Abstract
Anti-tissue transglutaminase 2 (anti-TG2) antibodies are present in the serum of the great majority of untreated coeliac disease (CD) patients. They are produced and deposited in the small intestinal mucosa. Potential CD patients present serum anti-TG2 antibodies higher than cut-off, but a normal duodenal mucosa where mucosal deposits of anti-TG2 are not always detectable. The aim of our work was to investigate the presence of anti-TG2 intestinal antibodies in patients with potential CD, and identify the most sensitive test to detect them. Twelve active CD patients, 28 potential CD patients and 39 non-CD controls were enrolled. Biopsy fragments from all patients were analysed by double immunofluorescence to detect mucosal deposits of anti-TG2 antibodies. Fragments from the same subjects were also cultured for 24 h with medium in the presence or absence of gliadin peptides. Anti-TG2 autoantibodies secreted into supernatants were measured by enzyme-linked immunosorbent assay. All active CD, 68% of potential CD patients and 20% of non-CD controls showed mucosal deposits of immunoglobulin (Ig)A anti-TG2; at the same time 100, 96 and 8% of active CD, potential CD and non-CD control patients secreted these antibodies in culture supernatants, respectively. Our data showed that, to detect intestinal anti-TG2 antibodies, the measurement of antibodies secreted into culture supernatants has higher sensitivity and specificity (97·5 and 92·3%, respectively) than the detection of mucosal deposits (77·5 and 80·0%, respectively). The measurement of intestinal anti-TG2 antibodies may prove useful in clinical practice to predict evolution towards mucosal atrophy in potential coeliac patients and identify patients with gluten sensitivity.
Keywords: anti-tissue transglutaminase2, gluten sensitivity, intestinal deposits, potential coeliac disease, secreted antibodies
Introduction
Coeliac disease (CD) is an immune-mediated systemic disorder elicited, in genetically susceptible individuals, by wheat gluten and related proteins of barley and rye [1]. CD is characterized by the presence of a variable combination of gluten-dependent clinical manifestations, CD-specific antibodies, human leucocyte antigen (HLA)-DQ2 and DQ8 haplotypes and enteropathy [2]. It is now clear that, as well as a spectrum of clinical presentations, there is a wide spectrum of alterations of the jejunal histology, ranging from an infiltrative lesion to villous atrophy [3]. Gluten is able to induce the production of autoantibodies that can be found in the serum of CD patients and that have, from a diagnostic viewpoint, a very high sensitivity and specificity [4]. These autoantibodies recognize an endomysial antigen known today as transglutaminase 2 (TG2) [5]. It is also well known that in CD patients anti-TG2 autoantibodies are produced at intestinal level [6], and can be deposited on the extracellular TG2 in the mucosa of small intestine [7], even before passing into circulation and being measurable in serum. These deposits are localized below the basement membrane, along the villous and crypt and around mucosal vessels [7]. In our paediatric population, deposits of immunoglobulin (Ig)A anti-TG2 are present in 96% of coeliac patients with overt disease [8].
Patients with serum positivity for anti-TG2 and normal duodenal mucosa, or with slight signs of inflammation, are defined today as potential coeliac patients [2,9]. In this group of patients intestinal deposits of IgA anti-TG2 are found less often [8,10]. However, their presence is relevant, as it has been associated with the risk of developing frank villous atrophy [10].
Several studies have reported that when specimens of duodenal mucosa are cultured for 24–48 h in medium alone and with peptic–tryptic digest of gliadin (PTG) [11,12] or with peptide 31–43 (P31–43) [13,14], there is a production of anti-endomysial antibodies in organ culture supernatants in CD patients, but not in controls.
More recently, similar data have been obtained measuring IgA anti-TG2 antibodies in supernatants of cultured biopsies from CD patients untreated and on a gluten-free diet [15–17]. This test has been proposed for diagnostic purposes, particularly in patients with normal mucosa. However, in this regard the information on potential CD patients is poor and scanty.
The aim of our work was to investigate the presence of anti-TG2 intestinal antibodies in patients with potential CD, and identify the most suitable detection test for this purpose. We compared two assays: the search for intestinal deposits of IgA anti-TG2 and measurement of the same antibodies in the supernatants after organ culture of duodenal biopsies.
Patients and methods
Patients
Our study involved 40 patients (29 females, median age 7 years, range 2–17 years) who underwent a small intestinal biopsy at the Department of Pediatrics, University Federico II in Naples for suspicion of CD. All patients presented increased serum levels of anti-TG2 antibodies. Twelve patients showed total villous atrophy (Marsh classification grade IIIc) and received a diagnosis of CD on the basis of diagnostic criteria established by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [2]. Twenty-eight of 40 patients showed architecturally normal small intestinal mucosa (11 with grade 0 and 17 with Marsh classification grade I); they were classified as potential CD patients. Thirty-nine subjects without coeliac disease (22 females, median age 8 years, range 1–16 years) were enrolled as a control group. All non-CD patients presented normal serum levels of anti-TG2 antibodies and architecturally normal small intestinal mucosa (30 with grade 0 and 9 with Marsh classification grade I). Twelve of 39 subjects had type 1 diabetes (T1D). The final diagnoses of the remaining 27 subjects were iron deficiency anaemia, failure to thrive, gastroesophageal reflux and recurrent abdominal pain. IgA deficiency was excluded.
Written informed consent was obtained by the parents of the children enrolled. The study protocol was approved by the Ethical Committee of the University ‘Federico II’ Naples, Italy (CE 230/05).
Duodenal biopsy and organ culture system
During upper gastrointestinal endoscopy, at least five duodenal biopsies were taken from all patients. Two fragments were fixed in 10% formalin, embedded in paraffin, and then treated for histological analysis. One of the duodenal specimens was embedded in an optimal cutting temperature compound (OCT; Tissue-Tek, Sakura Finetek Europe BV, Zoeterwoude, the Netherlands) and stored in liquid nitrogen until used. The remaining two fragments were cultured for 24 h at 37°C in either the presence or absence of P31–43 (0·1 mg/ml)/peptic–tryptic gliadin digest (PTG; 0·5 mg/ml). Organ culture was performed as reported previously [18]. After 24 h of culture, the tissues were embedded in OCT and stored in liquid nitrogen. The culture supernatants were collected and stored at −80°C until they were analysed.
Intestinal deposits of anti-TG2 IgA antibodies
The presence of intestinal deposits of anti-TG2 IgA was investigated before and after 24 h of organ culture with P31–43 or PTG or medium alone. Five μm cryostat sections were stained using a double-immunofluorescence method, as described previously [19]. The stained sections were evaluated using a confocal microscope (LSM510; Zeiss MicroImaging Inc., Milan, Italy).
Measurement of anti-TG2 IgA antibodies secreted into culture supernatants
Mucosal anti-TG2 IgA antibodies secreted into culture supernatants were measured in undiluted supernatants by enzyme-linked immunosorbent assay (ELISA; EU-tTG IgA kit; Eurospital S.p.A, Trieste, Italy), according to the manufacturer's instructions. When the value of anti-TG2 was higher than the last point of standard curve, supernatants were diluted 1:2, 1:4, 1:10 and 1:20 in culture medium. The cut-off value for anti-TG2 IgA antibodies in culture supernatants was calculated using supernatants from cultures with medium alone from the 39 controls and was established as the mean ± 2 standard deviations (SD) (2·8 U/ml).
Statistics
Statistical analysis was performed using GraphPad Prism 4 for Windows, version 4·03. Data with a Gaussian distribution were compared by Student's t-test; Pearson's χ2 test was used for non-normal data; Pearsons' correlation test was used to compare titres of anti-TG2 IgA in serum and supernatants in potential CD patients. A P-value of < 0.05 was considered to be significant. The sensitivity and specificity of the two methods examined were calculated by standard statistical formulae.
Results
Intestinal deposits of anti-TG2 IgA
Mucosal deposits of anti-TG2 IgA were searched in small intestinal fragments from 12 untreated CD, 28 potential CD and 30 control patients. All CD patients (100%) in the active phase of disease, 19 of 28 (67·8%) potential CD and six of 30 (20%) non-CD patients were positive. Among the six positive non-CD subjects, five were T1D patients and one affected by gastroesophageal reflux. In most potential positive patients and in the six positive controls a patchy distribution of mucosal deposits was observed. The value of serum anti-TG2 in those potential CD patients (nine of 28) without deposits ranged from 7 to 20·4 U/ml. Moreover, we confirmed that titres of serum anti-TG2 were statistically lower in patients negative for intestinal deposits (mean ± SD: 13·6 ± 5·0 U/ml) than in those positive (29·8 ± 30·3 U/ml, P < 0.05), as shown previously [10].
We also looked for the presence of intestinal deposits in duodenal fragments after 24 h of in vitro challenge with P31–43 or PTG, to investigate if gliadin peptides were able to induce the production and consequently the deposition of anti-TG2 antibodies. Culture experiments were performed with duodenal fragments from four of 12 untreated CD (one cultured in the presence of P31–43 and three in the presence of PTG), 20 of 28 potential CD (16 with P31–43 and four with PTG) and 14 of 30 control patients (11 with P31–43 and three with PTG) (Table 1). We found no significant change in the intensity of mucosal IgA deposits in biopsies from active CD cultured with P31–43/PTG when compared to those cultured with medium alone or in samples before culture. In 15 of 20 (75%) potential CD patients a higher intensity of mucosal IgA deposits was seen in fragments cultured with P31–43/PTG if compared to medium alone. Moreover, in fragments cultured with P31–43, 18 of 20 (90%) patients showed mucosal deposits, compared to 12 of 20 (60%; P < 0.02) patients in fragments cultured in medium alone (Fig. 1). Our data also showed that after 24 h culture with medium alone a lower intensity of mucosal deposits was seen in eight of 20 (40%) potential CD patients compared to what was seen before culture.
Table 1.
Detection of mucosal deposits and measurement of supernatant anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A in the study population
| Fragments showing IgA anti-TG2 mucosal deposits | Supernatants with anti-TG2 higher than cut-off* | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients | n | Before culture | n | After medium 24 h | After P31–43/PTG 24 h | n | Medium | P31–43/PTG |
| Active CD | 12 | 12/12 | 4 | 4/4 | 4/4 | 12 | 12/12 | 12/12 |
| Potential CD | 28 | 19/28 | 20 | 12/20 | 18/20 | 28 | 27/28 | 26/28 |
| Non-CD | 30 | 6/30 | 14 | 1/14 | 5/14 | 39 | 3/39 | 2/39 |
Cut-off = 2·8 U/ml. CD: coeliac disease; PTG: peptic–tryptic digest of gliadin.
Fig. 1.
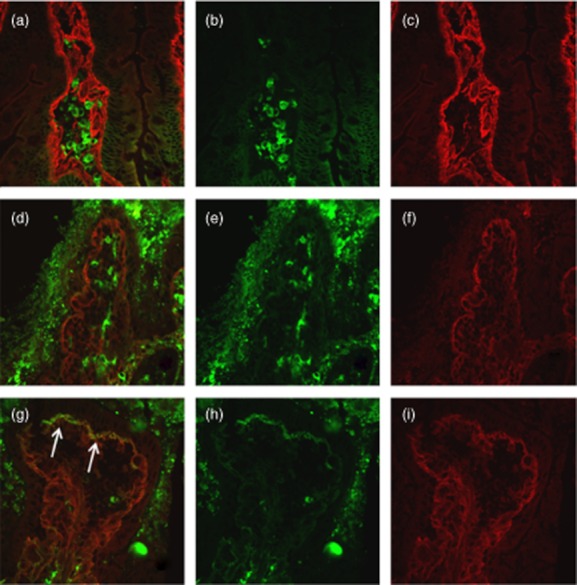
Duodenal mucosa from potential coeliac disease (CD) patient negative for mucosal deposits of immunoglobulin (Ig)A anti-transglutaminase 2 (TG2) before (a–c) and after 24 h culture with medium alone (d–f). Mucosal deposits of IgA anti-TG2 (in yellow) is lightly visible after 24-h culture with P31–43 (arrows) (g–i). IgA secreted by plasma cells are visible in green (b,e,h); TG2 with a subepithelial localization is shown in red (c,f,i).
IgA anti-TG2 in organ culture supernatants
We investigated the presence of anti-TG2 IgA in supernatants obtained after 24-h culture of biopsies from 12 active CD, 28 potential CD and 39 non-CD patients (Table 1). Anti-TG2 IgA antibodies in supernatants of biopsies cultured with medium only were higher than cut-off in 12 of 12 (100%) active CD, 27 of 28 (96·4%) potential CD and three of 39 (7·6%) non-CD patients. All active CD patients secreted very high titres, ranging from 287·2 to 2020·3 U/ml. Titres of secreted anti-TG2 in potential CD patients were variable, ranging from 5·75 to 1005 U/ml (Fig. 2), and were correlated with serum anti-TG2 titres (Pearson's r = 0·68, P < 0.0001) (Fig. 3). Of the three positive non-CD patients, one was a first-degree relative, the second was affected by T1D and the third was affected by gastroesophageal reflux disease (GERD) and headache. They secreted low amounts of anti-TG2 (range 3·33–5·02 U/ml). Among these three non-CD patients the detection of mucosal deposits of anti-TG2 IgA antibodies in fragments before culture was positive in only one of three (the T1D patient).
Fig. 2.
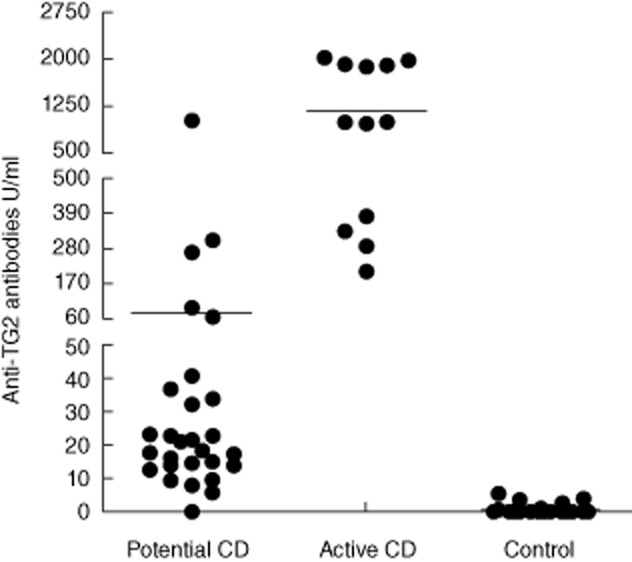
Titres of anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A, expressed as U/ml, in medium culture supernatants of all culture experiments. Horizontal lines represent mean values.
Fig. 3.
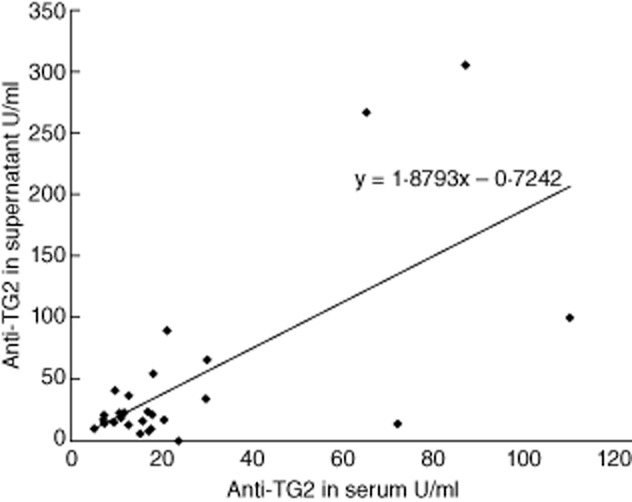
Correlation between titres of anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A (expressed as U/ml) in serum and in culture supernatants of intestinal fragments from 28 potential coeliac disease (CD) patients. Equation for the line: y = 1·8793x–0·7242 (R2 = 0·4596).
In cultured supernatants of all active CD patients after 24 h P31–43 or PTG in vitro stimulation, anti-TG2 antibodies titres were comparable to those of medium alone. Potential CD patients showed a wide spectrum of responses after P31–43/PTG in vitro challenge: the titres of supernatants anti-TG2 IgA were reduced or stable in six of 28 and 16 of 28, respectively; we found a more than doubled increase in only six of 28 patients. Among controls, all 36 patients who were negative for production of anti-TG2 antibodies in medium alone confirmed their negativity even after stimulation with P31–43/PTG; two of three control patients positive for secreted antibodies into medium culture were also positive after PTG stimulation with levels of anti-TG2 IgA comparable to those of medium alone.
The measurement of anti-TG2 titres in culture supernatants seems to be more sensitive and specific than the detection of mucosal deposits to reveal mucosal production of anti-TG2 antibodies in coeliac disease, showing a sensitivity and specificity of 97·5 and 92·3% versus 77·5 and 80% of mucosal deposits, respectively. Moreover, the rate of concordance of the two methods compared with serum anti-TG2 autoantibodies was 78·6% for intestinal deposits and 94·9% for supernatants anti-TG2, respectively (P < 0.01).
Discussion
The presence of anti-TG2 antibodies in serum represents a CD marker [4]. However, intestinal anti-TG2 antibodies may be even more relevant from a clinical viewpoint. These antibodies are produced at intestinal level [6], where they could be deposited even before they appear in circulation [7,20]. Two aspects can make intestinal anti-TG2 antibodies relevant: the first is their suggested ability in potential coeliac patients to predict evolution towards a clear enteropathy; the second is their possible role in revealing a condition of gluten sensitivity in patients with absence of CD-associated autoantibodies in their serum [21,22]. Therefore, it is particularly important to find the most suitable assay to detect and measure these antibodies.
In the last 20 years different assays have been used: measurement in faeces [23] or in intestinal fluids [24], search in supernatants of cultured biopsy fragments [11–17] or detection in the same biopsies of deposited antibodies [16], or expression in phage libraries of RNA, obtained from biopsies, coding for the antibodies [6,22]. Detection of anti-endomysium antibodies (EMA) and anti-TG2 IgA antibodies in faecal supernatants from CD patients was determined to be unreliable as a diagnostic test [25]. The search for anti-TG2 antibodies in culture supernatants of intestinal biopsy from CD patients by ELISA was proposed in particular to improve the accuracy of CD diagnosis in subjects with mild enteropathy and in patients negative for serum antibodies [15–17]. More recently, using immunofluorescence, Korponay-Szabo et al. [7] showed that IgA deposited in the intestine of CD patients are directed against TG2. This assay, while having high sensitivity and specificity for the diagnosis, requires frozen intestinal samples and highly experienced operators. By means of phage display libraries, Marzari et al. [6] showed that anti-TG2 IgA antibodies are synthesized primarily by specific B lymphocytes in the small intestinal mucosa and that there is a preferential use of heavy chain variable regions belonging to the VH5 gene family in antibodies from coeliac patients. The intestinal production of anti-TG2 antibodies has been confirmed recently by the finding of a high abundance of plasma cells secreting TG2-specific IgA autoantibodies with limited somatic hypermutation in CD intestinal lesions [26]. In identifying patients with gluten sensitivity, the phage display libraries technique seems to be more sensitive than detection of mucosal deposits of anti-TG2 antibodies using double immunofluorescence assay [22]. At the same time, it must be emphasized that, because of its complexity, this test cannot be proposed for routine use.
In this study, not using faeces detection and considering the phage library technique too demanding, we compared the diagnostic efficiency of the detection of intestinal deposits of these antibodies and their measurement in biopsy culture supernatants, particularly in patients with potential CD. We detected mucosal deposits of anti-TG2 antibodies in 68% of potential CD patients, confirming our previous findings [8]; at the same time, we showed in the same patients that a higher number (96%) secreted these antibodies into culture supernatants. The titres of secreted anti-TG2 antibodies of potential CD patients were lower than those observed in active CD patients and correlated significantly with serum titres. It is likely that a correlation exists between intestinal antibody titres and severity of mucosal damage. In our hands, the assay showed a sensitivity and specificity of 97·5% and 92·3%, respectively, differing from the search of mucosal deposits that showed sensitivity and specificity of 77·5% and 80%, respectively, in the same population. Furthermore, the measurement of anti-TG2 antibodies secreted into culture supernatants is performed by ELISA, so it is objective and not influenced by the operator's ability for the detection of mucosal deposits.
Our data also showed that gliadin peptides do not influence intestinal anti-TG2 antibody production in patients with active CD and, as expected, in control subjects. Moreover, the intensity of mucosal deposits seemed to increase in potential CD patients in response to culture with gliadin peptides, while in the majority of patients the titres of secreted autoantibodies did not show a substantial increase. Stimulation of specific B lymphocytes could induce production of the anti-TG2 antibodies that bind the tissue-transglutaminase and increase deposits signalling without increasing the titres in culture supernatants.
As mentioned previously, the clinical relevance of detecting intestinal anti-TG2 antibodies resides in their ability to predict evolution to mucosal damage and in identifying subjects with gluten sensitivity. In the first case, the presence of intestinal deposits of anti-TG2 seems to be a marker of forthcoming overt CD [10,27] in patients with normal intestinal mucosa. This ability, if confirmed by larger studies, could help to solve the vexed question of whether or not potential CD patients must be put onto a gluten-free diet or remain on a gluten-containing diet. Currently, our centre's policy is to leave asymptomatic potential CD patients on a gluten-containing diet and follow them over time. With regard to the state of gluten sensitivity, important observations have been made measuring antibodies in intestinal fluids and using the phage library technique. Using the first approach, patients with gluten sensitivity were identified among patients with irritable bowel syndrome who presented these antibodies in intestinal fluids, but not in serum [28]. Recently, Not et al. [22] have reported that a large proportion of DQ2/DQ8-positive relatives of CD patients with normal mucosa and without measurable serum levels of anti-TG2 produce these antibodies in the intestine in response to gluten. We showed that two of the three patients in the control group who secreted anti-TG2 into culture supernatants were, in one case, a first-degree relative and in the second case a patient affected by type 1 diabetes. This last finding is not surprising, as we have already demonstrated that patients with type 1 diabetes, without serum anti-TG2 and with normal duodenal mucosa, produce and deposit anti-TG2 antibodies in their small intestinal mucosa [29]. Finally, in the third case, there was little clinical evidence to support a condition of non-coeliac gluten sensitivity (NCGS). All the patients had normal anti-gliadin levels. So far, this is the only candidate marker of such a condition [30]. In this context, the presence of intestinal autoantibodies against TG2 should be assessed.
In conclusion, in this work we have demonstrated that to detect intestinal anti-TG2 antibodies, the sensitivity and specificity of the anti-TG2 supernatant are higher than those of mucosal deposits. This test could prove useful in clinical practice to predict evolution to mucosal atrophy in potential coeliac patients and to identify patients with gluten sensitivity who lack CD-associated serum autoantibodies.
Acknowledgments
This work was supported by Fondazione Celiachia.
Disclosure
The authors have no conflicts of interest.
References
- 1.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 2.Husby S, Koletzko S, Korponay-Szabó IR, et al. for the ESPGHAN Working Group on Coeliac Disease Diagnosis, on behalf of the ESPGHAN Gastroenterology Committee, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 3.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Giersiepen K, Lelgemann M, Stuhldreher N, et al. for the ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54:229–241. doi: 10.1097/MPG.0b013e318216f2e5. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 6.Marzari R, Sblattero D, Florian F, et al. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 7.Korponay-Szabo IR, Halttunen T, Szalai Z, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maglio M, Tosco A, Auricchio R, et al. Intestinal deposits of anti-tissue transglutaminase IgA in childhood celiac disease. Dig Liver Dis. 2011;43:604–608. doi: 10.1016/j.dld.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2012 doi: 10.1136/gutjnl-2011-301346. in press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosco A, Salvati MV, Auricchio R, et al. Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol. 2010;9:320–325. doi: 10.1016/j.cgh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Picarelli A, Maiuri L, Frate A, et al. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with coeliac disease. Lancet. 1996;348:1065–1067. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- 12.Vogelsang H, Schwarzenhofer M, Granditsch G, et al. In vitro production of endomysial antibodies in cultured duodenal mucosa from patients with celiac disease. Am J Gastroenterol. 1999;94:1057–1061. doi: 10.1111/j.1572-0241.1999.01014.x. [DOI] [PubMed] [Google Scholar]
- 13.Carroccio A, Iacono G, D'Amico D, et al. Production of anti-endomysial antibodies in cultured duodenal mucosa: usefulness in coeliac disease diagnosis. Scand J Gastroenterol. 2002;37:32–38. doi: 10.1080/003655202753387329. [DOI] [PubMed] [Google Scholar]
- 14.Picarelli A, Di Tola M, Sabbatella L, et al. Usefulness of the organ culture system in the in vitro diagnosis of coeliac disease: a multicentre study. Scand J Gastroenterol. 2006;41:186–190. doi: 10.1080/00365520510024151. [DOI] [PubMed] [Google Scholar]
- 15.Carroccio A, Di Prima L, Pirrone G, et al. Anti-transglutaminase antibody assay of the culture medium of intestinal biopsy specimens can improve the accuracy of celiac disease diagnosis. Clin Chem. 2006;52:1175–1180. doi: 10.1373/clinchem.2005.061366. [DOI] [PubMed] [Google Scholar]
- 16.Stenman SM, Lindfors K, Korponay-Szabo IR, et al. Secretion of celiac disease antibodies after in vitro gladin challenge is dependent on small-bowel mucosal transglutaminase2-specific IgA deposits. BMC Immunol. 2008;9:6. doi: 10.1186/1471-2172-9-6. doi: 10.1186/1471-2172-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picarelli A, Libanori V, De Nitto D, et al. Organ culture system as a means to detect celiac disease. Ann Clin Lab Sci. 2010;40:85–87. [PubMed] [Google Scholar]
- 18.Mazzarella G, Maglio M, Paparo F, et al. An immunodominant DQ8 restricted gliadin peptide activates small intestinal immune response in in vitro cultured mucosa from HLA-DQ8 positive but not HLA-DQ8 negative coeliac patients. Gut. 2003;52:57–62. doi: 10.1136/gut.52.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosco A, Maglio M, Paparo F, et al. Immunoglobulin A anti-tissue transglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J Pediatr Gastroenterol Nutr. 2008;47:293–298. doi: 10.1097/MPG.0b013e3181677067. [DOI] [PubMed] [Google Scholar]
- 20.Salmi TT, Collin P, Korponay-Szabó IR, et al. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55:1746–1753. doi: 10.1136/gut.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med. 2011;269:582–590. doi: 10.1111/j.1365-2796.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 22.Not T, Ziberna F, Vatta S, et al. Cryptic genetic gluten intolerance revealed by intestinal antitransglutaminase antibodies and response to gluten-free diet. Gut. 2011;60:1487–1493. doi: 10.1136/gut.2010.232900. [DOI] [PubMed] [Google Scholar]
- 23.Picarelli A, Sabbatella L, Di Tola M, et al. Antiendomysial antibody detection in fecal supernatants: in vivo proof that small bowel mucosa is the site of antiendomysial antibody production. Am J Gastroenterol. 2002;97:95–98. doi: 10.1111/j.1572-0241.2002.05426.x. [DOI] [PubMed] [Google Scholar]
- 24.Mawhinney H, Love AH. Anti-reticulin antibody in jejunal juice in coeliac disease. Clin Exp Immunol. 1975;21:394–398. [PMC free article] [PubMed] [Google Scholar]
- 25.Kappler M, Krauss-Etschmann S, Diehl V, et al. Detection of secretory IgA antibodies against gliadin and human tissue transglutaminase in stool to screen for coeliac disease in children: validation study. BMJ. 2006;332:213–214. doi: 10.1136/bmj.38688.654028.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Niro R, Mesin L, Zheng NY, et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med. 2012;26:441–445. doi: 10.1038/nm.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmi TT, Collin P, Jarvinen O, et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment Pharmacol Ther. 2006;24:541–552. doi: 10.1111/j.1365-2036.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 28.Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–850. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Maglio M, Florian F, Vecchiet M, et al. Majority of children with type 1 diabetes produce and deposit anti-tissue transglutaminase antibodies in the small intestine. Diabetes. 2009;58:1578–1584. doi: 10.2337/db08-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–685. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]


