role in autoimmune diseases. Our study aimed to explore the role of leptin and T helper type 17 (Th17) cells in Hashimoto's thyroiditis patients. Twenty-seven patients with Hashimoto's thyroiditis (HT) and 20 healthy controls were enrolled into the current study. A modest increase of plasma leptin in HT patients and the CD4+ T cell-derived leptin from HT patients was stronger than that from healthy controls. In HT patients, there are no statistically significant correlations between plasma leptin concentrations and the percentage of Th17 cells or the level of retinoic acid-related orphan receptor γt (RORγt), but strong positive correlations were observed between CD4+ T cell-derived leptin and the percentage of Th17 cells or the level of RORγt mRNA, and additionally significantly up-regulated leptin, interleukin (IL)17 and RORγt mRNA levels in the thyroid tissue. Furthermore, neutralization of leptin decreases the frequency of Th17 cells in vitro. Current study has revealed an increased leptin involvment in Hashimoto's thyroiditis associated with an increased number of Th17 cells.
Keywords: Hashimoto's thyroiditis, leptin, Th17 cells
Introduction
Hashimoto's thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is an organ-specific autoimmune disease characterized by the presence of goitre, lymphocytic infiltration and serum thyroid autoantibodies. HT is a complex disease caused by overt autoimmune response, multiple gene susceptibility and environmental factors. Previous reports have shown that autoreactive CD4+ T cells against thyroid antigens, especially interleukin (IL)-12-dependent T helper type 1 (Th1) cells, are involved in the disease progression of HT [1]. Furthermore, several reports, including our recent studies, have described that increased CD4+ Th17 cells might be involved in the pathogenesis of HT [2,3]. However, the mechanisms leading to increased Th17 cells in HT patients remain poorly understood.
Leptin is a 16 kDa non-glycosylated polypeptide encoded by the obese (ob) gene, consisting of four interconnected anti-parallel α-helices, which is in high similarity to members of the long-chain helical cytokines, such as IL-6, IL-11, IL-12 and granulocyte–colony-stimulating factor (G-CSF) [4–6]. As an adipocyte-derived hormone, leptin regulates energy homeostasis [7], neuroendocrine function [8], reproduction [9], angiogenesis [10] and haematopoiesis [11]. Many studies have characterized a critical role of leptin in T cell activation and function. We have shown recently that leptin plays an indispensable role in the maturation and function of dendritic cells and natural killer cells [12,13]. Accumulating evidence suggests that leptin acts as a proinflammatory cytokine in immune responses, which is involved in the pathogenesis of various autoimmune diseases [6]. Importantly, it has been reported that leptin is implicated in the pathogenesis of multiple sclerosis (MS) patients and experimental autoimmune encephalomyelitis (EAE) mice by altering the balance of Th1/Th2 and suppression of CD4+CD25+ regulatory T cell (Treg) proliferation [2,14,15]. However, little is known regarding the role of leptin in the disease pathogenesis of HT.
In this report, we investigate the change of plasma leptin and CD4+ T cell-derived leptin in HT patients, as well as the relationship between leptin and Th17 cells. We found that leptin neutralization affected the formation of Th17 cells in vitro. Our findings will provide further understanding regarding the role of leptin in the disease pathogenesis of HT.
Materials and methods
Individuals and samples
A total of 27 patients with Hashimoto's thyroiditis (HT) were enrolled into the study. The main clinical data of these patients are shown in Table 1. Body mass index (BMI), defined as weight in kilograms (kg) divided by the square of height in metres (m2), was calculated [16]. The serum concentrations of thyroid hormone, anti-thyroglobulin (Tg) and anti-thyroperoxidase (TPO) antibodies were measured by chemiluminescent immunoassay (Maglumi 2000 Plus) according to the manufacturer's protocol. Twenty age- and sex-matched healthy subjects were included as controls. Peripheral blood samples were obtained from all patients and healthy controls.
Table 1.
Clinical features of Hashimoto's thyroiditis (HT) patients and healthy controls included in the study
| HT patients | Healthy controls | Range | P | |
|---|---|---|---|---|
| Number | 27 | 20 | ||
| Sex | Female | Female | ||
| Age (years) | 49·96 ± 10·94 | 45·85 ± 10·14 | n.s. | |
| BMI (kg/m2) | 22·94 ± 2·81 | 23·67 ± 3·17 | n.s. | |
| FT3 (pmol/l) | 4·33 ± 0·94 | 4·72 ± 0·80 | 3·10–6·80 | n.s. |
| FT4 (pmol/l) | 16·37 ± 2·60 | 15·79 ± 2·25 | 12·00–22·00 | n.s. |
| Tg antibodies (IU/ml) | 272·1 ± 182·4 | 7·1 ± 2·1 | <30 | <0·001 |
| TPO antibodies (IU/ml) | 364·2 ± 275·8 | 15·7 ± 5·3 | <10 | <0·001 |
Data correspond to the arithmetic mean ± standard deviation, BMI: body mass index; n.s.: not significant; Tg: thyroglobulin; TPO: thyroperoxidase.
Thyroid glands were obtained from six HT patients who were undergoing thyroidectomy. All the patients were positive for Tg-antibody and TPO-antibody and had normal hormone levels, except for one patient (FT4: 7·92 pmol/l). Two of the patients were bilateral goitre; others were unilateral. Lymphocytic infiltration was detected in the goitres. Thyroid tissue from the patient with simple goitre was used as control.
Ethical approval was obtained from the Affiliated People's Hospital of Jiangsu University, and informed consent was obtained from all individuals.
Enzyme-linked immunosorbent assay (ELISA)
Levels of plasma leptin and CD4+ T cells-derived leptin were measured using a human leptin ELISA immunoassay (R&D Systems, Minneapolis, MN, USA), following the manufacturer's protocol.
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMCs) were isolated by standard density-gradient centrifugation over Ficoll-Hypaque solution. Plasma samples were collected through centrifugation and stored at –80°C for measurement.
Human CD4+ T cells were purified from PBMCs by magnetic beads using a CD4+ T Cell Isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), with purity routinely higher than 95%. CD4+ T cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. For leptin detection, CD4+ T cells were cultured with anti-human CD3 monoclonal antibody (mAb) (10 μg/ml) and anti-human CD28 mAb (2 μg/ml) for 72 h. Supernatants were then used to detect the levels of leptin by ELISA.
For in-vitro blocking experiments, 10 μg/ml human leptin-neutralizing mAb (R&D Systems) was administered in CD4+ T cell culture in the presence of soluble anti-human CD3 mAb (10 μg/ml) and anti-human CD28 mAb (2 μg/ml); the irrelevant isotype-matched antibody was used as control.
Thyroid specimens were minced and then digested with collagenase II (Sigma-Aldrich, St Louis, MO, USA) for 1–2 h at 37°C and then isolated by density-gradient centrifugation. Finally, thyroid mononuclear cells (TMCs) were obtained. The viability of cells was found to be higher than 95%.
Flow cytometric analysis
For CD4+ Th17 cell detection, PBMCs were washed and stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) in the presence of monensin (2 μM) for 5 h, and then stained with phycoerythrin-cyanin 5 (PE-Cy5)-conjugated anti-human CD3 mAb and fluorescein isothiocyanate (FITC)-conjugated anti-human CD8 mAb (eBiosciences, San Diego, CA, USA), fixed and permeabilized using an intracellular staining kit (Invitrogen, Carlsbad, CA, USA), followed by staining with PE-conjugated anti-human IL-17 mAb (eBiosciences). Immunostained cells were analysed using a fluorescence activated cell sorter [(FACS)Calibur, Becton Dickinson, San Jose, CA, USA]. Analysis of the Th17 cell population was performed by gating on CD3+CD8– T cells.
RNA isolation and reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from PBMCs or TMCs using TRIzol reagent (Invitrogen). Total RNA was isolated and reverse transcription was performed according to the manufacturer's instructions (Toyobo, Osaka, Japan).
Quantitative real-time PCR was performed by triplicate using Bio-Rad SYBR green super mix (Bio-Rad, Hercules, CA, USA). Primer sequences were as follows: retinoic acid-related orphan receptor γt (RORγt), sense, 5′-CCTGGGCTCCTCGCCTGACC-3′, anti-sense, 5′-TCTCTCTGCCCTCAGCCTTGCC-3′; and β-actin, sense, 5′-CACGAAACTACCTTCAACTCC-3′, anti-sense, 5′-CATACTCCTGCTTGCTGATC-3′. Samples were run in triplicate, and their relative expression was determined by normalizing to the expression level of β-actin. Data were analysed using Bio-Rad CFX Manager software.
In the case of TMCs, leptin, IL-17 and RORγt cDNA products were amplified by PCR with the following primer sequences: leptin, sense, 5′-TCCTGGGCTCCACCCCATCC-3′, anti-sense, 5′-TGCAGAGACCCCTGCAGCCT-3′; and IL-17, sense, 5′-CAAGACTGAACACCGACTAAG-3′, anti-sense, 5′-TCTCCAAAGGAAGCCTGA-3′.
Amplified products were electrophoresed on 2% agarose gel (Invitrogen), stained with ethidium bromide and visualized with ultraviolet transilluminator.
Statistical analysis
One-way analysis of variance (anova) was performed to determine whether there was an overall statistically significant change among the groups, and the post-test comparison was carried out using Bonferroni's test. Student's unpaired t-test was performed as appropriate. Correlations between variables were determined by Spearman's correlation coefficient. Data were analysed with GrapPad Prism version 5 software.
Results
Increased levels of plasma leptin and CD4+ T cell-derived leptin in HT patients
We first compared the basal plasma leptin levels of 27 female HT patients with 22 age-, sex- and BMI-matched female healthy controls. It was found that HT patients showed an increase of leptin which was at the border of statistical significance (P = 0·06, Fig. 1a). Subsequently, we analysed the correlation between the level of plasma leptin and BMI in HT patients and healthy controls. The results showed that plasma leptin correlated positively with BMI in healthy controls, but no correlation was observed in HT patients (Fig. 1b,c). Furthermore, the level of leptin in culture of CD4+ T cells from HT patients was higher than that from healthy controls (Fig. 1d).
Fig. 1.
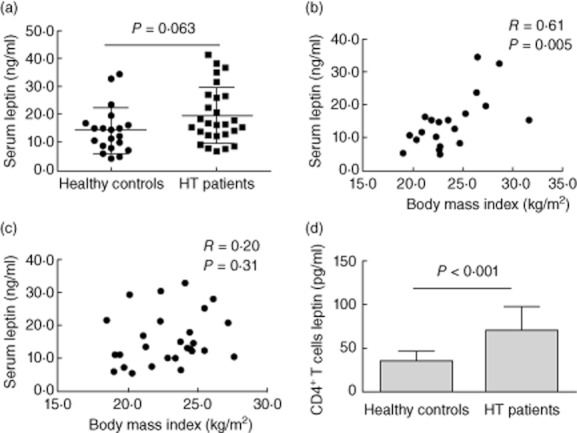
Increased serum and CD4+ T cell-derived leptin in Hashimoto's thyroiditis (HT) patients. Peripheral blood was obtained from 27 HT patients and 20 healthy controls. (a) Plasma leptin levels were determined by enzyme-linked immunosorbent assay (ELISA) from HT patients and healthy controls. Correlation between the level of plasma leptin and body mass index (BMI) in healthy controls (b) and HT patients (c), respectively. (d) The level of leptin in culture of CD4+ T cells from HT patients was determined by ELISA from HT patients and healthy controls. Each data point represents an individual subject; results are expressed as mean ± standard deviation, Student's unpaired t-test.
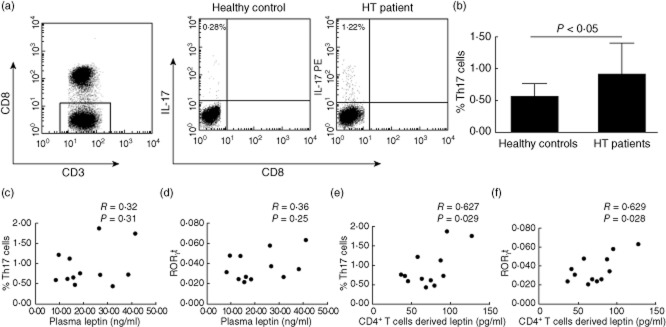
A positive correlation between CD4+ T cell-derived leptin and Th17 cells in HT patients
Flow cytometric analysis revealed that an increased proportion of Th17 cells from peripheral blood mononuclear cells (PBMCs) was observed in HT patients compared with healthy controls (Fig. 2a,b). There were no statistically significant correlations between plasma leptin concentrations and the percentage of Th17 cells or the level of RORγt in HT patients (Fig. 2c,d). However, strong positive correlations were observed between CD4+ T cell-derived leptin and the percentage of Th17 cells or the level of RORγt in HT patients (Fig. 2e,f).
Fig. 2.
The correlation between leptin and T helper type 17 (Th17) cells in Hashimoto's thyroiditis (HT) patients. (a) The percentages of CD3+CD8− Th17 cells were analysed by flow cytometry, representative flow cytometry plots of Th17 cells. (b) Collective analysis of results from HT patients and control groups. Results are expressed as mean ± standard deviation, n = 12, Student's unpaired t-test. (c,d) Correlation between plasma leptin concentrations and the percentage of Th17 cells or the level of retinoic acid-related orphan receptor γt (RORγt) in HT patients. (e,f) Correlation between CD4+ T cell-derived leptin concentrations and the percentage of Th17 cells or the level of RORγt in HT patients.
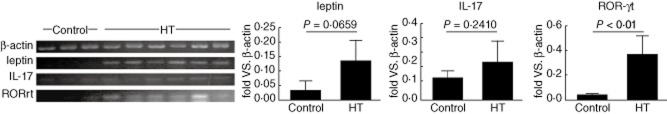
Expression of leptin, IL17 and RORγt mRNA in the thyroid tissue
As an organ-specific autoimmune disease, lymphoid infiltration is a significant feature of HT. To determine whether leptin, IL-17 and RORγt mRNA expression were also expressed in local thyroid tissue, we detected significantly up-regulated levels of leptin, IL-17 and RORγt transcripts in the thyroid tissue of six HT patients by PCR analysis (Fig. 3).
Fig. 3.
Expression of leptin, interleukin (IL)-17 and retinoic acid-related orphan receptor γt (RORγt) mRNA in thyroid tissue. Expression of leptin, IL-17 and RORγt mRNA in thyroid mononuclear cells (TMCs) from six Hashimoto's thyroiditis (HT) patients compared with three patients with simple goitre (control). The levels of β-actin, leptin, IL-17 and RORγt mRNA were determined by polymerase chain reaction (PCR). Levels of greyscale were then tested and corresponding values are shown.
Neutralization of leptin decreases the frequency of Th17 cells in vitro
To investigate a potential role of leptin in the development of Th17 cells in vitro, we treated CD4+ T cells from HT patients with neutralizing leptin monoclonal antibody in the presence of anti-CD3 and anti-CD28 mAb. As shown in Fig. 4, we detected a substantially decreased frequency of CD4+ Th17 cells and RORγt mRNA expression among naive CD4+ T cells cultured in the presence of anti-leptin mAb.
Fig. 4.
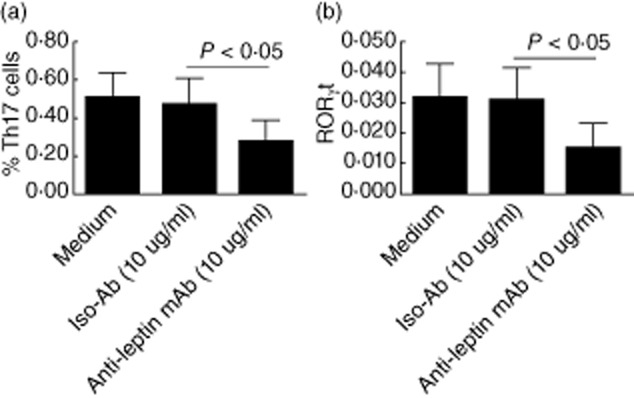
Neutralization of leptin decreases the percentage of T helper type 17 (Th17) cells in vitro. Human CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) by magnetic beads and cultured with RPMI-1640 medium supplemented with 10% fetal bovine serum; 10 μg/ml human leptin-neutralizing monoclonal antibody (mAb) was administered in CD4+ T cell culture in the presence of soluble anti-human CD3 mAb and anti-human CD28 mAb; the irrelevant isotype-matched antibody was used as control. The percentages of CD4+ Th17 cells were analysed by flow cytometry (a); retinoic acid-related orphan receptor γt (RORγt) mRNA were determined by quantitative PCR (b).
Discussion
Accumulating data indicate that leptin acts as a proinflammatory cytokine in autoimmune disease animal model, such as EAE [17], non-obese diabetic (NOD) mice [18]and experimental arthritis [19]. In human autoimmune thyroid diseases, the role of leptin seems to be more complicated. It has been reported that high levels of plasma leptin in women developed postpartum thyroiditis, suggesting a relationship between leptin and postpartum thyroid disease [16]. However, Sieminska and colleagues showed that concentrations of leptin were not altered in postmenopausal women with Hashimoto's thyroiditis [20]. The differences between these reported findings may be due to patient age and different disease stages. In the present study, our group showed a modest increased level of plasma leptin in HT patients compared to healthy controls, with a positive correlation between plasma leptin and BMI. Previous studies report that activated T lymphocytes could synthesize and produce leptin as an autocrine/paracrine cytokine [14,21,22]. Interestingly, the data presented here provide evidence that CD4+ T cell-derived leptin is increased in HT patients. Our results are consistent with a previous study on MS patients showing that activated T cells from relapsing–remitting MS patients secreted consistent amounts of leptin in the culture medium [15].
Extensive investigations have elucidated an important role of the T cell-mediated autoimmune response in enhancing autoimmune thyroid disease. A large amount of intrathyroidal lymphocytes in patients are CD4+ T cells, which have been proposed to be involved in the pathogenesis of HT diseases. The previous report showed that Th1/Th2 skew led to inflammatory factor and infiltrated Th1 cells destroy the thyroid gland in HT patients [1]. However, increasing evidence supports that the Th17 cell (IL-23/IL-17) pathway, rather than the Th1 cell [IL-12/interferon (IFN)-γ) pathway, is critical for the development of autoimmune inflammatory diseases [23,24]. In patients with HT, the percentage of Th17 cells and relative cytokines levels was elevated significantly in the peripheral blood and thyroid tissue compared with healthy controls [2,3]. A question remains about the possible source of increased Th17 cells in HT patients. As an important proinflammatory mediator, leptin could stimulate the proliferation of T lymphocytes and promote the Th1 phenotype immune response [25]. Moreover, some recent studies indicate that leptin signalling controls the proliferation of CD4+CD25+ Treg cells through an autocrine pathway, because Treg cells produce higher levels of leptin and express high leptin receptors [22]. In agreement with this observation, significantly increased Tregs are found in both the leptin deficiency (ob/ob) and leptin receptor deficiency (db/db) mice; administration with the leptin blockade could delay the onset and progression of EAE, which shows an inverse correlation between the concentration of leptin and the percentage of Treg cells [15]. These findings provide strong evidence that leptin signalling modulates a balance between effector T cells (Teff) and Treg cells.
Because IL-6 plays an important role in regulating the balance between IL-17-producing Th17 cells and Tregs [26,27] and the leptin signalling pathway shares the highest structural similarity and signalling capability with those of the IL-6-type cytokine receptors [5], we hypothesized that high levels of leptin may partly modulate Th17 cells involved in the pathogenesis of HT disease. In the present study, we provide direct evidence that plasma leptin and CD4+ T cell-derived leptin were higher in HT patients compared with healthy controls. Neither the percentage of Th17 cells nor the level of Th17 cell-specific transcription factor RORγt correlated with plasma leptin, but the percentage of Th17 cells or the level of RORγt correlated positively with CD4+ T cell-derived leptin in HT patients. In addition, we have detected up-regulated levels of leptin, IL-17 and RORγt expression in TMCs from HT patients compared to a patient with simple goitre. To address a direct role of leptin in modulating Th17 cells, we found that neutralization of leptin decreases Th17 cells in vitro. Together, our results provide direct evidence that T cell-derived leptin, but not plasma leptin, may contribute to the pathogenic role of increased Th17 cells in HT patients. Thus, further studies are warranted to characterize the molecular mechanism of leptin-mediated modulation of Th17 cells.
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant no. 30871193, 81072453, 30972748, 31100648, 30910103087), Health Department Foundation of Jiangsu Province (grant no.H200952), Graduate Student Research and Innovation Program of Jiangsu Province (CXLX11_0608, CXZZ12_0710), Jiangsu Province Qinglan Project and Top Talent Program of Jiangsu University.
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19:495–501. doi: 10.1089/thy.2008.0423. [DOI] [PubMed] [Google Scholar]
- 2.Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2010;95:953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Wang H, Su Z, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto's thyroiditis. Scand J Immunol. 2010;72:250–255. doi: 10.1111/j.1365-3083.2010.02425.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 7.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 8.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 10.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 11.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 12.Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–3130. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- 13.Lo CK, Lam QL, Yang M, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. 2009;6:353–360. doi: 10.1038/cmi.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanna V, Giacomo AD, La Cava A, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matarese G, Carrieri PB, La Cava A, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazziotti G, Parkes AB, Lage M, Premawardhana LD, Casanueva FF, Lazarus JH. High leptin levels in women developing postpartum thyroiditis. Clin Endocrinol (Oxf) 2004;60:208–213. doi: 10.1046/j.1365-2265.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 17.De Rosa V, Procaccini C, La Cava A, et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matarese G, Sanna V, Lechler RI, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 19.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 20.Sieminska L, Wojciechowska C, Kos-Kudla B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto's thyroiditis. Endokrynol Pol. 2010;61:112–116. [PubMed] [Google Scholar]
- 21.Siegmund B, Sennello JA, Jones-Carson J, et al. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965–972. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 26.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 27.Taflin C, Favier B, Baudhuin J, et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2011;108:2891–2896. doi: 10.1073/pnas.1011811108. [DOI] [PMC free article] [PubMed] [Google Scholar]