Abstract
In first-degree relatives of type 1 diabetic patients, we investigated whether diabetes risk assessment solely based on insulinoma antigen 2 (IA-2) and zinc transporter 8 (ZnT8) antibody status (IA-2A, respectively, ZnT8A) is as effective as screening for three or four autoantibodies [antibodies against insulin (IAA), glutamate decarboxylase 65 kDa (GAD) glutamate decarboxylase autoantibodies (GADA) and IA-2A with or without ZnT8A] in identifying children, adolescents and adults who progress rapidly to diabetes (within 5 years). Antibodies were determined by radiobinding assays during follow-up of 6444 siblings and offspring aged 0–39 years at inclusion and recruited consecutively by the Belgian Diabetes Registry. We identified 394 persistently IAA+, GADA+, IA-2A+ and/or ZnT8A+ relatives (6·1%). After a median follow-up time of 52 months, 132 relatives developed type 1 diabetes. In each age category tested (0–9, 10–19 and 20–39 years) progression to diabetes was significantly quicker in the presence of IA-2A and/or ZnT8A than in their joint absence (P < 0·001). Progression rate was age-independent in IA-2A+ and/or ZnT8A+ relatives but decreased with age if only GADA and/or IAA were present (P = 0·008). In the age group mainly considered for immune interventions until now (10–39 years), screening for IA-2A and ZnT8A alone identified 78% of the rapid progressors (versus 75% if positive for ≥ 2 antibodies among IAA, GADA, IA-2A and ZnT8A or versus 62% without testing for ZnT8A). Screening for IA-2A and ZnT8A alone allows identification of the majority of rapidly progressing prediabetic siblings and offspring regardless of age and is more cost-effective to select participants for intervention trials than conventional screening.
Keywords: IA-2 antibodies, prediction, prevention, type 1 diabetes, zinc transporter 8 antibodies
Introduction
Immune intervention trials using anti-T or anti-B lymphocyte antibodies, co-stimulatory blockade or an antigen-specific vaccine have reported efficacy in transiently preserving residual beta cell function in recent-onset type 1 diabetes [1–4,5]. The best results were obtained in subgroups of participants with a higher functional beta cell mass at diagnosis, short duration of clinically overt diabetes or younger age [1–7], thereby providing a strong argument to plan future interventions at a preclinical stage [8].
Because immune-modulating strategies carry the risk for acute or long-term side effects [1–5], it is important that their testing in non-diabetic subjects is restricted to those with a high risk of developing diabetes in the short term [9]. Also, from a practical standpoint, enrolling individuals with a homogeneously high risk should allow conclusions to be reached more rapidly as to the efficacy of the tested intervention [8]. Considering the number of subjects needed per trial [10–12], screening for islet autoantibodies would need to be performed in thousands of first-degree relatives of type 1 diabetic patients, or in a 10–20 times larger group without family history of diabetes [13–15]. Individuals with a high antibody-inferred diabetes risk could then be stratified further according to risk using standardized tests that assess residual beta cell function [8,16,17]. To avoid many high-risk individuals progressing to diabetes before the actual start of an intervention study, potential participants should be identified within a relatively short interval.
At least five different types of molecularly defined diabetes-associated antibodies have been used to stratify diabetes risk [18–21]. However, their frequency – and hence that of multiple antibody positivity – tends to decline with age at diagnosis, except for antibodies against glutamate decarboxylase (GADA) [22–24]. Moreover, the overall progression rate to diabetes decreases with increasing age at first antibody positivity [25,26]. Because immune intervention trials are expected to be launched first in adults before extending inclusions to adolescents and children [27], antibody screening for secondary prevention trials will be conducted in a first phase in this older age category and antibody-inferred risk should be age-independent. Time constraints and cost-efficiency reasons raise the need to select a limited number of antibody markers.
Antibodies against insulinoma antigen 2 (IA-2A) and zinc transporter 8 (ZnT8A) have been shown to appear later, in general, during the subclinical disease process and to herald more rapid progression to hyperglycaemia than antibodies against insulin (IAA) or GADA [21,26]. The present paper investigates whether diabetes risk assessment based solely on testing for IA-2A and ZnT8A is equally effective in identifying the majority of rapid progressors to diabetes among children, adolescents and adults with a type 1 diabetic sibling or parent, and could thus represent a cost-effective age-independent strategy for enrolment of participants in secondary prevention trials based on immunointervention.
Materials and methods
Participants
Between March 1989 and August 2011, the Belgian Diabetes Registry (BDR) recruited consecutively siblings or offspring (under age 40 years at entry) of type 1 diabetic probands according to previously defined criteria [28]. The probands are considered representative of the Belgian population of type 1 diabetic patients [22]. After obtaining written informed consent from each relative or their parents, a short questionnaire with demographic, familial and personal information was completed at each visit and blood samples were taken at entry and yearly thereafter. Only relatives with two or more contacts during follow-up (number of individuals = 6444), the last being at diagnosis in the case of progression to diabetes, were included into this study. This allowed unambiguous ascertainment of the clinical status of relatives at this last time-point. Diabetes was diagnosed according to the American Diabetes Association criteria [29].
The study was conducted in accordance with the guidelines in the Declaration of Helsinki, as revised in 2008 (http://www.wma.net/en/30publications/10policies/b3/index.html, accessed 17 April 2012), and approved by the ethics committees of the BDR and the participating university hospitals. Random blood samples were collected for sera and buffy coats, and aliquots were stored at −80°C until analysed for diabetes-associated autoantibodies and HLA-DQ genotype, respectively, as described previously [21]. Relatives were not prescreened for islet cell cytoplasmic antibodies (ICA), nor were ICA results analysed in the present study. Antibody positivity was defined as persistent if their next sample after baseline was also positive for at least one antibody type. During follow-up, development of diabetes was ascertained through repeated contacts with Belgian endocrinologists and paediatricians, self-reporting through yearly questionnaires and a link with the BDR patient database, where newly diagnosed patients under 40 years of age are registered. Follow-up ended at the time of the last blood sampling or, in the case of progression to diabetes, at clinical onset. Body mass index (BMI) was expressed as a standard deviation score (BMI-SDS) by comparison with an age- and sex-matched cohort [30].
Analytical methods
IAA, GADA, IA-2A and ZnT8A were determined by liquid-phase radiobinding assays [21] and HLA-DQ polymorphisms by allele-specific oligonucleotide genotyping [31], as described previously. Antibody levels were expressed as the percentage binding of added tracer [10 000 counts per minute (cpm)/tube] [21]. cDNAs for the preparation of radio-ligands by in-vitro transcription–translation were kind gifts from Å. Lernmark (when at University of Washington, Seattle, WA, USA) for full-length 65 kDa glutamate decarboxylase, M. Christie (King's College School of Medicine and Dentistry, London, UK) for the intracellular portion of insulinoma-associated protein 2 (IA-2) and J. C. Hutton (Barbara Davis Center for Childhood Diabetes, Aurora, CO, USA) for the dimeric hybrid ZnT8 construct generated by fusion of CR and CW (zinc transporter-8 carboxy-terminal constructs carrying, respectively, Arg325 and Trp325) (CRCW). In the 2009 Diabetes Autoantibody Standardisation Program (DASP) Workshop diagnostic sensitivity and specificity were, respectively, 74 and 97% for GADA, 40 and 98% for IAA, 66 and 99% for IA-2A and 68 and 100% for ZnT8A (CRCW). Cut-off values for positive antibodies were determined as the 99th percentile of antibody levels in 761 non-diabetic controls, and amounted to ≥ 0·6% tracer binding for IAA, ≥ 2·6% for GADA and ≥ 0·44% for IA-2A. As ZnT8A levels tended to decrease slightly with age in control subjects, cut-off values were calculated separately for the age groups 0–14 years (≥ 1·28%) and 15–39 years (≥ 1·02%) for ZnT8A [24]. Between-day coefficients of variation determined for serum pools within the normal range and within the moderately elevated range were, respectively, 35% (0·3% tracer binding) and 12% (6·9% tracer binding) for IAA, 12% (2·1% tracer binding) and 10% (7·1% tracer binding) for GADA, 18% (0·3% tracer binding) and 9% (2·3% tracer binding) for IA-2A and 21% (0·7% tracer binding) and 6% (3·9% tracer binding) for ZnT8A. Proinsulin (PI), C-peptide (CP) and the PI/CP ratio were determined as before [32].
Statistical analysis
Statistical differences between groups were assessed by χ2 test, with Yates' correction or Fisher's exact test for categorical variables and Mann–Whitney U-test for continuous data. Kaplan–Meier analysis was used to estimate diabetes-free survival. The survival curves were compared using the log-rank test. Follow-up started at the time of the first antibody-positive sample and ended at the last contact with the relative or at clinical onset, whichever came first. Two-tailed statistical tests were performed by SPSS for Windows version 20·0 (IBM SPSS Statistics, Chicago, IL, USA). GraphPad Prism version 5·00 for Windows (San Diego, CA, USA) was used for the figures.
Results
Overall progression to diabetes
We followed 6444 offspring or siblings of a type 1 diabetic patient and identified 394 who were persistently IAA+, GADA+, IA-2A+ and/or ZnT8A+. The degree of overlap between the four antibody types at baseline is shown in Fig. S1. At the time of first antibody positivity the majority (n = 249 or 63%) were IA-2A– and ZnT8A– (positive only for IAA and/or GADA); the others (n = 145 or 37%) presented IA-2A and/or ZnT8A with or without the other two antibodies. After a median (interquartile range; IQR) follow-up time of 63 (31–110) months, 34% of the antibody-positive relatives (n = 132) had developed diabetes [onset after 52 (25–84) months follow-up]. Most (n = 81, 61%) originated from the smaller subgroup with IA-2A+ and/or ZnT8A+, which consequently had a much higher risk of diabetes (81 of 145, 56% progression) than the larger IA-2A– and ZnT8A– subgroup (51 of 249, 21%; P < 0·001). Similar results were obtained when the analysis was conducted on antibody-positive relatives who developed diabetes within a 5-year follow-up period: 55 of the 74 rapid progressors (74%) were IA-2A+ and/or ZnT8A+ and the fraction of IA-2A+ and/or ZnT8A+ relatives who progressed within this short period was again significantly higher (55 of 145, 38%) than that of GADA+ and/or IAA+ relatives at first antibody positivity (19 of 249, 8%; P < 0·001). The general characteristics of IA-2A+ and/or ZnT8A+ relatives compared to those negative for both antibodies are shown in Table 1. Overall, relatives positive for IA-2A and/or ZnT8A had a higher PI/CP ratio (P = 0·001) and a shorter follow-up time (P < 0·001), as expected from their higher fraction of rapid progressors. They carried the HLA-DQ8 risk haplotype (P < 0·001) more often, but not the highest-risk HLA-DQ2/DQ8 genotype P = 0·233). They tended to be less frequently positive for HLA-DQ2 (P = 0·076) and neutral or protective genotypes (P = 0·005). As could be expected, they had a much lower frequency of solitary antibody positivity at baseline (P < 0·001) (Table 1).
Table 1.
General characteristics of the study population according to positivity for insulinoma antigen 2A (IA-2A) and/or zinc transporter 8 autoantibodies (ZnT8A)
| Characteristic | IA-2A+ and/or ZnT8A+ (n = 145) | IA-2A− and ZnT8A− (n = 249) | P-value |
|---|---|---|---|
| Age, years* | 11 (5–17) | 13 (7–19) | 0·046 |
| Sex, male/female (ratio) | 83/62 (1·3) | 136/113 (1·2) | 0·613 |
| BMI, kg/m2* | 17·9 (16·0–21·4) | 19·2 (16·5–23·5) | 0·019 |
| BMI SDS* | 0·05 (-0·75–0·85) | 0·32 (-0·62–1·36) | 0·075 |
| PI/CP, ratio* | 2·8 (1·7–2·4) | 2·1 (1·5–3·2) | 0·001 |
| Glucose, mmol/l* | 4·8 (4·3–5·3) | 4·8 (4·4–5·2) | 0·596 |
| Follow-up time, months* | 45 (24–81) | 75 (38–125) | < 0·001 |
| Relationship with proband | |||
| Sibling, n (%) | 84 (58) | 133 (53) | 0·385 |
| Offspring M, n (%) | 20 (14) | 61 (24) | 0·011 |
| Offspring F, n (%) | 41 (28) | 55 (22) | 0·168 |
| HLA genotype | |||
| DQ2/DQ8, n (%) | 41 (28) | 57 (23) | 0·233 |
| Non-DQ2/DQ8, n (%) | 70 (48) | 71 (28) | < 0·001 |
| DQ2/non-DQ8, n (%) | 23 (16) | 77 (31) | 0·001 |
| Non-DQ2/non-DQ8, n (%) | 11 (8) | 44 (18) | 0·005 |
| HLA haplotype | |||
| DQ8, n (%) | 111 (77) | 128 (51) | < 0·001 |
| DQ2, n (%) | 64 (44) | 134 (54) | 0·076 |
| Antibody positivity | |||
| Solitary antibody+ (1 of 4) | 18 (12) | 201 (81) | < 0·001 |
Data are median (interquartile range); BMI: body mass index; SDS: standard deviation score; PI: proinsulin; CP: C-peptide; offspring M: offspring of a T1D father; offspring F: offspring of a T1D mother; threshold for significance P < 0·05/17 or P < 0·0029 (Bonferroni correction).
Progression to diabetes according to age and antibody type
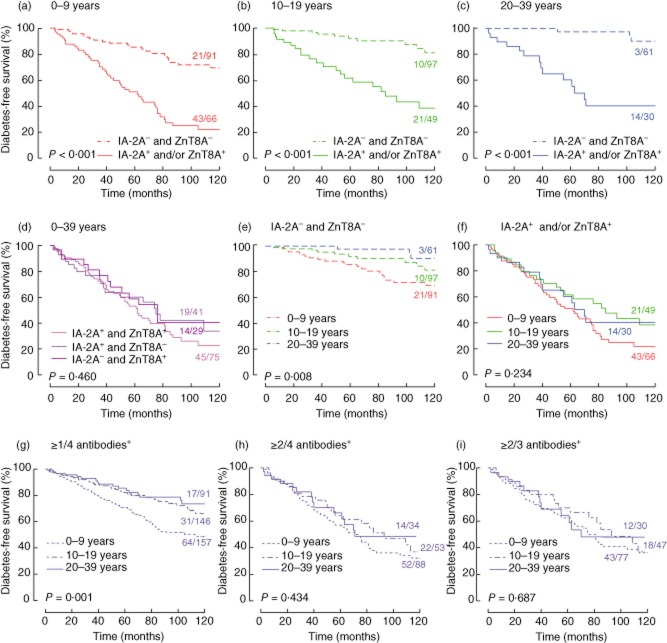
In children aged 0–9 years, in adolescents aged 10–19 years and in adults aged 20–39 years progression to diabetes was significantly more rapid in the presence of IA-2A and/or ZnT8A at baseline than in their joint absence (Fig. 1a–c; P < 0·001). The risk conferred by IA-2A+ and by ZnT8A+ was not additive (Fig. 1d; P > 0·05), but screening for ZnT8A in addition to IA-2A increased the number of high-risk individuals identified from 116 to 145 and the number of prediabetic relatives from 64 to 78 (Fig. 1d). When tested for four antibodies, relatives with solitary positivity for IA-2A or ZnT8A were rarely observed and progressed overall less rapidly to diabetes than IA-2A+ or ZnT8A+ individuals presenting with at least one other antibody (Fig. S2; P = 0·044 both for IA-2A+ and for ZnT8A+ relatives). In the latter group, the progression rate was, overall, not affected by the number of additional antibodies present (n = 1, 2 or 3; P = 0·831 for IA-2A+ relatives, P = 0·556 for ZnT8A+ relatives in Fig. S2). Hence, additional determination of other diabetes antibodies (IAA and GADA) is indicated only if IA-2A+ or ZnT8A+ are detected during initial screening for the latter two antibodies. As IA-2A and ZnT8A tend to cluster together [21], this is only a small fraction (18 of 145, 12%) of the relatives positive for either of the two antibodies.
Fig. 1.
Diabetes-free survival of persistently antibody-positive relatives as a function of time after first antibody-positive sample. (a) Age group 0–9 years: insulinoma antigen 2A (IA-2A)– and zinc transporter 8 autoantibodies (ZnT8A)– relatives (n = 91 at time 0) versus IA-2A+ and/or ZnT8A+ relatives (n = 66 at time 0). (b) Age group 10–19 years: IA-2A– and ZnT8A– relatives (n = 97 at time 0) versus IA-2A+ and/or ZnT8A+ relatives (n = 49 at time 0). (c) Age group 20–39 years: IA-2A– and ZnT8A– relatives (n = 61 at time 0) versus IA-2A+ and/or ZnT8A+ relatives (n = 30 at time 0). (d) Age group 0–39 years: IA-2A+ and ZnT8A+ relatives (n = 75 at time 0) versus IA-2A+ and ZnT8A– relatives (n = 41 at time 0) versus IA-2A– and/or ZnT8A+ relatives (n = 29 at time 0). (e) IA-2A– and ZnT8A– relatives: 0–9 years versus 10–19 years versus 20–39 years. (f) IA-2A+ and/or ZnT8A+ relatives: 0–9 years versus 10–19 years versus 20–39 years. (g) Relatives positive for ≥1 antibody among insulin autoantibodies (IAA), glutamate decarboxylase autoantibodies (GADA), IA-2A and ZnT8A: 0–9 years versus 10–19 years versus 20–39 years. (h) Relatives positive for ≥2 antibodies among IAA, GADA, IA-2A and ZnT8A: 0–9 years versus 10–19 years versus 20–39 years. (i) Relatives positive for ≥2 antibodies among IAA, GADA and IA-2A: 0–9 years versus 10–19 years versus 20–39 years. P-values by log-rank test. The fraction of relatives progressing to diabetes during follow-up is indicated above each arm in the nine panels.
The progression rate to diabetes decreased with increasing age at first antibody positivity only if IAA and/or GADA were present at baseline (Fig. 1e; P = 0·008), or in the case of positivity for more than one of four antibodies (Fig. 1g; P = 0·001), but was age-independent in the case of IA-2A+ and/or ZnT8A+ (Fig. 1f; P > 0·05) or in the case of positivity for more than two to three or two to four antibodies (Fig. 1h,i; P > 0·05). Although IA-2A and ZnT8A titres tended to increase with the number of antibodies present (not shown), progression to diabetes according to tertiles of antibody levels was not significantly different for both antibodies (P > 0·05; Fig. S3). In line with previous findings [33], our data show that relatives carrying the HLA-DQ2/DQ8 high-risk genotype progressed significantly faster than those at lower genetic risk both in the IA-2A+ and/or ZnT8A+ group (P < 0·001) and in antibody positive relatives lacking these two antibodies (P = 0·003, not shown). However, after stratifying the IA-2A+ and/or ZnT8A+ groups according to age at first antibody positivity, significance was reached only after age 10 (P = 0·038 and P = 0·003, respectively, between 10–19 and 20–39 years), despite the lower numbers of individuals in these age groups.
Table 2 confirms that the 5-year progression rate to diabetes and the sensitivity of screening for prediabetes remained age-independent in the case of IA-2A+ and/or ZnT8A+ at baseline, while they tended to decrease with age in the absence of both antibodies. Exclusion of relatives with protective HLA-DQ genotypes [26] or with a diabetic mother decreased the number of high-risk groups to be followed overall by about 20% and increased progression rates by 10–20% at the expense of <10% loss in sensitivity (Table 2). When the diagnostic performance of screening for IA-2A and/or ZnT8A positivity was compared to that of all other possible combinations for two types of antibodies (e.g. IAA and/or ZnT8A, GADA and/or IA-2A, …), the overall 5-year progression rate was highest for IA-2A+ and/or ZnT8A+, however, at the expense of a lower fraction of prediabetic relatives identified (Table S1).
Table 2.
Five-year progression to type 1 diabetes according to age and antibody type
| All relatives | Relatives without protective factors* | ||||||
|---|---|---|---|---|---|---|---|
| Age group | Antibody profile | n | Progression rate % (95% CI)† | Fraction T1D‡ % (n) | n | Progression rate % (95% CI) | Fraction T1D % (n) |
| 0–39 years | ≥ 1 antibody+ | 394 | 22 (17–27) | 100 (74) | 259 | 29 (23–35) | 100 (64) |
| IA-2A− and ZnT8A− | 249 | 9 (5–13) | 26 (19) | 145 | 13 (6–19) | 23 (15) | |
| IA-2A+ and/or ZnT8A+ | 145 | 44 (35–53) | 74 (55) | 114 | 49 (39–59) | 77 (49) | |
| ≥ 2/4 antibodies+ | 175 | 40 (32–47) | 82 (61) | 139 | 44 (35–53) | 86 (55) | |
| ≥ 2/3 antibodies+§ | 154 | 35 (27–43) | 65 (48) | 123 | 42 (32–51) | 72 (46) | |
| 0–9 years | ≥ 1 antibody+ | 157 | 29 (22–37) | 100 (42) | 106 | 36 (26–45) | 100 (35) |
| IA-2A− and ZnT8A− | 91 | 15 (7–22) | 29 (12) | 52 | 19 (8–30) | 26 (9) | |
| IA-2A+ and/or ZnT8A+ | 66 | 49 (36–62) | 71 (30) | 54 | 51 (37–65) | 74 (26) | |
| ≥ 2/4 antibodies+ | 88 | 43 (32–54) | 86 (36) | 68 | 49 (37–61) | 91 (32) | |
| ≥ 2/3 antibodies+ | 77 | 38 (27–49) | 67 (28) | 61 | 45 (32–58) | 77 (27) | |
| 10–19 years | ≥ 1 antibody+ | 146 | 18 (11–25) | 100 (21) | 98 | 24 (15–34) | 100 (19) |
| IA-2A− and ZnT8A− | 97 | 8 (2–14) | 29 (6) | 60 | 11 (2–20) | 26 (5) | |
| IA-2A+ and/or ZnT8A+ | 49 | 39 (23–54) | 71 (15) | 38 | 46 (28–64) | 74 (14) | |
| ≥ 2/4 antibodies+ | 53 | 36 (26–45) | 71 (15) | 43 | 41 (24–58) | 74 (14) | |
| ≥ 2/3 antibodies+ | 47 | 30 (15–45) | 52 (11) | 38 | 37 (19–55) | 58 (11) | |
| 20–39 years | ≥ 1 antibody+ | 91 | 15 (7–23) | 100 (11) | 55 | 23 (10–35) | 100 (10) |
| IA-2A− and ZnT8A− | 61 | 2 (0–7) | 9 (1) | 33 | 5 (0–14) | 10 (1) | |
| IA-2A+ and/or ZnT8A+ | 30 | 40 (20–59) | 91 (10) | 22 | 47 (24–70) | 90 (9) | |
| ≥ 2/4 antibodies+ | 34 | 34 (16–52) | 91 (10) | 28 | 37 (17–57) | 90 (9) | |
| ≥ 2/3 antibodies+ | 30 | 36 (17–56) | 82 (9) | 24 | 40 (18–62) | 80 (8) | |
Exclusion of carriers of protective HLA-DQ genotypes [30] and offspring of diabetic mothers;
95% confidence interval (CI);
type 1 diabetes (T1D);
screening for insulin autoantibodies (IAA), glutamate decarboxylase autoantibodies (GADA) and insulinoma antigen 2A (IA-2A). ZnT8A: zinc transporter 8 autoantibodies.
The fraction of IA-2A+ and/or ZnT8A+ relatives who progressed to diabetes within 5 years did not decrease with age at first antibody positivity (Table 2). Above age 10 years, screening for IA-2A and ZnT8A was at least as sensitive to detect rapid progressors as screening for positivity for more than two antibodies among IAA, GADA and IA-2A (with or without ZnT8A). Furthermore, the smaller group of IA-2A+ and/or ZnT8A+ adolescents or adults tended to have a 10–20% higher progession rate than the subgroup with more than two positive antibodies. Under age 10 years, screening for four antibodies, with or without exclusion of relatives with protective factors, detected up to 20% more rapid progressors but at the expense of following up to 30% more relatives. In addition, their overall progression rate was slower (Table 2). Of the 36 prediabetic relatives identified on the basis of positivity for more than two of the four antibodies tested, seven were IA-2A– and ZnT8A– at baseline. Of these seven, only three were older than 8 years – the usual lower age limit for inclusion in immunointervention [5] – and two of these three developed IA-2A and/or ZnT8A at a later time-point before diagnosis. One prediabetic child was recognized only by his positivity for IA-2A, and not by positivity for more than two antibodies. Under age 10 years, screening for ZnT8A and IA-2A remained more sensitive in detecting rapid progessors than screening for more than two or three positive antibodies among IAA, GADA and IA-2A.
Discussion
Regardless of age, screening for IA-2A and ZnT8A alone identified the majority (74%) of siblings and offspring with a family history of type 1 diabetes who developed diabetes within 5 years, compatible with the knowledge that both antibodies generally occur later during the preclinical disease phase than GADA, and in particular IAA [26,34]. The screening sensitivity and overall progression rate of IA-2A+ and/or ZnT8A+ relatives compared well with that of various combinations of multiple positive antibodies, at least for the age group older than 10 years at baseline. In relatives aged 0–9 years, additional measurement of IAA and GADA further improved screening sensitivity for rapid progressors, in line with observations from the Diabetes Autoimmunity Study in the Young (DAISY) on the importance of age at seroconversion and IAA levels in the prediction of diabetes in young children [34]. However, to a large extent this age group is – at least at present – not included in immunointervention trials [1–4]. Therefore, screening only for IA-2A and ZnT8A can be generalized as a cost-effective strategy to identify potential participants in secondary immunomodulatory prevention trials with similar antibody-inferred risk in children, adolescents and adults. Omission of relatives with genetic [31] and/or maternal [35] protective factors further improves screening efficiency in all age groups.
The strengths of the present study include the registry-based nature of the group of relatives, its wide age range, including many adults, and the lack of preselection based on ICA positivity. Although ICA have been measured in all samples, they were not taken into account in this study as they are not completely independent of GADA, IA-2A and ZnT8A, which are all believed to contribute to ICA [23]. Moreover, solitary ICA+ is rare and associated with a low risk of diabetes [36]. Our study population was selected among relatives of type 1 diabetic patients who represent only 10–15% of all newly diagnosed diabetic patients [37]. This is a limitation of our study. However, familial and non-familial cases of type 1 diabetes were shown not to differ in genetic, autoimmune and metabolic characteristics [37]; neither were there differences in disease-specific sensitivity of antibody markers between first-degree relatives and the general population [38]. Therefore, it is likely that our results in relatives can be extrapolated to the general population.
Immune interventions before clinical onset of type 1 diabetes require identification of adults and children with a high risk of diabetes in the short term [1–10]. For this type of prevention trial, a high positive predictive value of the marker combinations used – if necessary obtained at the expense of the sensitivity of identifying all prospective diabetic patients – is key to minimize the numbers needed to treat to reach conclusions within a few years [39,40]. The proposed screening strategy based on IA-2A and ZnT8A testing identifies a group of relatives with an overall 5-year diabetic risk that is higher than what can be achieved by any other screening combination for two autoantibodies. Moreover, the antibody-inferred risk by the proposed strategy is age-independent, but this property is related to the presence of more than two antibodies – which is frequently the case when IA-2A or ZnT8A can be detected – rather than to the type of circulating antibodies. Nevertheless, the screening sensitivity of the proposed approach for rapid progressors is equal or superior to detection of double antibody positivity through screening for three (at any age) or four autoantibodies (in the age group 10–39 years). Our data also indicate that the proposed antibody combination is not a surrogate for HLA-inferred risk or high antibody levels. Rather, these risk factors could complement each other in prediction models [13,19,33,34].
The proposed strategy allows reduction of the numbers of antibody-positive relatives who should undergo standardized beta cell function tests evaluating insulin or C-peptide release after acute or prolonged nutrient stimulation before their final enrolment in secondary prevention trials [2,6–8,16,17]. Indeed, decreased first- and/or second-phase C-peptide release (less than percentile 10 of healthy controls) during hyperglycaemic clamp identified the subgroup of IA-2A+ relatives with rapid progression to diabetes within 2 years [8]. As the second-phase C-peptide release of prediabetic relatives [median (IQR): 58% (44–73) of controls] was still higher than that of recent-onset patients (22% [15–29] of controls) [8,27], these relatives are likely candidates for participation in immune interventions [2,27].
Finally, a triple chimeric protein has been produced containing the intracellular domains of IA-2 (amino acids 605–979) and of the two common polymorphysms of ZnT8 (amino acids 268–369 with arginine or tryptophan at position 325) [41]. With this chimeric protein antigen, IA-2–ZnT8WR, a radiobinding assay was shown to detect autoantibodies to IA-2A and both major forms of ZnT8A with the same sensitivity and specificity as assays using the individual proteins as antigen [36]. Using such an assay would halve the cost and amount of serum needed for the screening strategy we propose here.
In conclusion, screening for IA-2A and ZnT8A allows efficient identification of relatives synchronized in the later stages of subclinical islet autoimmunity and to facilitate the identification of individuals with already compromised beta cell function who are likely to benefit most from immune interventions. Their enrolment may minimize the numbers needed to treat to observe significant effects within a reasonable period of 2–3 years, while avoiding the unnecessary exposure of non-progressors to the risk of side effects. If the lower age limit for participation in immune intervention trials is to be lowered in the future, additional determination of IAA and GADA should be considered at under age 10 years. Less aggressive prevention strategies may require other prediction models.
Acknowledgments
F. G. designed research, obtained funding, supervised the study, analysed and interpreted data, wrote and edited the manuscript. E. V. B. researched data, provided statistical analysis, analysed and interpreted data, reviewed and edited the manuscript. I. V., S. D., A. V. D., O. C., H. D., S. T., T. M., C. D. B., P. G. and K. D. researched data, reviewed and edited the manuscript. J. M. W., J. C. H. and D. G. P. researched data, contributed essential reagents, contributed to the discussion, reviewed and edited the manuscript. I. W. researched data, provided statistical analysis and advice, contributed to the discussion and reviewed and edited the manuscript. All authors approved the submitted version. The present work was supported by grants from the Juvenile Diabetes Research Foundation (JDRF Center Grant 4–2005-1327), the European Union (FP-7 project 241883), the Belgian Fund for Scientific Research (FWO Vlaanderen projects G.0319·01, G.0514·04, G.0311·07, G.0374·08 and G.0868·11; senior clinical research fellowship for K. D. and I. W.), the research council of the Brussels Free University (projects OZR1150, 1149 and 1615) and the Willy Gepts Fund (projects 3–2005 and 3/22–2007; University Hospital Brussels – UZ Brussel). J. C. H. acknowledges the University of Washington Diabetes Endocrinology Research Center (DERC) [National Institute of Health (NIH) grant P30-DK-57516], NIH grant R01-DK-052068 and JDRF grant 4–2007-1056. The Belgian Diabetes Registry was sponsored by the Belgian National Lottery, the Ministries of Public Health of the Flemish and French Communities of Belgium, Hippo and Friends, Weight Watchers, Ortho-Clinical Diagnostics, Novo Nordisk Pharma, Lifescan, Roche Diagnostics, Bayer and Eli Lilly. The expert technical assistance of co-workers at the central unit of the Belgian Diabetes Registry, Free University Brussels – VUB, Brussels (V. Baeten, G. De Block, T. De Mesmaeker, H. Dewinter, N. Diependaele, S. Exterbille, T. Glorieux, P. Goubert, C. Groven, T. Haulet, A. Ivens, D. Kesler, F. Lebleu, E. Quartier, G. Schoonjans, U. Vandevelde, M. Van Molle, S. Vanderstraeten, K. Verhaeghen and A. Walgrave) is gratefully acknowledged. We would also like to thank the different university teams of co-workers for their excellent assistance in collecting samples and organizing the fieldwork in University Hospital Antwerp – UZA, Antwerp (L. Van Gaal, C. De Block, R. Braspenning, J. Michiels and J. Vertommen), at University Hospital Brussels – UZ Brussel, Brussels (B. Keymeulen, K. Decochez, T. De Mesmaeker, S. Exterbille, P. Goubert, C. Groven, V. Kemels, C. Tettelin, S. Vanderstraeten, U. Van de Velde, A. Walgrave), at University Hospital Ghent – UZ Gent, Ghent (J. M. Kaufman, J. Ruige, A. Hutse, A. Rawoens, N. Steyaert) and in University Leuven – KUL, Leuven (C. Mathieu, P. Gillard, M. Carpentier, M. Robijn, K. Rouffé, A. Schoonis and H. Morobé). We sincerely thank all members of the Belgian Diabetes Registry who contributed to the recruitment of relatives for the present study. The list of members is given in Appendix S1.
Disclosure
The authors have no conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Overlap of positivity for the four autoantibody specificities tested at baseline. Prevalence data are expressed as percentage of the total number of relatives (n = 394). Reference: Oliveros, J. C. (2007) VENNY. An interactive tool for comparing lists with Venn diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html
Fig. S2. Diabetes-free survival of relatives positive for insulinoma antigen 2A (IA-2A) (a) or zinc transporter 8 autoantibodies (ZnT8A) (b) according to the number of other antibody specificities present (n = 0: purple, n = 1: red, n = 2: blue, n = 3: green). The fraction of relatives progressing to diabetes during follow-up is indicated above each arm in the two panels. *P-value for comparison of IA-2A+ (a) or ZnT8A+ (b) relatives presenting one, two or three additional antibody specificities (log-rank test).**P-value for comparison of solitary IA-2A+ (panel a) or ZnT8+ (panel b) relatives versus relatives positive for at least one additional antibody (n = 1, n = 2 and n = 3) taken together (log-rank test).
Fig. S3. Diabetes-free survival of relatives positive for insulinoma antigen 2A (IA-2A) (a) and those positive for zinc transporter 8 autoantibodies (ZnT8A) (b) according to the titre of IA-2A and ZnT8A, respectively: tertile 1 (green), tertile 2 (blue), tertile 3 (red). P-values by log-rank test. The fraction of relatives progressing to diabetes during follow-up is indicated above each arm in the two panels.
Table S1. Five-year progression to type 1 diabetes according to antibody type in antibody-positive relatives (0–39 years).
Appendix S1. List of the current members of the Belgian Diabetes Registry who participated in the recruitment of relatives and the handling of samples.
References
- 1.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 3.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skyler JS, Ricordi C. Stopping type 1 diabetes: attempts to prevent or cure type 1 diabetes in man. Diabetes. 2011;60:1–8. doi: 10.2337/db10-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach JF. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet. 2011;378:459–460. doi: 10.1016/S0140-6736(11)60980-X. [DOI] [PubMed] [Google Scholar]
- 7.Chatenoud L. Diabetes: type 1 diabetes mellitus – a door opening to a real therapy? Nat Rev Endocrinol. 2011;7:564–566. doi: 10.1038/nrendo.2011.148. [DOI] [PubMed] [Google Scholar]
- 8.Vandemeulebroucke E, Keymeulen B, Decochez K, et al. Hyperglycaemic clamp test for diabetes risk assessment in IA-2-antibody-positive relatives of type 1 diabetic patients. Diabetologia. 2010;53:36–44. doi: 10.1007/s00125-009-1569-3. [DOI] [PubMed] [Google Scholar]
- 9.Rewers M, Gottlieb P. Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care. 2009;32:1769–1782. doi: 10.2337/dc09-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skyler JS, Brown D, Chase HP, et al. Effect of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 11.Gale EAM, Bingley PJ, Knip M, et al. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised intervention before onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 12.Lachin JM, McGee PL, Greenbaum CJ, et al. Sample size requirements for studies of treatment effects on beta cell function in newly diagnosed type 1 diabetes. PLoS ONE. 2011;6:e26471. doi: 10.1371/journal.pone.0026471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingley PJ, Bonifacio E, Gale EA. Can we really predict IDDM? Diabetes. 1993;42:213–220. doi: 10.2337/diab.42.2.213. [DOI] [PubMed] [Google Scholar]
- 14.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010;33:1206–1212. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simell O, Winter WE, Schatz D. Enhancing the understanding of pre-type 1 diabetes in the general population. Diabetes Care. 2010;33:1403–1405. doi: 10.2337/dc10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56:2420–2424. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
- 17.Sosenko JM, Palmer JP, Rafkin LE, et al. Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care. 2010;33:620–625. doi: 10.2337/dc09-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orban T, Sosenko JM, Cuthbertson D, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach P, Bonifacio E, Koczwara K, et al. Natural history of type 1 diabetes. Diabetes. 2005;54(Suppl. 2):S25–S31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 20.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 21.De Grijse J, Asanghanwa M, Nouthe B, et al. Predictive power of screening for antibodies against insulinoma-associated protein 2 beta (IA-2beta) and zinc transporter-8 to select first-degree relatives of type 1 diabetic patients with risk of rapid progression to clinical onset of the disease: implications for prevention trials. Diabetologia. 2010;53:517–524. doi: 10.1007/s00125-009-1618-y. [DOI] [PubMed] [Google Scholar]
- 22.Gorus FK. Diabetes registries and early biological markers of insulin-dependent diabetes mellitus. Belgian Diabetes Registry. Diabetes Metab Rev. 1997;13:247–274. doi: 10.1002/(sici)1099-0895(199712)13:4<247::aid-dmr196>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Wenzlau JM, Frisch LM, Gardner TJ, et al. Novel antigens in type 1 diabetes: the importance of ZnT8. Curr Diabetes Rep. 2009;9:105–112. doi: 10.1007/s11892-009-0019-4. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen I, Weets I, Asanghanwa M, et al. Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care. 2011;34:1760–1765. doi: 10.2337/dc10-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie RD. Predicting adult-onset autoimmune diabetes: clarity from complexity. Diabetes. 2010;59:330–331. doi: 10.2337/db09-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen I, Weets I, Costa O, et al. An important minority of prediabetic first-degree relatives of type 1 diabetic patients derives from seroconversion to persistent autoantibody positivity after 10 years of age. Diabetologia. 2012;55:413–420. doi: 10.1007/s00125-011-2376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 28.Vandewalle CL, Coeckelberghs MI, De Leeuw IH, et al. Epidemiology, clinical aspects, and biology of IDDM patients under age 40 years. Comparison of data from Antwerp with complete ascertainment with data from Belgium with 40% ascertainment. The Belgian Diabetes Registry. Diabetes Care. 1997;20:1556–1561. doi: 10.2337/diacare.20.10.1556. [DOI] [PubMed] [Google Scholar]
- 29.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 30.Weets I, Van Autreve J, Van der Auwera BJ, et al. Male-to-female excess in diabetes diagnosed in early adulthood is not specific for the immune-mediated form nor is it HLA-DQ restricted: possible relation to increased body mass index. Diabetologia. 2001;44:40–47. doi: 10.1007/s001250051578. [DOI] [PubMed] [Google Scholar]
- 31.Van der Auwera BJ, Schuit FC, Weets I, et al. Relative and absolute HLA-DQA1–DQB1 linked risk for developing type 1 diabetes before 40 years of age in the Belgian population: implications for future prevention studies. Hum Immunol. 2002;63:40–50. doi: 10.1016/s0198-8859(01)00362-7. [DOI] [PubMed] [Google Scholar]
- 32.Truyen I, De Pauw P, Jørgensen PN, et al. Proinsulin levels and the proinsulin: C-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia. 2005;48:2322–2329. doi: 10.1007/s00125-005-1959-0. [DOI] [PubMed] [Google Scholar]
- 33.Decochez K, Truyen I, Van der Auwera B, et al. Combined positivity for HLA DQ2/DQ8 and IA-2 antibodies defines population at high risk of developing type 1 diabetes. Diabetologia. 2005;48:687–694. doi: 10.1007/s00125-005-1702-x. [DOI] [PubMed] [Google Scholar]
- 34.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes. Diabetes Autoimmunity Study in the Young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifacio E, Pflüger M, Marienfeld S, et al. Maternal type 1 diabetes reduces the risk of islet autoantibodies: relationships with birthweight and maternal HbA(1c) Diabetologia. 2008;51:1245–1252. doi: 10.1007/s00125-008-1022-z. [DOI] [PubMed] [Google Scholar]
- 36.Long AE, Gooneratne AT, Rokni S, et al. The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab. 2012;97:632–637. doi: 10.1210/jc.2011-1952. [DOI] [PubMed] [Google Scholar]
- 37.Veijola R, Reijonen H, Vähäsalo P, et al. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. Childhood Diabetes in Finland (DiMe) Study Group. J Clin Invest. 1996;98:2489–2495. doi: 10.1172/JCI119067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siljander HT, Veijola R, Reunanen A, Virtanen SM, Åkerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272–2275. doi: 10.1007/s00125-007-0799-5. [DOI] [PubMed] [Google Scholar]
- 39.Mahon JL, Dupre J. The limitations of clinical trials for preventions of IDDM. Diabetes Care. 1997;20:1027–1033. doi: 10.2337/diacare.20.6.1027. [DOI] [PubMed] [Google Scholar]
- 40.Gorus FK, Pipeleers DG. Belgian Diabetes Registry. Prospects for predicting and stopping the development of type 1 diabetes. Best Pract Res Clin Endocrinol Metab. 2001;15:371–389. doi: 10.1053/beem.2001.0152. [DOI] [PubMed] [Google Scholar]
- 41.Yu L, Liu Y, Miao D, et al. Triple chimeric islet autoantigen IA-2-ZnT8WR to facilitate islet autoantibody determination. J Immunol Methods. 2010;353:20–23. doi: 10.1016/j.jim.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.