Abstract
Although a number of studies have examined potential differences in temperature regulation between males and females during heat stress, conclusions have remained limited as to whether reported differences are due to confounding physical characteristics or to actual differences in the physiological variables of temperature regulation. Recent observations suggest that sex differences in temperature regulation, particularly in sudomotor activity, go beyond those associated with physical characteristics. Females have recently been shown to have a lower sudomotor activity, as well as a lower thermosensitivity of the response compared to males during exercise performed at a fixed rate of metabolic heat production. Furthermore, sex differences in local and whole-body sudomotor activity are only evident above a certain combination of environmental conditions and rate of metabolic heat production. In contrast, both the onset threshold and thermosensitivity of cutaneous vasodilatation are similar between males and females. In theory, differences in the thermosensitivity of sudomotor activity could be related to either a central (neural activity/integration) and/or peripheral (effector organ) modulation of temperature regulation. Based on recent findings, sex differences in sudomotor activity appear to be mediated peripherally, although a central modulation has yet to be conclusively ruled out. Here we present a brief yet comprehensive review of the current state of knowledge pertaining to sex differences in temperature regulation during exercise in the heat.
Daniel Gagnon (left) is a doctoral candidate under the supervision of Dr Glen Kenny at the University of Ottawa. His research focuses on sex-related differences in human temperature regulation, with an emphasis on trying to eliminate confounding biophysical factors to isolate potential physiological sex differences. His work has been recognised by the American Physiological Society's Environmental and Exercise Physiology Section (Predoctoral Recognition Award), as well as the American College of Sports Medicine (Charles M. Tipton National Student Award). Glen P. Kenny (right) is Professor of Exercise Physiology at the University of Ottawa and holds a University Research Chair in Environmental Physiology. His unique calorimeter-based research is directed at evaluating the physiological mechanisms governing human thermoregulatory control during heat stress with an emphasis on understanding the thermal and non-thermal contributions to human heat balance. His work also includes studying the physiological effects and consequences of heat stress in at-risk subpopulations with conditions that render them particularly vulnerable to heat injury, such as ageing, obesity, diabetes and related disorders.
 |
Introduction
A consistent growth in publications investigating sex differences in temperature regulation during heat stress began during the 1960s (Wyndham et al. 1965; Morimoto et al. 1967; Weinman et al. 1967). These studies aimed to determine if males and females could tolerate prolonged exercise in the heat to a similar extent pre- and post-acclimatisation, and led to the contradictory notions that females thermoregulate both less (pre-acclimatisation) and more (post-acclimatisation) effectively than males. These findings were summarised in three review articles, all of which raised the possibility that reported sex differences in temperature regulation could have been due to confounding differences in physical characteristics and/or fitness (Nunneley, 1978; Burse, 1979; Kenney, 1985). A number of subsequent studies therefore attempted to control for either or both of these variables. Overall, these later studies led to the general consensus that sex differences in temperature regulation can be explained by differences in physical characteristics and aerobic fitness (Sawka et al. 1996; Havenith, 2001b). Recent studies (Gagnon & Kenny, 2011, 2012) which have attempted to isolate the effect of sex from those of differences in physical characteristics and rate of metabolic heat production suggest that true physiological differences in temperature regulation may exist between males and females, particularly in maximal sweating as observed during exercise performed in environments that allow its full evaporation.
The purpose of the current review is to summarise these recent findings and to provide a brief yet exhaustive review of the current knowledge pertaining to sex differences in local and whole-body heat loss responses. To do so, the current review will first provide a brief overview of the physiological and physical variables governing temperature regulation during exercise in the heat. This will serve to provide a background against which to assess potential physiological sex differences in sweating and/or skin blood flow.
Physiology of temperature regulation
The neural control of body temperature is achieved through thermoreceptors which detect changes in body temperature both centrally within the central nervous system (Hammel et al. 1960), as well as peripherally in the skin (Pierau, 1996) and core (Stitt, 1993). The peripheral thermoreceptors are responsible for transmitting thermoafferent information to the central nervous system, particularly in the region of the preoptic anterior hypothalamus where most of thermal integration is thought to occur (Boulant, 1996). Integration of thermal information ultimately results in the central nervous system sending thermoefferent signals, via the autonomic nervous system, to the appropriate effector organs which control and alter rates of heat exchange within the body and from the body to the environment (Werner, 1980, 1981). The relation between neural integration and effector output is best described by a mean body temperature onset threshold beyond which effector responses increase proportionally to the change in core/skin temperatures (Fig. 1). During exercise in the heat, cutaneous vasodilatation and increased sudomotor activity serve to dissipate heat from the body for the proper regulation of body temperature (Hardy, 1961). Since both responses are affected by core and skin temperatures (Fusco et al. 1961; Nadel et al. 1971a; Wenger et al. 1985), the change in effector response is often analysed as a function of the change in mean body temperature (Gisolfi & Wenger, 1984), calculated using a weighted summation of core and mean skin temperatures (i.e. X × core + (1 – X) × mean skin). It should be noted that the influence of core temperature is heavily weighted (X= 0.8–0.9) during heat stress, such that it represents the main variable driving the relationship between mean body temperature and thermoeffector output. Therefore, studies have also analysed changes in the onset threshold and thermosensitivity of thermoeffector responses as a function of changes in core temperature only, while examining the influence that skin temperature may have upon this relationship (Nadel et al. 1971a,b). When mean body temperature is used as the independent variable, the onset threshold is determined as the mean body temperature at which a sustained increase in thermoeffector output occurs, while the rate at which the thermoeffector output changes as a function of the increase in mean body temperature is known as the thermosensitivity (Hammel, 1968; Cheuvront et al. 2009). Since the interpretation of thermoefferent activity, namely skin sympathetic nerve activity, is problematic between individuals or over separate days (Young et al. 2009), the onset threshold and the thermosensitivity of thermoeffector responses currently represent the only viable means by which the physiology of human temperature regulation can be evaluated (Fig. 1). Although both variables can represent a central and/or peripheral modulation of temperature regulation (Hammel, 1968), it has been suggested that a parallel shift in the onset threshold of both effector responses must occur to be representative of a central modulation (Gisolfi & Wenger, 1984). As such, changes in the thermosensitivity of an effector response, without parallel changes in the onset threshold, probably imply a peripheral modulation. In fact, changes in onset threshold have traditionally been used as an indicator of central modulation of temperature regulation, while the thermosensitivity has been used to describe peripheral adaptations in effector responses (Nadel et al. 1971b, 1974).
Figure 1. Schematic representation of the thermoeffector output-to-mean body temperature relationship during heat stress.
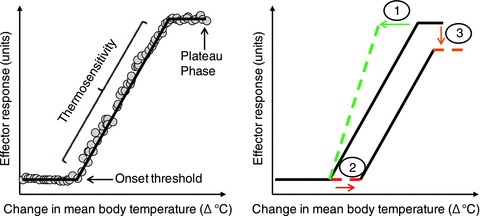
Left panel: an increase in mean body temperature occurs before the effector response is activated at a given onset threshold. The effector output subsequently increases proportionally to the increase in mean body temperature, the linear portion of which represents the thermosensitivity of the response. Once the effector response reaches maximal values, a flattening of the line is observed, whereby no further increase in effector output occurs despite increasing mean body temperature. It should be noted that this relationship has been demonstrated using local, whole-body, and whole-limb measurements of thermoeffector responses. Right panel: examples of how the parameters of the thermoeffector output-to-mean body temperature relationship can change: (1) the thermosensitivity of the response is increased, such that a greater change in effector output occurs for a given change in mean body temperature; (2) the onset threshold of the response is shifted to the right, such that a greater change in mean body temperature is required to initiate the activation of effector output; (3) the plateau phase of the effector output is reduced, such that lower maximal values are attained for a given change in mean body temperature.
Temperature regulation and exercise in the heat
At the onset of dynamic exercise, there is an instant and rapid elevation in the rate of metabolic heat production with a time constant of ∼5 min (Kenny et al. 2008). The increased heat production at the onset of exercise is not immediately matched by an increase in whole-body heat loss (Webb, 1995). Mean body temperature therefore rises beyond onset threshold values, activating the heat loss responses of cutaneous vasodilatation and sweating (Benzinger, 1969; Werner, 1993; Sawka et al. 1996). Together, both serve to increase whole-body heat loss (Fig. 2), with a time constant of ∼10 min (Webb et al. 1970; Kenny et al. 2008). While the onset threshold at which heat loss responses are activated and the rate at which they increase (thermosensitivity) depend upon changes in mean body temperature, the level of whole-body heat loss achieved during exercise in the heat depends upon the required evaporation for heat balance (Ereq), which is the sum of metabolic heat production and dry heat exchange. Since evaporation of sweat represents the main component of total heat loss during exercise, particularly in the heat, the level of sudomotor activity achieved is therefore driven by the evaporation needed to achieve heat balance (Fig. 2). When the maximal evaporation possible within a given environment (Emax) does not limit an individual's ability to achieve heat balance (i.e. compensable; Emax ≥ Ereq), the level of sudomotor activity achieved during exercise is driven by the required evaporation for heat balance (Jay et al. 2011; Cramer et al. 2012). However, when the combination of environmental conditions and metabolic heat production exceed the individual's maximum evaporative capacity (Esk,max), therefore creating an uncompensable heat stress scenario (i.e. Esk,max < Ereq), the level of sudomotor activity will be driven by the individual's maximum sweating rate (Fig. 2). It should be noted, however, that these scenarios assume that all of the sweat produced by the body is evaporated (i.e. 100% efficiency). In reality, sweating efficiency decreases as the required evaporation for heat balance approaches the maximum evaporative capacity of the individual within a given environment (Candas et al. 1979). Consequently, the level of sudomotor activity achieved is more precisely related to the ratio of required evaporation for heat balance and the individual's maximum evaporative capacity in that environment (Shapiro et al. 1982; Bain et al. 2011).
Figure 2. Schematic illustration of the relationship between the required evaporation for heat balance (Ereq) and evaporative heat loss (Esk) during exercise.
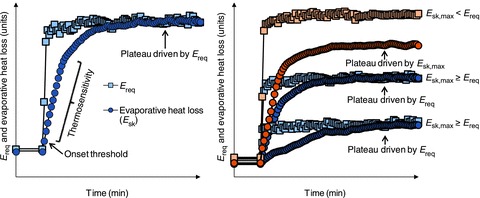
Left panel: the initial increase in evaporative heat loss during exercise is dictated by the onset threshold for sweating, with the subsequent linear increase in evaporative heat loss a function of the change in mean body temperature (thermosensitivity). However, the level of evaporative heat loss attained during exercise is determined by the required evaporation for heat balance (Ereq). Right panel: when the required evaporation for heat balance does not exceed the individual's maximal evaporative (sweating) capacity (Esk,max ≥ Ereq), the level of evaporative heat loss attained during exercise is determined by the required evaporation for heat balance (blue colour). However, when the required evaporation for heat balance exceeds the individual's maximum evaporative capacity (Esk,max < Ereq), the level of evaporative heat loss attained during exercise is determined by the individual's maximal evaporative (Esk,max) capacity (orange colour).
Physical factors influencing sex-related differences in temperature regulation
The main physical characteristics influencing temperature regulation during exercise in the heat are body mass and surface area (Anderson, 1999; Havenith, 2001b). Briefly, body mass represents the capacity of the body to store heat, such that individuals with a greater body mass typically have smaller increases in core temperature during heat stress (Havenith et al. 1995, 1998). In contrast, the amount of body surface area exposed to the external environment determines the rate of heat exchange between the skin and the ambient air, such that a high body surface area is generally associated with a lower core temperature during heat stress (Havenith & van Middendorp, 1990; Havenith et al. 1995, 1998).
The possible impact sex differences in physical characteristics may have upon temperature regulation was put forward by Burse (1979). As a population, females are smaller and have less body surface area compared to males which makes it difficult to differentiate whether a different core temperature and/or heat loss response is attributed to either simple differences in physical characteristics, differences in the physiological variables of temperature regulation, or a combination of the two. Studies which examine sex differences in temperature regulation during exercise in the heat have generally failed to consider sex differences in physical characteristics when examining both sexes as a whole. In some studies (Shapiro et al. 1980; McLellan, 1998), the potential impact of such differences was examined by comparing subgroups of males and females with similar physical characteristics. In either case, most studies have relied upon core temperature as an indicator of thermoregulatory function, the majority of which report greater end-exercise core temperatures in females. Although sex differences in core temperature might intuitively suggest differences in the physiology of temperature regulation (e.g. altered sweating response), we have shown that females can exhibit greater core, skin and active muscle temperatures despite a similar whole-body heat loss response compared to males (Gagnon et al. 2009). Therefore, core temperature alone cannot be reliably used to gain insight into potential physiological differences in temperature regulation when males and females are not matched for particular physical characteristics.
Another important physical characteristic to consider when comparing heat loss responses between sexes during exercise is maximum oxygen consumption (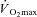 ). In the studies performed during the 1960s, greater heart rates in females led to the suggestion that physical fitness should be considered when comparing males and females during exercise in the heat (Drinkwater et al. 1976, 1977). To account for potential differences in physical fitness, subsequent studies either compared males and females during treadmill exercise at a fixed external workload (Davies, 1979; Avellini et al. 1980a,b; Shapiro et al. 1980; McLellan, 1998; Moran et al. 1999), or during exercise performed at a given percentage of
). In the studies performed during the 1960s, greater heart rates in females led to the suggestion that physical fitness should be considered when comparing males and females during exercise in the heat (Drinkwater et al. 1976, 1977). To account for potential differences in physical fitness, subsequent studies either compared males and females during treadmill exercise at a fixed external workload (Davies, 1979; Avellini et al. 1980a,b; Shapiro et al. 1980; McLellan, 1998; Moran et al. 1999), or during exercise performed at a given percentage of 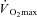 (Paolone et al. 1978; Frye & Kamon, 1981; Horstman & Christensen, 1982; Keatisuwan et al. 1996; Ichinose-Kuwahara et al. 2010). In some cases, an attempt was made to compare males and females with similar aerobic capacities expressed relative to body weight (Avellini et al. 1980a; Frye & Kamon, 1981; Moran et al. 1999; Ichinose-Kuwahara et al. 2010). In general, it was found that physically active females had similar cardiovascular responses and heat tolerance times despite lower sweat rates compared to males when treadmill exercise was performed at a given external workload. On the other hand, when both sexes exercised at the same percentage of
(Paolone et al. 1978; Frye & Kamon, 1981; Horstman & Christensen, 1982; Keatisuwan et al. 1996; Ichinose-Kuwahara et al. 2010). In some cases, an attempt was made to compare males and females with similar aerobic capacities expressed relative to body weight (Avellini et al. 1980a; Frye & Kamon, 1981; Moran et al. 1999; Ichinose-Kuwahara et al. 2010). In general, it was found that physically active females had similar cardiovascular responses and heat tolerance times despite lower sweat rates compared to males when treadmill exercise was performed at a given external workload. On the other hand, when both sexes exercised at the same percentage of 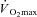 , it was generally found that females had lower end-exercise core temperatures despite having lower sweat rates. However, both exercise protocols contain inherent methodological issues due to the fact that males and females were not matched for body mass.
, it was generally found that females had lower end-exercise core temperatures despite having lower sweat rates. However, both exercise protocols contain inherent methodological issues due to the fact that males and females were not matched for body mass.
Metabolic energy expenditure is proportional to body mass during weight-bearing exercise such as treadmill walking (Austin & Lansing, 1986). Consequently, treadmill walking at a fixed external workload elicits a lower rate of metabolic heat production in females due to their lower body mass (Havenith, 2001a). This results in a lower required evaporation for heat balance (Ereq), which itself can explain the lower sweat rates observed. Similarly, exercise at a given percentage of 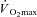 also elicits a lower rate of metabolic heat production in females. This occurs both when females have a lower absolute
also elicits a lower rate of metabolic heat production in females. This occurs both when females have a lower absolute 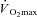 (in l min−1) as well as when both sexes are matched for relative
(in l min−1) as well as when both sexes are matched for relative 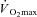 (either per unit of body mass or of fat-free mass) if females have a lower body mass (see calculation example in Appendix). While this problem has been recognised previously (Kenney, 1985; Bar-Or, 1998), and again more recently (Schwiening et al. 2011), this protocol was employed in most studies because it was thought that exercise at a given rate of metabolic heat production would cause undue physiological strain to females since they would be working at a greater percentage of
(either per unit of body mass or of fat-free mass) if females have a lower body mass (see calculation example in Appendix). While this problem has been recognised previously (Kenney, 1985; Bar-Or, 1998), and again more recently (Schwiening et al. 2011), this protocol was employed in most studies because it was thought that exercise at a given rate of metabolic heat production would cause undue physiological strain to females since they would be working at a greater percentage of 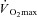 (Bar-Or, 1998). Nonetheless, when males and females exercise at the same percentage of
(Bar-Or, 1998). Nonetheless, when males and females exercise at the same percentage of 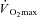 , the lower rate of metabolic heat production in females results in a lower rate of whole-body sudomotor activity (Gagnon et al. 2008; Gagnon & Kenny, 2011). Since whole-body sudomotor activity is determined by local sweat production, it follows that a lower rate of metabolic heat production will elicit a lower sweat rate in females (Fig. 3). As such, protocols based either on treadmill exercise performed at a fixed external workload, or on exercise performed at a given percentage of
, the lower rate of metabolic heat production in females results in a lower rate of whole-body sudomotor activity (Gagnon et al. 2008; Gagnon & Kenny, 2011). Since whole-body sudomotor activity is determined by local sweat production, it follows that a lower rate of metabolic heat production will elicit a lower sweat rate in females (Fig. 3). As such, protocols based either on treadmill exercise performed at a fixed external workload, or on exercise performed at a given percentage of 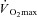 , result in a lower required evaporation for heat balance in females when males and females are not matched for body mass. This limitation explains the lower sweat rates in females reported in many studies, and conclusions therefore remained limited as to whether sex actually modulates the physiological variables of temperature regulation during exercise in the heat.
, result in a lower required evaporation for heat balance in females when males and females are not matched for body mass. This limitation explains the lower sweat rates in females reported in many studies, and conclusions therefore remained limited as to whether sex actually modulates the physiological variables of temperature regulation during exercise in the heat.
Figure 3. Schematic illustration of the relationship between sex differences in metabolic heat production (M-W) and total whole-body heat loss (THL).
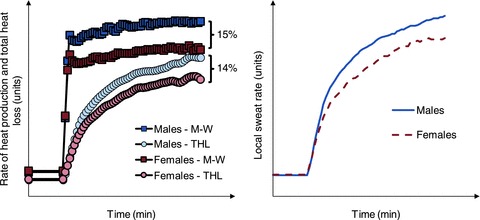
Left panel: exercise performed at a fixed percentage of maximum oxygen consumption results in a rate of metabolic heat production (squares) that is ∼15% greater in males compared to females. Consequently, rate of whole-body heat loss is proportionately greater in males, such that the differences in whole-body heat loss between sexes are of the same order (∼14%) as those for rate of metabolic heat production. Right panel: the greater rate of whole-body heat loss is reflected by a greater rate of local sweat production in males compared to females. In this situation, the greater rate of local sweat production can be attributed to the differences in rate of metabolic heat production elicited by employing an experimental protocol in which exercise is performed at a given percentage of maximum oxygen consumption. The left panel is re-drawn with kind permission of Springer Science+Business Media from Gagnon et al. (2008).
Physiological sex-related differences in temperature regulation
The majority of studies which have examined physiological differences in temperature regulation in females have generally focused on those related to the female menstrual cycle. The rise in plasma concentrations of progesterone and oestrogen during the luteal phase of the menstrual cycle shifts resting body temperature by ∼0.3–0.5°C relative to the follicular phase (Stephenson & Kolka, 1993). In parallel, the core temperature onset thresholds for cutaneous vasodilatation (Stephenson & Kolka, 1985; Kolka & Stephenson, 1997) and sweating (Stephenson & Kolka, 1985) are similarly shifted to core temperatures which are ∼0.3–0.5°C greater relative to the follicular phase. In contrast, the thermosensitivity of each response is generally unchanged. This upward shift in temperature regulation is similarly affected in females taking oral contraceptives by the synthetic progesterone and oestrogen contained in combination contraceptives (Charkoudian & Johnson, 1997). For more specific differences in temperature regulation across the menstrual cycle, the reader is referred to excellent reviews on this topic (Stephenson & Kolka, 1993; Marsh & Jenkins, 2002; Charkoudian & Joyner, 2004). However, these findings emphasise the need to consider the phase of the menstrual cycle in females when examining sex differences in temperature regulation. In the recent findings highlighted below, females were tested between the first and tenth day after the onset of their self-reported menses or towards the end of the no pill/placebo period of oral contraceptive use.
In contrast to the influence of sex hormones, no experiments have examined a possible influence of sex on the onset threshold and thermosensitivity of sweating and cutaneous vasodilatation, independent of differences in physical characteristics and rate of metabolic heat production. Recently, we sought to determine whether such differences were apparent during exercise in the heat (Gagnon & Kenny, 2011). Males and females, matched for body mass (∼66–67 kg) and surface area (∼1.76–1.79 m2), performed 90 min of exercise in a warm (35°C) and dry (12% relative humidity) environment at either 50% of 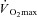 or at a fixed rate of metabolic heat production of 500 W. As expected, rate of metabolic heat production was significantly greater in males throughout exercise performed at 50% of
or at a fixed rate of metabolic heat production of 500 W. As expected, rate of metabolic heat production was significantly greater in males throughout exercise performed at 50% of 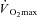 , which was paralleled by a greater rate of whole-body sudomotor activity, as well as a greater thermosensitivity of the whole-body sudomotor and local cutaneous vascular responses. Importantly, more than 80% of the variance in whole-body sudomotor activity between sexes was explained by differences in rate of metabolic heat production. Furthermore, previous studies have shown that the thermosensitivity of both sudomotor activity and cutaneous vascular conductance increases at greater rates of metabolic heat production (Montain et al. 1995; Kondo et al. 1998). Therefore, the lower rate of whole-body sudomotor activity in females during this condition, as well as the lower thermosensitivity of the response, was mainly attributed to the lower rate of metabolic heat production associated with the experimental protocol. However, when exercise was performed at a fixed rate of metabolic heat production, whole-body sudomotor activity and the thermosensitivity of the response were nonetheless lower in females. In contrast, no sex differences in the onset threshold for whole-body sudomotor activity, as well as in cutaneous vascular conductance as a whole, were observed during exercise performed at a fixed rate of metabolic heat production. These observations suggest a sex difference in sudomotor activity that is not associated with differences in physical characteristics and rate of metabolic heat production.
, which was paralleled by a greater rate of whole-body sudomotor activity, as well as a greater thermosensitivity of the whole-body sudomotor and local cutaneous vascular responses. Importantly, more than 80% of the variance in whole-body sudomotor activity between sexes was explained by differences in rate of metabolic heat production. Furthermore, previous studies have shown that the thermosensitivity of both sudomotor activity and cutaneous vascular conductance increases at greater rates of metabolic heat production (Montain et al. 1995; Kondo et al. 1998). Therefore, the lower rate of whole-body sudomotor activity in females during this condition, as well as the lower thermosensitivity of the response, was mainly attributed to the lower rate of metabolic heat production associated with the experimental protocol. However, when exercise was performed at a fixed rate of metabolic heat production, whole-body sudomotor activity and the thermosensitivity of the response were nonetheless lower in females. In contrast, no sex differences in the onset threshold for whole-body sudomotor activity, as well as in cutaneous vascular conductance as a whole, were observed during exercise performed at a fixed rate of metabolic heat production. These observations suggest a sex difference in sudomotor activity that is not associated with differences in physical characteristics and rate of metabolic heat production.
In terms of physiological control, a lower thermosensitivity in females of the whole-body sudomotor response could be due to differences in: (1) thermoafferent information from peripheral thermoreceptors; (2) neural integration of thermoafferent activity, (3) thermoefferent neural activity, (4) thermoeffector response for a given level of thermoefferent activity, or (5) a combination of these possibilities. In theory, if sex modulates sudomotor activity centrally through differences in neural activity and/or integration, sex differences in sweat rate might be expected to be observed at any combination of ambient conditions and rate of metabolic heat production. In contrast to this possibility, a study by Ichinose-Kuwahara et al. (2010) suggests that sex differences in sweat gland function improvements elicited by exercise training are intensity-dependent. Although they used an exercise protocol in which fixed percentages of 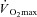 were employed, they only report a greater thermosensitivity of the local sweating response in trained males compared to trained females. In contrast, no differences were observed between untrained males and untrained females. Despite the discussed limitations associated with the experimental protocol (Gagnon et al. 2008; Schwiening et al. 2011), the fact that differences in local sudomotor thermosensitivity were only observed between the males and females who exercised at the greatest external workloads (and therefore rates of metabolic heat production), led us to believe that the threshold at which the requirement for heat loss exceeds the capacity of the sweat gland to contribute to temperature regulation might be lower in females.
were employed, they only report a greater thermosensitivity of the local sweating response in trained males compared to trained females. In contrast, no differences were observed between untrained males and untrained females. Despite the discussed limitations associated with the experimental protocol (Gagnon et al. 2008; Schwiening et al. 2011), the fact that differences in local sudomotor thermosensitivity were only observed between the males and females who exercised at the greatest external workloads (and therefore rates of metabolic heat production), led us to believe that the threshold at which the requirement for heat loss exceeds the capacity of the sweat gland to contribute to temperature regulation might be lower in females.
We therefore examined sex differences in local and whole-body sudomotor activity during exercise performed at progressive increments in the requirement for heat loss (Gagnon & Kenny, 2012). Males and females performed three successive 30 min periods of cycling exercise at fixed rates of metabolic heat production equal to 200, 250 and 300 W m−2 of body surface area. The ambient temperature (40°C) and relative humidity (10–20%) were fixed which, combined with fixed rates of metabolic heat production, elicited the same requirement for heat loss in males and females. We observed that whole-body sudomotor activity did not significantly differ between males and females during the first two exercise periods, becoming greater in males during the last exercise period. Similarly, consistent sex differences in local sudomotor activity were only evidenced at the highest requirement for heat loss employed. Furthermore, the thermosensitivity of whole-body and local sudomotor activity was generally similar between males and females at the lower requirements for heat loss, only becoming greater in males at the highest requirement for heat loss. In contrast, no sex differences were observed in the onset thresholds for sudomotor activity (local and whole-body), as well as in local and whole-limb cutaneous vasodilatation at all requirements for heat loss. These observations emphasise that sex differences in sudomotor activity are only evidenced above a certain combination of environmental conditions and rate of metabolic heat production. Taken together, the observed differences in sudomotor thermosensitivity and lack of differences in the onset threshold, combined with the lack of differences in cutaneous blood flow as a whole, are inconsistent with a central modulation of sudomotor activity in females, and point toward a peripheral modulation of the thermoeffector organ.
A peripheral modulation of the thermoeffector organ could stem from differences in the physical properties of the sweat gland itself (e.g. size), the sensitivity of the sweat gland to a given concentration of neurotransmitters released from sudomotor nerve terminals, and/or in the breakdown of acetylcholine within the sudomotor junction. The lower local sweat rates observed in females at the highest requirement for heat loss were solely due to differences in sweat gland output, as sweat gland recruitment was generally greater in females (Gagnon & Kenny, 2012). These findings are consistent with previous studies during passive heating (Bar-Or et al. 1969; Knip, 1969; Inoue et al. 2005), and exercise (Morimoto et al. 1967; Frye & Kamon, 1983; Ichinose-Kuwahara et al. 2010). Furthermore, we observed a clear levelling off of sudomotor activity in females at the highest requirement for heat loss (Gagnon & Kenny, 2012), which provides evidence for a lower maximal sweating capacity. Since variations in sweat gland output (Sato & Dobson, 1970) and the maximal capacity of the gland to produce sweat (Sato & Sato, 1983) have been used to evaluate differences in the physical properties of the sweat gland, these findings hint towards the possibility of sex differences in the physical properties of the sweat gland itself. Furthermore, studies have shown a lower sweat response in females to locally administered acetylcholine (Kahn & Rothman, 1942; Gibson & Shelley, 1948) and pilocarpine (Madeira et al. 2010), which points towards a sex difference in the cholinergic sensitivity of the sweat gland. Taken together, these findings support the hypothesis that sex differences in sudomotor activity are mediated peripherally through differences in the thermoeffector organ, rather than centrally though potential differences in neural activity.
Future directions
The recent findings summarised in this review article (Fig. 4) are limited to the follicular phase (days 1–10) of the menstrual cycle or the low hormone period of oral contraceptive use. Furthermore, comparisons were performed in environments which permit full evaporation of the sweat produced. Future work is therefore needed to determine whether these recent findings will hold true as a function of changes in hormonal status and in a variety of environmental conditions.
Figure 4. Schematic summary of sex differences in temperature regulation as a function of the requirement for heat loss during exercise in the heat.
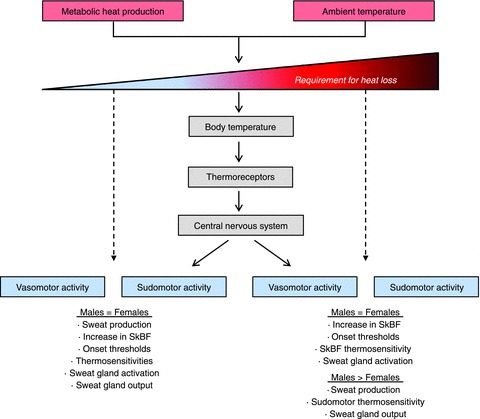
The sum of metabolic heat production and dry heat exchange from the ambient temperature form the requirement for heat loss and drive increases in body (core and skin) temperatures. Increases in body temperature are sensed by thermoreceptors (central and peripheral) which transmit thermoafferent information to the central nervous system where thermal integration occurs. The central nervous system sends thermoefferent signals to the effector organs (cutaneous blood vessels and sweat glands) to initiate vasomotor and sudomotor activity. At low requirements for heat loss (left dashed arrow) no sex differences are evidenced for increases in skin blood flow (SkBF) and sweat production, as well as in the physiological variables (onset threshold, thermosensitivity, sweat gland activation, sweat gland output) of vasomotor and sudomotor activity. At high requirements for heat loss (right dashed arrow), there are also no sex differences in SkBF, onset thresholds for both vasomotor and sudomotor activity, SkBF thermosensitivity, and sweat gland activation. In contrast, a greater sweat production, sudomotor thermosensitivity and sweat gland output is observed in males. The precise requirement for heat loss at which sex differences in sudomotor activity are evidenced remains to be determined.
In terms of hormonal status, the sex differences in sweat rate highlighted in the current review article would not be expected to differ across the female menstrual cycle. As discussed previously, the main change in thermoregulatory function that occurs over the course of the female menstrual cycle is a shift in resting body temperature, with a parallel shift in the onset threshold for sweating and cutaneous vasodilatation (Stephenson & Kolka, 1985; Kolka & Stephenson, 1997). In contrast, however, the thermosensitivity of each response is generally not affected. Since we consistently did not observe any sex differences in the onset threshold for sweating (local and whole body), but observed a lower thermosensitivity of the response in females (Gagnon & Kenny, 2011, 2012), we would expect similar observations if females in the luteal phase of the menstrual cycle/high hormone phase of oral contraceptive use were compared to males. It is important to note that the absolute onset temperature threshold would be expected to be greater in females during the luteal phase of the menstrual cycle/high hormone phase of oral contraceptive use, but this would simply result from a higher resting core temperature. If the change in core temperature were examined as the onset threshold, there is no evidence to suggest that it would significantly differ from males.
An important avenue of future research is to examine whether the observed sex differences in sweat rate hold true in a variety of environmental conditions, particularly those which do not permit full evaporation of the sweat produced. A greater sweat rate in males at high requirements for heat loss may not be evidenced in hot and humid environments, as the skin surface would become saturated at sweat rates which do not approach maximum due to the relatively low vapour pressure gradient. In these scenarios, it is therefore possible that males and females may exhibit a similar sweat rate, as it would be limited in males by the low vapour pressure gradient between the skin surface and the environment, as opposed to being limited in females by potential peripheral differences in sweating. A few studies have investigated sex-related differences as a function of environmental conditions, generally reporting lower sweat rates in females whether the environment was considered dry or humid (Morimoto et al. 1967; Shapiro et al. 1980; Keatisuwan et al. 1996). However, the conclusions from these studies are equally confounded by the aforementioned limitations based on differences in physical characteristics and particularly in rate of metabolic heat production between males and females. Examining sex differences in sweat rate at a given requirement for heat loss in relation to whether the environment is dry or humid is therefore an important avenue of future research.
While recent findings provide a strong case for a peripheral modulation of sudomotor activity in females, studies are required to directly evaluate a possible central modulation. Recent advances in the use of skin sympathetic nerve activity to evaluate the cutaneous vascular response to heat stress (Kamijo et al. 2011) may hold promise in evaluating possible sex differences in skin sympathetic nerve activity. Regardless of a possible central modulation, the specific mechanisms responsible for the peripheral modulation of sweating in females also need to be further examined. Although previous studies have demonstrated a lower cholinergic sensitivity of the sweat gland in females, it is unclear whether this response is mediated through differences in receptor sensitivity or through differences in acetylcholinesterase activity. Studies are also needed to determine whether differences in the physical properties of the sweat gland are indeed apparent. Finally, temperature regulation is intricately linked to the cardiovascular system, particularly during exercise in the heat (González-Alonso et al. 2008). Future studies should therefore consider examining whether the lower sudomotor activity in females has any implications on the cardiovascular response during exercise in the heat.
Summary
Until recently, conclusions remained limited as to whether or not true physiological differences in human temperature regulation exist between males and females or if previously observed differences were solely due to confounding differences in physical characteristics and rate of metabolic heat production. Recent observations clearly establish sex differences in sudomotor activity during exercise, independently of differences in physical characteristics and rate of metabolic heat production. The findings imply a lower maximal sweat rate in females during exercise, which can only be evidenced at a requirement for heat loss that exceeds the maximal capacity of the sweat gland in females, within an environment that ensures full evaporation of the sweat produced. Furthermore, current evidence points towards a peripheral modulation of sudomotor activity in females, although a central modulation has yet to be conclusively ruled out. In contrast, the cutaneous vascular response to exercise in the heat does not appear to differ between sexes. These recent findings provide a rationale for future studies to consider sex as an independent modulator of human temperature regulation during exercise in the heat.
Acknowledgments
D.G. is supported by an Alexander Graham Bell Canadian Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada. Work presented in this article was supported by the Natural Sciences and Engineering Research Council (RGPIN-298159-2009) and Leaders Opportunity Fund from the Canada Foundation for Innovation (22529). G.P.K. is supported by a University of Ottawa Research Chair in Environmental Physiology.
Appendix
Example calculation of sex differences in metabolic heat production during exercise at a fixed percentage of maximum oxygen consumption
If a male and female perform exercise at 50% of their same relative 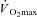 of 50 ml kg−1 min−1, exercise oxygen consumption would equal 25 ml kg−1 min−1 in both cases. Assuming that the male weighs 70 kg and the female 60 kg, absolute oxygen consumption will equal 1750 ml min−1 for the male and 1500 ml min−1 for the female. Assuming a respiratory exchange ratio of 0.85 and the same external workload, the 250 ml min−1 difference between sexes will result in a rate of metabolic heat production that is ∼80–85 W greater in the male. Such a difference in rate of metabolic heat production has been shown to result in proportional differences in whole-body heat loss (Gagnon et al. 2008; Gagnon & Kenny, 2011).
of 50 ml kg−1 min−1, exercise oxygen consumption would equal 25 ml kg−1 min−1 in both cases. Assuming that the male weighs 70 kg and the female 60 kg, absolute oxygen consumption will equal 1750 ml min−1 for the male and 1500 ml min−1 for the female. Assuming a respiratory exchange ratio of 0.85 and the same external workload, the 250 ml min−1 difference between sexes will result in a rate of metabolic heat production that is ∼80–85 W greater in the male. Such a difference in rate of metabolic heat production has been shown to result in proportional differences in whole-body heat loss (Gagnon et al. 2008; Gagnon & Kenny, 2011).
References
- Anderson GS. Human morphology and temperature regulation. Int J Biometeorol. 1999;43:99–109. doi: 10.1007/s004840050123. [DOI] [PubMed] [Google Scholar]
- Austin DM, Lansing MW. Body size and heat tolerance: a computer simulation. Hum Biol. 1986;58:153–169. [PubMed] [Google Scholar]
- Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol. 1980a;49:254–261. doi: 10.1152/jappl.1980.49.2.254. [DOI] [PubMed] [Google Scholar]
- Avellini BA, Shapiro Y, Pandolf KB, Pimental NA, Goldman RF. Physiological responses of men and women to prolonged dry heat exposure. Aviat Space Environ Med. 1980b;51:1081–1085. [PubMed] [Google Scholar]
- Bain AR, Deren TM, Jay O. Describing individual variation in local sweating during exercise in a temperate environment. Eur J Appl Physiol. 2011;111:1599–1607. doi: 10.1007/s00421-010-1788-9. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Effects of age and gender on sweating pattern during exercise. Int J Sports Med. 1998;19(Suppl. 2):S106–107. doi: 10.1055/s-2007-971970. [DOI] [PubMed] [Google Scholar]
- Bar-Or O, Lundegren HM, Buskirk ER. Heat tolerance of exercising obese and lean women. J Appl Physiol. 1969;26:403–409. doi: 10.1152/jappl.1969.26.4.403. [DOI] [PubMed] [Google Scholar]
- Benzinger TH. Heat regulation: homeostasis of central temperature in man. Physiol Rev. 1969;49:671–759. doi: 10.1152/physrev.1969.49.4.671. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic neurons regulating body temperature. In: Fregly M, Blatteis C, editors. Handbook of Physiology, section 4, Environmental Physiology. New York, NY, USA: Oxford University Press; 1996. pp. 105–126. [Google Scholar]
- Burse RL. Sex differences in human thermoregulatory response to heat and cold stress. Hum Factors. 1979;21:687–699. doi: 10.1177/001872087912210606. [DOI] [PubMed] [Google Scholar]
- Candas V, Libert JP, Vogt JJ. Human skin wettedness and evaporative efficiency of sweating. J Appl Physiol. 1979;46:522–528. doi: 10.1152/jappl.1979.46.3.522. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Johnson JM. Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol. 1997;83:2012–2018. doi: 10.1152/jappl.1997.83.6.2012. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ. Physiologic considerations for exercise performance in women. Clin Chest Med. 2004;25:247–255. doi: 10.1016/j.ccm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, Degroot DW, Sawka MN, Montain SJ. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107:69–75. doi: 10.1152/japplphysiol.00250.2009. [DOI] [PubMed] [Google Scholar]
- Cramer MN, Bain AR, Jay O. Local sweating on the forehead, but not forearm, is influenced by aerobic fitness independently of heat balance requirements during exercise. Exp Physiol. 2012;97:572–582. doi: 10.1113/expphysiol.2011.061374. [DOI] [PubMed] [Google Scholar]
- Davies CT. Thermoregulation during exercise in relation to sex and age. Eur J Appl Physiol. 1979;42:71–79. doi: 10.1007/BF00421907. [DOI] [PubMed] [Google Scholar]
- Drinkwater BL, Denton JE, Kupprat IC, Talag TS, Horvath SM. Aerobic power as a factor in women's response to work in hot environments. J Appl Physiol. 1976;41:815–821. doi: 10.1152/jappl.1976.41.6.815. [DOI] [PubMed] [Google Scholar]
- Drinkwater BL, Kupprat IC, Denton JE, Horvath SM. Heat tolerance of female distance runners. Ann NY Acad Sci. 1977;301:777–792. doi: 10.1111/j.1749-6632.1977.tb38246.x. [DOI] [PubMed] [Google Scholar]
- Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol. 1981;50:65–70. doi: 10.1152/jappl.1981.50.1.65. [DOI] [PubMed] [Google Scholar]
- Frye AJ, Kamon E. Sweating efficiency in acclimated men and women exercising in humid and dry heat. J Appl Physiol. 1983;54:972–977. doi: 10.1152/jappl.1983.54.4.972. [DOI] [PubMed] [Google Scholar]
- Fusco MM, Hardy JD, Hammel HT. Interaction of central and peripheral factors in physiological temperature regulation. Am J Physiol. 1961;200:572–580. doi: 10.1152/ajplegacy.1961.200.3.572. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Dorman LE, Jay O, Hardcastle SG, Kenny GP. Core temperature differences between sexes during intermittent exercise: physical considerations. Eur J Appl Physiol. 2009;105:453–461. doi: 10.1007/s00421-008-0923-3. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Kenny GP. Sex modulates whole-body sudomotor thermosensitivity during exercise. J Physiol. 2011;589:6205–6217. doi: 10.1113/jphysiol.2011.219220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D, Kenny GP. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol. 2012;113:746–757. doi: 10.1152/japplphysiol.00637.2012. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Jay O, Lemire B, Kenny GP. Sex-related differences in evaporative heat loss: the importance of metabolic heat production. Eur J Appl Physiol. 2008;104:821–829. doi: 10.1007/s00421-008-0837-0. [DOI] [PubMed] [Google Scholar]
- Gibson TE, Shelley WB. Sexual and racial differences in the response of sweat glands to acetylcholine and pilocarpine. J Invest Dermatol. 1948;11:137–142. doi: 10.1038/jid.1948.78. [DOI] [PubMed] [Google Scholar]
- Gisolfi CV, Wenger CB. Temperature regulation during exercise: old concepts, new ideas. Exerc Sport Sci Rev. 1984;12:339–372. [PubMed] [Google Scholar]
- González-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol. 2008;586:45–53. doi: 10.1113/jphysiol.2007.142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT. Regulation of internal body temperature. Ann Rev Physiol. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol. 1960;198:481–486. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- Hardy JD. Physiology of temperature regulation. Physiol Rev. 1961;41:521–606. doi: 10.1152/physrev.1961.41.3.521. [DOI] [PubMed] [Google Scholar]
- Havenith G. Human surface to mass ratio and body core temperature in exercise heat stress – a concept revisited. J Therm Biol. 2001a;26:387–393. [Google Scholar]
- Havenith G. Individualized model of human thermoregulation for the simulation of heat stress response. J Appl Physiol. 2001b;90:1943–1954. doi: 10.1152/jappl.2001.90.5.1943. [DOI] [PubMed] [Google Scholar]
- Havenith G, Coenen JM, Kistemaker L, Kenney WL. Relevance of individual characteristics for human heat stress response is dependent on exercise intensity and climate type. Eur J Appl Physiol. 1998;77:231–241. doi: 10.1007/s004210050327. [DOI] [PubMed] [Google Scholar]
- Havenith G, Luttikholt VG, Vrijkotte TG. The relative influence of body characteristics on humid heat stress response. Eur J Appl Physiol. 1995;70:270–279. doi: 10.1007/BF00238575. [DOI] [PubMed] [Google Scholar]
- Havenith G, van Middendorp H. The relative influence of physical fitness, acclimatization state, anthropometric measures and gender on individual reactions to heat stress. Eur J Appl Physiol. 1990;61:419–427. doi: 10.1007/BF00236062. [DOI] [PubMed] [Google Scholar]
- Horstman DH, Christensen E. Acclimatization to dry heat: active men vs. active women. J Appl Physiol. 1982;52:825–831. doi: 10.1152/jappl.1982.52.4.825. [DOI] [PubMed] [Google Scholar]
- Ichinose-Kuwahara T, Inoue Y, Iseki Y, Hara S, Ogura Y, Kondo N. Sex differences in the effects of physical training on sweat gland responses during a graded exercise. Exp Physiol. 2010;95:1026–1032. doi: 10.1113/expphysiol.2010.053710. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Tanaka Y, Omori K, Kuwahara T, Ogura Y, Ueda H. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol. 2005;94:323–332. doi: 10.1007/s00421-004-1303-2. [DOI] [PubMed] [Google Scholar]
- Jay O, Bain AR, Deren TM, Sacheli M, Cramer MN. Large differences in peak oxygen uptake do not independently alter changes in core temperature and sweating during exercise. Am J Physiol Regul Integr Comp Physiol. 2011;301:R832–R841. doi: 10.1152/ajpregu.00257.2011. [DOI] [PubMed] [Google Scholar]
- Kahn D, Rothman S. Sweat response to acetylcholine. J Invest Dermatol. 1942;5:431–444. [Google Scholar]
- Kamijo Y, Okada Y, Ikegawa S, Okazaki K, Goto M, Nose H. Skin sympathetic nerve activity component synchronizing with cardiac cycle is involved in hypovolaemic suppression of cutaneous vasodilatation in hyperthermia. J Physiol. 2011;589:6231–6242. doi: 10.1113/jphysiol.2011.220251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatisuwan W, Ohnaka T, Tochihara Y. Physiological responses of men and women during exercise in hot environments with equivalent WBGT. Appl Hum Sci. 1996;15:249–258. doi: 10.2114/jpa.15.249. [DOI] [PubMed] [Google Scholar]
- Kenney WL. A review of comparative responses of men and women to heat stress. Environ Res. 1985;37:1–11. doi: 10.1016/0013-9351(85)90044-1. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Webb P, Ducharme MB, Reardon FD, Jay O. Calorimetric measurement of postexercise net heat loss and residual body heat storage. Med Sci Sports Exerc. 2008;40:1629–1636. doi: 10.1249/MSS.0b013e31817751cb. [DOI] [PubMed] [Google Scholar]
- Knip AS. Measurement and regional distribution of functioning eccrine sweat glands in male and female Caucasians. Hum Biol. 1969;41:380–387. [PubMed] [Google Scholar]
- Kolka MA, Stephenson LA. Effect of luteal phase elevation in core temperature on forearm blood flow during exercise. J Appl Physiol. 1997;82:1079–1083. doi: 10.1152/jappl.1997.82.4.1079. [DOI] [PubMed] [Google Scholar]
- Kondo N, Takano S, Aoki K, Shibasaki M, Tominaga H, Inoue Y. Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation. Acta Physiol Scand. 1998;164:71–78. doi: 10.1046/j.1365-201X.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- McLellan TM. Sex-related differences in thermoregulatory responses while wearing protective clothing. Eur J Appl Physiol. 1998;78:28–37. doi: 10.1007/s004210050383. [DOI] [PubMed] [Google Scholar]
- Madeira LG, da Fonseca MA, Fonseca IA, de Oliveira KP, Passos RL, Machado-Moreira CA, Rodrigues LO. Sex-related differences in sweat gland cholinergic sensitivity exist irrespective of differences in aerobic capacity. Eur J Appl Physiol. 2010;109:93–100. doi: 10.1007/s00421-009-1262-8. [DOI] [PubMed] [Google Scholar]
- Marsh SA, Jenkins DG. Physiological responses to the menstrual cycle: implications for the development of heat illness in female athletes. Sports Med. 2002;32:601–614. doi: 10.2165/00007256-200232100-00001. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Latzka WA, Sawka MN. Control of thermoregulatory sweating is altered by hydration level and exercise intensity. J Appl Physiol. 1995;79:1434–1439. doi: 10.1152/jappl.1995.79.5.1434. [DOI] [PubMed] [Google Scholar]
- Moran DS, Shapiro Y, Laor A, Izraeli S, Pandolf KB. Can gender differences during exercise-heat stress be assessed by the physiological strain index. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1798–R1804. doi: 10.1152/ajpregu.1999.276.6.R1798. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Slabochova Z, Naman RK, Sargent F. Sex differences in physiological reactions to thermal stress. J Appl Physiol. 1967;22:526–532. doi: 10.1152/jappl.1967.22.3.526. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating. J Appl Physiol. 1971a;31:80–87. doi: 10.1152/jappl.1971.31.1.80. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Mitchell JW, Saltin B, Stolwijk JA. Peripheral modifications to the central drive for sweating. J Appl Physiol. 1971b;31:828–833. doi: 10.1152/jappl.1971.31.6.828. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- Nunneley SA. Physiological responses of women to thermal stress: a review. Med Sci Sports Exerc. 1978;10:250–255. [PubMed] [Google Scholar]
- Paolone AM, Wells CL, Kelly GT. Sexual variations in thermoregulation during heat stress. Aviat Space Environ Med. 1978;49:715–719. [PubMed] [Google Scholar]
- Pierau F. Peripheral thermosensors. In: Fregly M, Blatteis C, editors. Handbook of Physiology, section 4, Environmental Physiology. New York, NY, USA: Oxford University Press; 1996. pp. 85–104. [Google Scholar]
- Sato K, Dobson RL. Regional and individual variations in the function of the human eccrine sweat gland. J Invest Dermatol. 1970;54:443–449. doi: 10.1111/1523-1747.ep12259272. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol Regul Integr Comp Physiol. 1983;245:R203–R208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Wenger CB, Pandolf KB. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. New York, NY, USA: Oxford University Press; 1996. pp. 157–186. [Google Scholar]
- Schwiening CJ, Mason MJ, Thompson M. Absolute power, not sex, promotes perspiration. Exp Physiol. 2011;96:556–558. doi: 10.1113/expphysiol.2010.055996. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Avellini BA, Pimental NA, Goldman RF. Physiological responses of men and women to humid and dry heat. J Appl Physiol. 1980;49:1–8. doi: 10.1152/jappl.1980.49.1.1. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Goldman RF. Predicting sweat loss response to exercise, environment and clothing. Eur J Appl Physiol Occup Physiol. 1982;48:83–96. doi: 10.1007/BF00421168. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Integr Comp Physiol. 1985;249:R186–R191. doi: 10.1152/ajpregu.1985.249.2.R186. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Thermoregulation in women. Exerc Sport Sci Rev. 1993;21:231–262. [PubMed] [Google Scholar]
- Stitt JT. Central regulation of body temperature. In: Gisofi CV, Lamb DR, Nadel ER, editors. Perspectives in Exercise Science and Sports Medicine, vol. 6, Exercise, Heat, and Thermoregulation. Dubuque, IA, USA: WCB Brown and Benchmark; 1993. pp. 1–47. [Google Scholar]
- Webb P. The physiology of heat regulation. Am J Physiol Regul Integr Comp Physiol. 1995;268:R838–R850. doi: 10.1152/ajpregu.1995.268.4.R838. [DOI] [PubMed] [Google Scholar]
- Webb P, Troutman SJ, Annis JF. Automatic cooling in water cooled space suits. Aerospace Med. 1970;41:269–277. [PubMed] [Google Scholar]
- Weinman KP, Slabochova Z, Bernauer EM, Morimoto T, Sargent F. Reactions of men and women to repeated exposure to humid heat. J Appl Physiol. 1967;22:533–538. doi: 10.1152/jappl.1967.22.3.533. [DOI] [PubMed] [Google Scholar]
- Wenger CB, Baily RB, Roberts MF, Nadel ER. Interaction of local and reflex thermal effects in control of forearm blood flow. J Appl Physiol. 1985;58:251–257. doi: 10.1152/jappl.1985.58.1.251. [DOI] [PubMed] [Google Scholar]
- Werner J. The concept of regulation for human body temperature. J Therm Biol. 1980;5:75–82. [Google Scholar]
- Werner J. Control aspects of human temperature regulation. Automatica. 1981;17:351–362. [Google Scholar]
- Werner J. Temperature regulation during exercise: an overview. In: Gisofi CV, Lamb DR, Nadel ER, editors. Perspectives in Exercise Science and Sports Medicine, vol. 6, Exercise, Heat, and Thermoregulation. Dubuque, IA, USA: WCB Brown and Benchmark; 1993. pp. 49–84. [Google Scholar]
- Wyndham CH, Morrison JF, Williams CG. Heat reactions of male and female Caucasians. J Appl Physiol. 1965;20:357–364. doi: 10.1152/jappl.1965.20.3.357. [DOI] [PubMed] [Google Scholar]
- Young CN, Keller DM, Crandall CG, Fadel PJ. Comparing resting skin sympathetic nerve activity between groups: caution needed. J Appl Physiol. 2009;106:1751–1752. doi: 10.1152/japplphysiol.91538.2008. [DOI] [PubMed] [Google Scholar]


