Despite many efforts over the past ∼40 years to determine the influence of ageing on exercise hyperaemia, fundamental questions remain regarding (1) the interactive influences of primary ageing and physical activity level and (2) whether or not attenuated blood flow and vascular conductance responses at a given work rate have metabolic or functional consequences (Proctor & Parker, 2006). Nowhere have these questions been more comprehensively addressed than in a study published in the current issue of The Journal of Physiology. In this study (Mortensen et al. 2012), Mortensen and colleagues at the Copenhagen Muscle Research Centre examined exercise- and pharmacologically-induced leg vascular and metabolic responses in three groups of men: young, lifelong sedentary, and lifelong physically active older men. They report that, during single leg knee extensor exercise at the same submaximal work rate, both sedentary and physically active older men exhibited blunted vasodilator responses compared with younger men. Similarly, leg blood flow during exercise was significantly (sedentary) or on average (physically active) lower in both groups of older men. However, in the sedentary older men, attenuated leg vascular responses were associated with greater lactate release (Fig. 3 in their paper), while similarly attenuated vascular responses in the lifelong active men were not. These findings provide evidence to suggest that lower blood flow to exercising leg muscles in sedentary older men may be associated with impaired local aerobic metabolism. Therefore, despite similar vasodilator and blood flow responses to dynamic exercise between the two older groups, the extent to which these responses are sufficient to meet the metabolic demand appear to differ depending on lifelong physical activity.
While the use of isolated leg ergometry models (e.g. knee extensor and stationary cycling) offer the advantage of measuring arterial inflow and assessing local vascular and metabolic responses within a well-defined group of muscles, it is important to note that whole limb blood flow and arterial-venous differences tell us little about the distribution of blood flow within the active limb(s). As seen in Fig. 3 of their paper, local noradrenaline release induced by tyramine lowered blood flow and vascular conductance but augmented (a–v) O2 difference in the exercising leg of sedentary older men despite unaltered leg 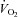 and lactate release. Taken together, these findings suggest that tyramine-induced constriction in the exercising leg of sedentary older men may be occurring to a greater extent within less metabolically active regions. These findings are in contrast to the younger and lifelong active older men who exhibited unaltered vascular and metabolic responses to tyramine. Collectively, these findings suggest that blood flow may be less effectively distributed within the exercising leg of sedentary, but not lifelong active, older men. Moreover, a less effective distribution of blood flow in the exercising leg of sedentary older men could possibly result from attenuated constriction within less metabolically active regions and/or impaired dilatation within active muscle fibres.
and lactate release. Taken together, these findings suggest that tyramine-induced constriction in the exercising leg of sedentary older men may be occurring to a greater extent within less metabolically active regions. These findings are in contrast to the younger and lifelong active older men who exhibited unaltered vascular and metabolic responses to tyramine. Collectively, these findings suggest that blood flow may be less effectively distributed within the exercising leg of sedentary, but not lifelong active, older men. Moreover, a less effective distribution of blood flow in the exercising leg of sedentary older men could possibly result from attenuated constriction within less metabolically active regions and/or impaired dilatation within active muscle fibres.
The paper by Mortensen et al. also significantly advances our understanding of how ageing and activity level may influence the specific vasodilator and constrictor mechanisms that regulate the distribution of flow in human skeletal muscle during exercise. Intra-arterial ATP induces a conducted vasodilator response that is resistant to sympathetically mediated vasoconstriction, and low 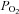 -induced release of ATP from erythrocytes has been proposed to control muscle perfusion during exercise (Sprague et al. 2010). In their study, Mortensen et al. found that ATP-induced vasodilatation was attenuated in the leg vasculature of sedentary older men, but was preserved in the lifelong active older men compared with the young men. Furthermore, Fig. 6 of their paper provides evidence to suggest that these differences may be related, at least in part, to differences in skeletal muscle P2Y2 receptor content, which was ∼3-fold lower in the older sedentary vs. young men. While the role of interstitial (i.e. extravascular) ATP on exercise hyperaemia in humans is unclear, putative roles include both vasodilator and vasoconstrictor functions by blunting local sympathetic vasoconstriction and augmenting sympathetic nerve activation, respectively. With regard to this latter function, it should be noted that α-adrenergic responsiveness is lower in leg blood vessels of older men (Smith et al. 2007). As such, it is reasonable to speculate that the augmented interstitial ATP concentrations in lifelong active older men (Fig. 5 in Mortensen et al.) may enhance sympathetically mediated constriction in less metabolically active muscle fibres, and provide a potential compensatory mechanism by which efficient distribution of flow within the active limb is preserved in lifelong physically active, but not sedentary, older men.
-induced release of ATP from erythrocytes has been proposed to control muscle perfusion during exercise (Sprague et al. 2010). In their study, Mortensen et al. found that ATP-induced vasodilatation was attenuated in the leg vasculature of sedentary older men, but was preserved in the lifelong active older men compared with the young men. Furthermore, Fig. 6 of their paper provides evidence to suggest that these differences may be related, at least in part, to differences in skeletal muscle P2Y2 receptor content, which was ∼3-fold lower in the older sedentary vs. young men. While the role of interstitial (i.e. extravascular) ATP on exercise hyperaemia in humans is unclear, putative roles include both vasodilator and vasoconstrictor functions by blunting local sympathetic vasoconstriction and augmenting sympathetic nerve activation, respectively. With regard to this latter function, it should be noted that α-adrenergic responsiveness is lower in leg blood vessels of older men (Smith et al. 2007). As such, it is reasonable to speculate that the augmented interstitial ATP concentrations in lifelong active older men (Fig. 5 in Mortensen et al.) may enhance sympathetically mediated constriction in less metabolically active muscle fibres, and provide a potential compensatory mechanism by which efficient distribution of flow within the active limb is preserved in lifelong physically active, but not sedentary, older men.
In summary, the findings presented by Mortensen et al. significantly advance our understanding of how ageing and physical activity influence the local regulation of blood flow during exercise. In particular, they highlight the importance of focusing less on absolute limb flow and conductance responses, and more on the extent to which these responses are sufficient to meet the metabolic demand in a particular subject group. In this context, vascular responses in lifelong physically active men, but not their sedentary peers, appear to provide sufficient oxygen delivery to the active muscles during exercise. Lastly, the novel insight gained by the study of Mortensen et al. supports the use of similar integrative approaches in other sub-groups exhibiting attenuated vascular responses during exercise, e.g. post-menopausal women (Moore et al. 2012) and other patient groups with elevated cardiovascular or metabolic disease risk.
References
- Moore DJ, Gonzales JU, Tucker SH, Elavsky S, Proctor DN. Appl Physiol Nutr Metab. 2012;37:418–424. doi: 10.1139/h2012-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K, Saltin B. J Physiol. 2012;590:6227–6237. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Bowles EA, Achilleus D, Ellsworth ML. Acta Physiol (Oxf) 2010;202:285–292. doi: 10.1111/j.1748-1716.2010.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


