Abstract
Young healthy adults exhibit an inverse linear relationship between muscle sympathetic nerve activity (MSNA) and α-adrenergic responsiveness. This balance may be reversed in metabolic syndrome (MetSyn) as animal models exhibit increased sympathetic activity and α-mediated vasoconstriction. We hypothesized humans with MetSyn would demonstrate increased α-adrenergic vasoconstriction and the inverse relationship between MSNA and adrenergic responsiveness would be lost. We measured MSNA (microneurography of the peroneal nerve) and forearm blood flow (FBF, Doppler ultrasound) in 16 healthy control subjects (31 ± 3 years) and 14 adults with MetSyn (35 ± 3 years; P > 0.05) during local administration of α-adrenergic agonists (phenylephrine (PE), α1; clonidine (CL), α2). MSNA was greater in MetSyn subjects than in healthy controls (P < 0.05). A group difference in vasoconstriction to PE was not detected (P= 0.08). The level of α1-mediated vasoconstriction was inversely related to MSNA in control subjects (r= 0.5, P= 0.04); this balance between MSNA and α1 responsiveness was lost in adults with MetSyn. MetSyn subjects exhibited greater vasoconstriction to CL infusion as compared with healthy controls (P < 0.01). A relationship between MSNA and α2-mediated vasoconstriction was not detected in either group. In summary, altered neurovascular control in human MetSyn is receptor specific. The observed uncoupling between MSNA and α1-adrenergic responsiveness and increased α2 vasoconstriction may lead to reduced FBF, altered flow distribution, and/or severe hypertension with the progression toward diabetes and cardiovascular disease.
Key points
Young healthy adults exhibit a balance between muscle sympathetic nerve activity (MSNA) and α-adrenergic-mediated vasoconstriction such that those with higher MSNA exhibit lower vascular-adrenergic responsiveness.
In contrast to healthy adults, the balance between MSNA and α1-adrenergic-mediated vasoconstriction is lost in adults with metabolic syndrome. In addition, adults with metabolic syndrome exhibit increased α2-adrenergic responsiveness.
This study uncovered some of the earliest sympathetic–haemodynamic changes in the progression from metabolic syndrome to cardiovascular disease and diabetes.
Considering metabolic syndrome subjects were relatively young and free of overt cardiovascular disease, it is reasonable to speculate as the disease progresses the observed uncoupling between MSNA and α-adrenergic responsiveness may lead to reduced whole-limb blood flow, altered blood flow distribution, reduced glucose delivery and/or increased hypertension severity.
Introduction
Adults with metabolic syndrome (MetSyn) are obese, hypertensive, hyperglycaemic, dyslipidaemic and at high risk of developing cardiovascular disease and type 2 diabetes (Ford, 2005). In addition, MetSyn adults exhibit increased muscle sympathetic nerve activity (MSNA), which has been linked to increased rates of cardiovascular morbidity and mortality (Lambert et al. 2007). The exact mechanisms behind this increase are unknown; however, a rise in MSNA may occur in response to changes in body composition, altered insulin signalling, changes in central autonomic regulation and/or stiffening of receptive fields (Scherrer & Sartori, 1997; Rumantir et al. 1999; Esler et al. 2006).
Chronic sympathetic activation can affect neurovascular coupling, resulting in altered noradrenaline release, α-adrenergic receptor number, receptor affinity and/or downstream signalling (Gurdal et al. 1995; Mita et al. 2010). Consistent with this concept, young healthy adults exhibit a balance between MSNA and α-adrenergic-mediated vasoconstriction at rest such that those with chronically higher MSNA exhibit lower vascular–adrenergic responsiveness (Hart et al. 2009). This inverse linear relationship may play an important role in blood flow control and blood pressure (BP) regulation in healthy humans. However, this adaptation appears to be lost in MetSyn. Animal models of MetSyn exhibit increased sympathetic activity in addition to increased basal α-adrenergic tone and responsiveness when compared with healthy control animals (Stepp & Frisbee, 2002; Naik et al. 2008). This altered relationship may have important clinical implications for the progression of MetSyn toward cardiovascular disease and diabetes; however, an understanding of neurovascular control and its functional significance in human MetSyn is unknown. To address this gap in knowledge, we aimed to determine whether adults with MetSyn exhibit increased α-adrenergic responsiveness despite higher MSNA. Based on animal models, we hypothesized adults with MetSyn would exhibit greater α-adrenergic vasoconstriction than age-matched, healthy control subjects. In addition, we hypothesized the inverse relationship between α-adrenergic vasoconstriction and MSNA observed in healthy adults would be lost in adults with MetSyn.
Methods
Ethical approval
All procedures were approved by the Institutional Review Board at the University of Wisconsin – Madison and conformed to the standards set by the latest revision of the Declaration of Helsinki. All drugs were approved by the United States Food and Drug Administration for investigational use in this project under Investigational New Drug Application no. 110253. Written informed consent was obtained from all subjects prior to study procedures.
Subjects
Two groups (MetSyn, healthy controls) of adult men and women were recruited from Madison and surrounding areas. Subjects were relatively young (18–55 years) to minimize the potentially confounding effects of ageing. All subjects completed a screening process in which physical activity and personal health history (including history of medications and family history of cardiovascular disease) were assessed. All control subjects were lean (waist circumference ≤102 cm for males and ≤88 cm for females), normotensive (resting BP <130/<85 mmHg) and otherwise healthy as determined by blood lipid and glucose levels. All subjects were non-smokers, were free from overt cardiovascular disease and neurological disorders, and were not taking any cardiovascular or glucose/lipid-lowering medications, as determined by self-report.
Adults were characterized as having MetSyn if subjects met at least three of the following National Cholesterol Education Program Adult Treatment Panel III criteria as modified by the American Diabetes Association: central obesity (waist circumference >102 cm males, >88 cm females), pre-hypertension (resting BP ≥130/≥85 mmHg), hypertriglyceridaemia (triglycerides ≥150 mg dl−1), hyperglycaemia (fasting glucose ≥100 mg dl−1) and/or dyslipidaemia (high-density lipoprotein (HDL) < 40 mg dl−1 in males, <50 mg dl−1 in females) (Alberti et al. 2009). Female subjects were premenopausal and had a negative urine pregnancy test on each study day. Given menstrual phase is known to modulate MSNA and adrenergic responsiveness (Minson et al. 2000; Limberg et al. 2010), all women were studied during the early follicular phase/placebo phase (days 1–6) of the menstrual cycle, as determined by self-report.
The study required one 90 min screening visit, two nights of sleep-disordered breathing monitoring and two study visits. All study visits began in the morning after a 10 h fast and were conducted at the same time within subjects. Subjects were instructed to refrain from exercise, non-steroidal anti-inflammatory drugs, alcohol and caffeine for 24 h prior to each visit.
Descriptive measurements
Descriptive measurements were conducted during the screening visit. After a 10 h fast, whole blood was collected and levels of HDL cholesterol, triglycerides and glucose were measured immediately (CardioChek; PTS Panels, Indianapolis, IN, USA). Additional blood samples were collected at rest and plasma was frozen at −80°C to be analysed at a later date for plasma insulin (radioimmunoassay) and leptin (enzyme immunoassay) concentrations (Wisconsin National Primate Research Center, Madison, WI, USA).
Body composition and central adiposity were determined based on waist circumference, body mass index (BMI) and dual-energy X-ray absorptometry (DEXA; GE Lunar Prodigy, Milwaukee, WI, USA). Lean forearm mass was analysed from whole-body DEXA scans using anatomical landmarks (Radegran, 1997). The Paffenbarger physical activity questionnaire was completed to examine physical activity (Paffenbarger et al. 1993). In addition, subjects wore a pulse oximeter (Pulsox-300i; Konica Minolta, Osaka, Japan) on the index finger during two nights of sleep for oximetry and cardiac monitoring, which provided a measure of haemoglobin desaturation events as an index of potential sleep apnoea (Collop et al. 2007).
Blood flow measurements
Studies were performed with subjects in the supine position with the non-dominant arm extended to the side (approximately 90 deg) at heart level. Forearm blood flow (FBF) was measured using Doppler ultrasound (Vivid 7, General Electric; Milwaukee, WI). FBF was determined as the product of mean blood velocity (cm s−1) and vessel cross-sectional area (π× radius2 with radius measured in cm) and values were multiplied by 60 to convert from ml s−1 to ml min−1. A 12 MHz linear array probe was placed approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle, with a probe insonation angle ≤60 deg and the sample volume was adjusted to cover the width of the artery using methods identical to those described by Schrage et al. (2007) and Limberg et al. (2010). Pulse-wave velocity was continuously assessed (beat-to-beat) and results were reported in 30 s intervals.
A commercial interface unit (Multigon Industries, Yonkers, NY, USA) processed the angle-corrected, intensity-weighted Doppler audio information from the GE Vivid ultrasound system into a flow velocity signal sampled in real time with signal processing software (PowerLab; ADinstruments, Colorado Springs, CO, USA). All haemodynamic data were digitized, stored on a computer at 400 Hz and analysed off-line using PowerLab. Post-processing using PowerLab's Chart application package yielded mean blood velocities.
Artery diameters were obtained from B-mode video images and measurements typically resulted in loss of pulse wave signal for 15 s. To determine vessel cross-sectional area, artery diameter was taken as the median of five measurements in late diastole. Arterial diameter was measured in the part of the artery running perpendicular to the ultrasound beam, identified by strong wall signals in a longitudinal section of the artery (measuring the distance between near and far intima-media interfaces). All measurements were made off-line by a trained operator.
Intra-arterial pharmacological intervention
Under aseptic conditions and after local anaesthesia (2% lidocaine), a physician placed an arterial catheter (20 gauge, 4.45 cm; Arrow International Inc, Reading, PA, USA) in the brachial artery of the non-dominant forearm in the antecubital fossa. The catheter was used for local administration of vasoactive drugs and beat-to-beat BP monitoring.
All drugs were infused via the brachial artery catheter using a Twin Syringe Infusion pump (Pump 33; Harvard Apparatus, Holliston, MA, USA). Infusions were adjusted for lean forearm size to normalize concentrations of each drug between subjects and to minimize systemic effects. The pump rate (ml min−1) for each drug infusion was calculated as: [Drug dose (μg dl−1 ml−1) × Lean forearm mass (ml)]/(100 × Drug concentration (μg min−1)] (Limberg et al. 2010).
α-Adrenergic agonists were infused at rest to evoke vasoconstriction and assess adrenergic responsiveness using previously published doses (Dinenno et al. 2005; Limberg et al. 2010). Phenylephrine (Baxter Healthcare Corp., Deerfield, IL, USA) is a selective α1-adrenergic agonist and was infused at a rate of 0.03125 μg dl−1 min−1. Clonidine (Anodyne Pharmaceuticals, Inc, Newport, KY, USA) is an α2-adrenergic agonist and was infused at a rate of 0.15 μg dl−1 min−1 (Dinenno et al. 2005; Limberg et al. 2010). MetSyn has been associated with reduced β-mediated vasodilatation (Lesniewski et al. 2008). To control for potential confounding effects of differences in β-adrenergic regulation between groups, a continuous infusion of a non-selective β-adrenergic receptor antagonist (propranolol) was given throughout the study. Propranolol (Ben Venue Laboratories, Inc, Bedford, OH, USA) was given at rest as a loading dose (20 μg dl−1 min−1 for 5 min) followed by a continuous maintenance dose (25 μg min−1) using doses published previously (Dinenno et al. 2002).
Microneurography
On a second study day, MSNA was assessed by microneurography (in a subset of subjects, n= 22; a clear recording could not be obtained from four subjects and four subjects declined participation in the procedure). With subjects in the supine position, multiunit, direct intraneural recordings of MSNA were obtained by percutaneous insertion of a unipolar tungsten microelectrode into muscle fascicles of the right peroneal nerve, posterior to the fibular head (Hanada et al. 2003; Charkoudian et al. 2006). Recording electrodes had a diameter of 200 μm in the shaft, tapering to 1–5 μm at the uninsulated tip (UNA32F2S; FHC, Bowdoin, ME, USA). A reference electrode was positioned subcutaneously approximately 4 cm from the recording electrode (UNR32FRS; FHC). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch resulted in the appearance of afferent activity, and no afferent neural response was evoked by skin stimulation.
Neural signals were amplified (20,000–50,000 times), filtered (bandwidth 700–2000 Hz), rectified and integrated (time constant, 0.1 s) to obtain mean voltage neurograms (Rys Systems, Milwaukee, WI, USA). Data were sampled in real time with signal-processing software (PowerLab) and analysed off-line. Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured semi-automatic analysis program. Burst identification was controlled visually by a single investigator. Nerve activity was quantified by determining the burst frequency (bursts per minute) and burst incidence (bursts per 100 heart beats) (Hanada et al. 2003).
Protocol
On the first study day, subjects were supine for catheter insertion, followed by three individual trials. Trials were separated by a minimum of 10 min of quiet rest and were conducted as follows. (1) β-Adrenergic blockade: a loading dose of propranolol was given during 5 min of quiet rest, after which the pump rate was reduced to achieve a continuous maintenance dose for the remainder of the study. (2) and (3) α-Adrenergic responsiveness: during the last 3 min of a 7 min resting period, subjects received intra-arterial infusion of either phenylephrine or clonidine (drug order was randomized). During each trial, BP (pressure transducer; ICU Medical Inc, San Clemente, CA, USA), heart rate (ECG; Datex-Ohmeda, Helsinki, Finland) and brachial artery blood velocity (Doppler ultrasound) were measured continuously. Steady-state measures of the aforementioned parameters were reported from the last 30 s of rest/drug infusion, followed by a measure of brachial artery diameter.
The second (MSNA) study visit was completed as soon as possible after the catheter visit (visits were separated by 10 ± 2 weeks, range 2 days to 9 months). Subject characteristics (BP, heart rate, FBF) were similar between study days (Supplementary Table S1). Subjects were instructed to avoid sympathoexcitatory manoeuvres including Valsalvas and prolonged expirations. Subject compliance was verified using strain-gauge pneumography positioned at the midchest level. Measures of resting MSNA were taken from the last 1 min of rest prior to three individual trials (data from which are not shown here) and are reported as an average (coefficient of variance = 10 ± 2%). Trials were separated by a minimum of 10 min of quiet rest and were designed to mirror baseline data collection time points from the catheter study day. When this analysis approach was compared with methods using an average over a continuous time period (by analysing five continuous minutes of MSNA from the initial rest period), measures of MSNA were not different (Table S2).
Data analysis and statistics
All data are presented as mean ± standard error of the mean (SEM) and were analysed using Minitab Version 16 (Minitab Inc., State College, PA, USA). All distributions for main outcome variables were approximately normal (P > 0.05). Subject characteristics were compared using an independent samples t test. To account for potential individual differences in lean muscle mass and perfusion pressures and to assess vasodilatation, FBF measurements were normalized for mean arterial BP and forearm lean mass (FBF/BP/lean mass) and are reported as lean vascular conductance (FVC; ml min−1 100 mmHg−1 100 g−1).
The two main outcome variables were MSNA and the relative change in lean FVC with infusion of adrenergic agonists (%FVC). The percentage reduction (%FVC) with drug infusion was calculated as: [(FVCpost-infusion– FVCpre-infusion)/(FVCpre-infusion) × 100%] (Dinenno et al. 2005). Haemodynamic variables were analysed using repeated measures analysis of variance to determine the significance of the fixed effect of group (MetSyn, Control) and condition (with/without adrenergic agonist) on the parameters of interest. Bonferroni post hoc comparisons were performed when one-tailed significant effects were observed at P≤ 0.05. The number of participants (minimum of 11 per group) was determined a priori by a power test equation with α= 0.05 and power = 0.80, using group differences from previously published research in older adults (Dinenno et al. 2005). Pearson's correlation coefficients were calculated to assess the relationship between resting MSNA and α-adrenergic responsiveness (%FVC) (Charkoudian et al. 2006). A post hoc power analysis showed a minimum of ten subjects would provide 76% power to detect a correlation of 0.65 (Dupont & Plummer, 1998).
Results
Subject characteristics
Subject characteristics are summarized in Table 1. Fourteen adults with MetSyn and 16 healthy control subjects participated in the current study (80% White non-Hispanic, 12% White Hispanic, 4% Asian, 4% African American). One subject reported diagnosed sleep apnoea, although he presented with an average of three desaturation events per hour, as determined by two nights of pulse oximetry. Given a threshold of 15 desaturation events has been linked to abnormal apnoea–hypopnoea indices (Netzer et al. 2001), the subject was included in the current study. Eight subjects (Control n= 5, MetSyn n= 3) were taking a daily vitamin supplement. All subjects reported exercising less than 3 h a week and when activity was assessed by questionnaire, the majority of participants reported physical activity ≤12560 KJ per week (Control n= 12, MetSyn n= 14). All female subjects (Control n= 5, MetSyn n= 5) reported having regular menses and were studied during the early follicular/placebo phase of the menstrual cycle (n= 3 using hormonal birth control).
Table 1.
Subject demographics
| Control (n= 16) | MetSyn (n= 14) | |
|---|---|---|
| Sex (M/F) | 11/5 | 9/5 |
| Age (years) | 31 ± 3 | 35 ± 3 |
| Weight (kg) | 69 ± 3 | 111 ± 8* |
| BMI (kg m−2) | 23 ± 1 | 35 ± 2* |
| Waist (cm) | 78 ± 3 | 111 ± 4* |
| Body fat (%) | 20 ± 2 | 40 ± 3* |
| Leptin (ng ml−1) | 4 ± 1 | 17 ± 3* |
| Glucose (mg dl−1) | 83 ± 3 | 89 ± 3 |
| Insulin (μU ml−1) | 11 ± 1 | 19 ± 2* |
| Triglycerides (mg dl−1) | 77 ± 9 | 153 ± 23* |
| HDL (mg dl−1) | 61 ± 4 | 43 ± 3* |
| Systolic blood pressure (mmHg) | 125 ± 2 | 140 ± 3* |
| Diastolic blood pressure (mmHg) | 70 ± 2 | 85 ± 2* |
| MSNA burst frequency (bursts min−1) | 23 ± 3 | 41 ± 6* |
| MSNA burst incidence (burst per 100 heart beats) | 40 ± 4 | 61 ± 7* |
| Lean forearm mass (g) | 930 ± 59 | 1067 ± 85 |
| Physical activity (kcal week−1) | 2526 ± 547 | 1772 ± 356 |
| Desaturation Event Index (events h−1) | 3 ± 1 | 9 ± 2* |
Data are presented as mean ± SEM. Control n= 16, MetSyn n= 14 unless otherwise noted (Desaturation Event Index: Control n= 15, MetSyn n= 13; MSNA measures: Control n= 12, MetSyn n= 10). *P < 0.05 vs. Control. Control 10576 ± 2290 KJ/WK, MetSyn 4906 ± 1490 KJ/WK.
Groups were not significantly different with regard to age or lean forearm mass (P > 0.05). On average, adults with MetSyn were clinically obese – displaying significantly higher weight, BMI, waist circumference and body fat – in addition to exhibiting greater triglycerides, BP and lower HDL cholesterol when compared with healthy controls (P < 0.05; Table 1). Of the adults with MetSyn, 13 met the criterion for waist circumference, 12 for BP, ten for HDL, eight for triglycerides and two for glucose. Healthy adults did not meet the criteria for MetSyn.
Muscle sympathetic nerve activity
A representative neurogram is given in Fig. 1. Adults with MetSyn exhibited higher MSNA burst frequency (bursts per minute) than healthy control subjects (P < 0.05). Similar results were observed when MSNA was analysed as burst incidence (Table 1).
Figure 1. Representative neurogram.
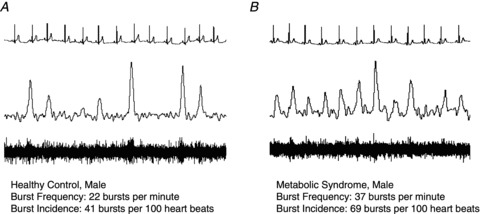
Data are presented from ∼15 s of quiet rest and include heart rate (ECG), integrated and raw voltage neurograms. A, healthy control; B, MetSyn.
α1-Adrenergic responsiveness
Results are summarized in Table 2 and Fig. 2 (Control n= 16, MetSyn n= 13; one MetSyn subject did not complete due to discomfort during initial phenylephrine infusion). Brachial artery diameter was similar between groups (main effect of group, P= 0.19). Mean arterial BP, heart rate, FBF and FVC were greater in MetSyn adults than in healthy controls irrespective of condition (main effect of group, P < 0.05).
Table 2.
Responses to phenylephrine and clonidine infusion at rest
| Phenylephrine (PE) | Clonidine (CL) | |||
|---|---|---|---|---|
| Control | MetSyn | Control | MetSyn | |
| Heart rate (beats min−1) | ||||
| Rest | 56 ± 2 | 64 ± 3* | 58 ± 2 | 64 ± 3* |
| Rest + Drug | 57 ± 2 | 64 ± 3* | 57 ± 2 | 65 ± 4* |
| Mean BP (mmHg) | ||||
| Rest | 90 ± 2 | 107 ± 3* | 91 ± 2 | 105 ± 4* |
| Rest + Drug | 92 ± 2 | 110 ± 3* | 92 ± 2 | 109 ± 4* |
| Diameter (cm) | ||||
| Rest | 0.43 ± 0.02 | 0.44 ± 0.02 | 0.42 ± 0.02 | 0.44 ± 0.02 |
| Rest + Drug | 0.43 ± 0.02 | 0.44 ± 0.02 | 0.42 ± 0.02 | 0.44 ± 0.02 |
| Blood flow (ml min−1) | ||||
| Rest | 58 ± 12 | 116 ± 21* | 76 ± 17 | 118 ± 16* |
| Rest + Drug | 48 ± 10a | 73 ± 9*a | 37 ± 7a | 42 ± 6*a |
| Vascular conductance (ml min−1 100 mmHg−1) | ||||
| Rest | 64 ± 13 | 107 ± 18* | 82 ± 18 | 110 ± 12 |
| Rest + Drug | 51 ± 11a | 66 ± 8*a | 40 ± 8a | 40 ± 6a |
| Lean blood flow (ml min−1 100 g−1) | ||||
| Rest | 6 ± 1 | 11 ± 2* | 8 ± 2 | 11 ± 1 |
| Rest + Drug | 5 ± 1a | 7 ± 1*a | 4 ± 1a | 4 ± 1a |
| Change with Drug (%) | −23 ± 4 | −31 ± 4 | −42 ± 5 | −61 ± 6* |
| Lean vascular conductance (ml min−1 100 mmHg−1 100 g−1) | ||||
| Rest | 7 ± 1 | 10 ± 2* | 8 ± 2 | 11 ± 1 |
| Rest + Drug | 5 ± 1a | 6 ± 1*a | 4 ± 1a | 4 ± 1a |
| Change with Drug (%) | −25 ± 4 | −33 ± 4 | −43 ± 4 | −62 ± 5* |
PE: Control n= 16, MetSyn n= 13. CL: Control n= 16, MetSyn n= 14. Data are presented as mean ± SEM. Main effect of group (*P < 0.05 vs. Control), main effect of condition (aP < 0.05 vs. Rest).
Figure 2. α1-Adrenergic vasoconstriction and the relationship between MSNA and α1-adrenergic responsiveness in healthy controls and adults with metabolic syndrome.
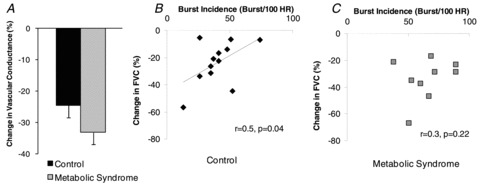
A, Control n= 16, MetSyn n= 13. Phenylephrine infusion resulted in a significant reduction in FVC at rest and relative responses were not different between groups (main effect of group, P = 0.08). B, an inverse linear relationship between resting α1-adrenergic responsiveness and MSNA was observed in young healthy adults (n= 12). C, a relationship was not detected in MetSyn subjects (n = 9).
Phenylephrine (an α1-adrenergic agonist) infusion resulted in a significant reduction in FBF and FVC (main effect of condition, P < 0.05). Relative responses to phenylephrine infusion were not different between groups (%FVC; main effect of group, P= 0.08; Fig. 2A), although MetSyn adults exhibited a trend for greater α1-mediated vasoconstriction as compared with controls. A post hoc power analysis suggested 38 subjects would be necessary to detect statistical significance.
An inverse linear relationship between resting α1-adrenergic responsiveness and MSNA was observed in young healthy adults (r= 0.5, P= 0.04; Fig. 2B) such that adults with higher MSNA exhibited blunted α1-vasoconstrictor responses. A relationship was not detected in MetSyn subjects (r= 0.3, P= 0.22; Fig. 2C).
α2-Adrenergic responsiveness
Results are summarized in Table 2 and Fig. 3. Brachial artery diameter was similar between groups (main effect of group, P= 0.19). Mean arterial BP, heart rate and FBF were greater in MetSyn adults than in healthy controls irrespective of condition (main effect of group, P < 0.05). Group differences in FVC were not detected (main effect of group, P= 0.13).
Figure 3. α2-Adrenergic vasoconstriction and the relationship between MSNA and α2-adrenergic responsiveness in healthy controls and adults with metabolic syndrome.
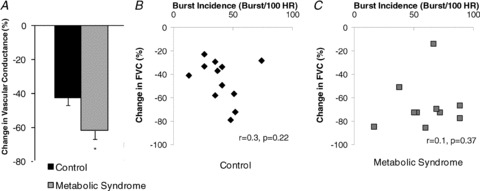
A, Control n= 16, MetSyn n = 14. Clonidine infusion resulted in a significant reduction in FVC, with relative responses greater in adults with MetSyn than in healthy controls (main effect of group, *P < 0.05). B and C, no relationship was observed between MSNA and α2-adrenergic responsiveness in either group (Control n = 12 (B), MetSyn n = 10 (C)).
Clonidine (an α2-adrenergic agonist) infusion resulted in a significant reduction in FBF and FVC at rest (main effect of condition, P < 0.05), with relative responses (%FBF, %FVC) greater in adults with MetSyn than in healthy controls (main effect of group, P < 0.01; Fig. 3A). No relationship was observed between MSNA and clonidine-mediated vasoconstriction at rest in either group (Control r= 0.3, P= 0.22; MetSyn r= 0.1, P= 0.37; Fig. 3B and C).
Discussion
We measured MSNA, adrenergic responsiveness and changes in FBF in human MetSyn. By combining key measures of neurovascular control, we were able to clearly examine the relationship between MSNA and FBF control in MetSyn. Several novel findings are worth noting: (1) a group difference in α1-adrenergic responsiveness is not apparent, although vasoconstriction tends to be greater in MetSyn adults; (2) the inverse relationship between MSNA and α1-adrenergic responsiveness seen in control subjects is lost in human MetSyn; (3) adults with MetSyn exhibit increased α2-adrenergic responsiveness; and (4) in contrast to α1-adrenergic responsiveness, no relationship is observed between MSNA and α2-adrenergic-mediated vasoconstriction in either group. Together, these results help to provide an understanding of receptor-specific alterations in the sympathetic–haemodynamic balance in MetSyn and may have important implications for FBF control and BP regulation in human MetSyn and the progression toward more severe cardiovascular disease.
α1-Adrenergic receptor responsiveness
Although data in humans are limited, α1-adrenoceptors are thought to be located primarily on large arterioles and regulate whole-muscle blood flow (Faber, 1988; Anderson & Faber, 1991). Animal models of MetSyn exhibit reduced whole-limb blood flow and increased α1-adrenergic responsiveness at rest (Stepp & Frisbee, 2002; Frisbee et al. 2011). In contrast, an increase in α1-adrenergic responsiveness was not detected in human MetSyn and FBF was increased compared with healthy controls, despite higher levels of MSNA (Tables 1 and 2, Fig. 2A).
MSNA is highly variable between individuals, with 5- to 10-fold differences in young, healthy adults (Sundlof & Wallin, 1977; Fagius & Wallin, 1993; Hart et al. 2009). This variability is thought to be integral to cardiovascular control (Joyner et al. 2010). Results from the current study confirm recent findings in young healthy adults demonstrating an inverse linear relationship between MSNA and resting vasoconstrictor responses to phenylephrine infusion (Fig. 2B; Charkoudian et al. 2006). This sympathetic–haemodynamic balance probably plays an important role in maintaining skeletal muscle perfusion and systemic BP in the face of high levels of MSNA. The exact mechanisms behind this relationship are unknown, although research supports adrenergic receptor desensitization and/or receptor downregulation in response to chronic neural firing (Hogikyan & Supiano, 1994).
Although an increase in α1-vasoconstrictor response to phenylephrine infusion was not detected in adults with MetSyn (Fig. 2A), the trend for increased responsiveness (P= 0.08) and the lack of a linear relationship between MSNA and α1-adrenergic-mediated vasoconstriction at rest suggests that MetSyn adults do not exhibit the same receptor desensitization and/or downregulation that may occur in healthy control subjects (Fig. 2C). Consistent with this concept, Dincer et al. (2006) demonstrated a lack of α1-adrenergic receptor downregulation in a canine model of MetSyn (Dincer et al. 2006). Altered sympathetically mediated vasoconstriction may result in increased BP and reduced oxygen and glucose delivery, and thus the uncoupling between MSNA and adrenergic responsiveness in MetSyn (Fig. 2C) may have implications for the progression toward cardiovascular disease and diabetes. In support of this idea, α-adrenergic responsiveness is maintained in healthy obesity (Agapitov et al. 2008), but is increased with type 2 diabetes (Hogikyan et al. 1999). Here we identified a trend for greater α1-adrenergic responsiveness in an intermediate condition, MetSyn (Fig. 2A), and thus filled an important gap in our understanding of neurovascular control from health to disease.
α2-Adrenergic receptor responsiveness
MetSyn adults exhibit increased α2-adrenergic vasoconstriction at rest when compared with healthy controls (Fig. 3A). To begin to understand the specific mechanisms underlying increased α2-mediated vasoconstriction in human MetSyn, we examined potential relationships between α2-adrenergic receptor responsiveness and MSNA. The lack of a linear relationship between MSNA and α2-adrenergic responsiveness (Fig. 3B and C) indicates – in contrast to α1-adrenoceptors – that factors other than MSNA probably play a more direct role in α2-mediated vasoconstriction (endothelial function, nitric oxide bioavailability, etc). Consistent with this, research in humans supports the presence of endothelial dysfunction in MetSyn (Steinberg et al. 1996). Taken together, if the integrity of the endothelium and/or nitric oxide bioavailability is altered in MetSyn, this may be a key contributor to the observed increase in α2-mediated vasoconstriction in human MetSyn.
Integrated neurovascular control
FBF is greater in MetSyn adults despite higher MSNA, greater α2-adrenergic responsiveness and a trend for increased α1-mediated vasoconstriction. There are many factors that contribute to FBF and in the context of current findings it is reasonable to speculate the higher MSNA observed in MetSyn may be combined with increased pre-synaptic inhibition of neurotransmitter release. Consistent with this concept, animal models of diabetes and hypertension support the presence of hyperactive pre-synaptic α2-adrenergic receptors and/or reduced norepinephrine overflow (Gando et al. 1993; Burgdorf et al. 2006). Moreover, obese women exhibit reduced norepinephrine spillover in forearm skeletal muscle (Coppack et al. 1998). In line with previous findings, results from the current study indicate plasma norepinephrine levels were similar between groups (Control 191 ± 22 pg ml−1, MetSyn 181 ± 28 pg mL−1; P= 0.39), despite ∼2-fold higher MSNA in MetSyn. However, it is important to note that plasma concentrations are an imprecise measure of catecholamine release (Esler, 1993) and future research will be necessary to directly examine the potential for altered norepinephrine release in human MetSyn. These findings emphasize the complexity of the sympathetic–haemodynamic balance and highlight the need for multiple physiological measures to appropriately interpret neurovascular adaptations occurring in MetSyn.
Experimental considerations
An important strength of the current study design was the use of receptor-specific pharmacological agonists under β-adrenergic blockade. Using previously published doses, we controlled for potentially confounding effects of differences in β-adrenergic regulation between groups (Johnsson, 1967; Eklund & Kaijser, 1976; Lesniewski et al. 2008). However, it is important to note that adrenergic agonists were delivered intra-arterially and may not reflect responses of adrenergic receptors normally stimulated by norepinephrine released from nerve endings (Jie et al. 1987).
MSNA was measured in the peroneal nerve on a second study day for subject comfort and to minimize potential conflicts with subject and equipment availability. This methodology should not limit the interpretation of relationships between MSNA and adrenergic responsiveness. First, upper extremity MSNA and lower extremity MSNA appear to be uniform under resting conditions in both healthy controls and obese adults (Agapitov et al. 2008). Second, MSNA has been shown to be repeatable between days and across years (Wallin & Charkoudian, 2007). Third, key subject characteristics (BP, heart rate, FBF) were similar between study days (Table S1), supporting maintained cardiovascular control across days/weeks. It is also important to acknowledge correlational analysis does not necessarily determine causation. Thus, future work will be necessary to elucidate potential mechanisms behind the observed relationships between MSNA and adrenergic responsiveness or lack thereof.
Conclusion
The current study combined multiple physiological measures to provide an understanding of receptor-specific neurovascular control of skeletal muscle blood flow in human MetSyn. The results revealed some of the earliest changes in the progression from MetSyn to cardiovascular disease and diabetes and emphasize the complexity of the sympathetic–haemodynamic balance in human health and disease. Considering MetSyn subjects were relatively young and free of overt cardiovascular disease, it is reasonable to speculate the observed uncoupling between MSNA and α1-adrenergic responsiveness and increased α2-mediated vasoconstriction may lead to reduced whole-limb blood flow, altered blood flow distribution and/or severe hypertension as the disease progresses.
Acknowledgments
We thank the subjects for their participation. In addition, we would like to thank John Harrell, Rebecca Johansson, Jessica Danielson, Meghan Crain and Edward McKenna for technical assistance and Dr John Dopp for equipment use. This study was supported by American Heart Association pre-doctoral awards #0815622G and #10PRE3870000 (to J.K.L.), WNPRC Assay Services and partially by grant RR000167 from the National Institutes of Health, and grant HL105820 (W.G.S.) from the National Institutes of Health.
Glossary
- BMI
body mass index
- BP
blood pressure
- DEXA
dual-energy X-ray absorptometry
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- HDL
high-density lipoprotein
- MetSyn
metabolic syndrome
- MSNA
muscle sympathetic nerve activity
Author contributions
Studies were performed at the Bruno Balke Biodynamics Laboratory at the University of Wisconsin – Madison. J.J.S., L.T.P., B.J.W.: placement of arterial catheters, medical supervision and consultation. M.W.E.: placement of arterial catheters, medical supervision, experimental design and interpretation. B.J.M.: equipment use, experimental design, data collection, analysis and interpretation. W.G.S.: conception and experimental design, data collection, interpretation, manuscript preparation. J.K.L.: conception and experimental design, data collection, analysis, interpretation and manuscript preparation. All authors approved the final version of the manuscript. There are no conflicts of interest to disclose.
Supplementry Material
References
- Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension. 2008;52:687–695. doi: 10.1161/HYPERTENSIONAHA.107.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Burgdorf C, Richardt G, Schütte F, Dendorfer A, Kurz T. Impairment of presynaptic alpha2-adrenoceptor-regulated norepinephrine overflow in failing hearts from Zucker diabetic fatty rats. J Cardiovasc Pharmacol. 2006;47:256–262. doi: 10.1097/01.fjc.0000202560.61667.3e. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- Coppack SW, Horowitz JF, Paramore DS, Cryer PE, Royal HD, Klein S. Whole body, adipose tissue, and forearm norepinephrine kinetics in lean and obese women. Am J Physiol Endocrinol Metab. 1998;275:E830–E834. doi: 10.1152/ajpendo.1998.275.5.E830. [DOI] [PubMed] [Google Scholar]
- Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary α-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006;13:587–595. doi: 10.1080/10739680600885228. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm post-junctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol. 1976;262:39–50. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res. 1988;62:37–50. doi: 10.1161/01.res.62.1.37. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Goodwill AG, Butcher JT, Olfert IM. Divergence between arterial perfusion and fatigue resistance in skeletal muscle in the metabolic syndrome. Exp Physiol. 2011;96:369–383. doi: 10.1113/expphysiol.2010.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando S, Hattori Y, Kanno M. Altered cardiac adrenergic neurotransmission in streptozotocin-induced diabetic rats. Br J Pharmacol. 1993;109:1276–1281. doi: 10.1111/j.1476-5381.1993.tb13761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdal H, Cai G, Johnson MD. Alpha 1-adrenoceptor responsiveness in the aging aorta. Eur J Pharmacol. 1995;274:117–123. doi: 10.1016/0014-2999(94)00717-l. [DOI] [PubMed] [Google Scholar]
- Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol. 2003;551:635–647. doi: 10.1113/jphysiol.2003.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogikyan RV, Galecki AT, Halter JB, Supiano MA. Heightened norepinephrine-mediated vasoconstriction in type 2 diabetes. Metabolism. 1999;48:1536–1541. doi: 10.1016/s0026-0495(99)90242-1. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV, Supiano MA. Arterial alpha-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab. 1994;266:E717–E724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Modulation of noradrenaline release by peripheral presynaptic alpha 2-adrenoceptors in humans. J Cardiovasc Pharmacol. 1987;9:407–413. doi: 10.1097/00005344-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Johnsson G. The effects of intra-arterially administered propranolol and H 56–28 on blood flow in the forearm–a comparative study of two beta-adrenergic receptor antagonists. Acta Pharmacol Toxicol (Copenh) 1967;25:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56:10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H1840–H1850. doi: 10.1152/ajpheart.00692.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. α-Adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol. 2010;109:1360–1368. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Mita M, Kuramoto T, Ito K, Toguchi-Senrui N, Hishinuma S, Walsh MP, Shoji M. Impairment of α1-adrenoceptor-mediated contractile activity in caudal arterial smooth muscle from type 2 diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol. 2010;37:350–357. doi: 10.1111/j.1440-1681.2009.05308.x. [DOI] [PubMed] [Google Scholar]
- Naik JS, Xiang L, Hodnett BL, Hester RL. α-Adrenoceptor-mediated vasoconstriction is not involved in impaired functional vasodilation in the obese Zucker rat. Clin Exp Pharmacol Physiol. 2008;35:611–616. doi: 10.1111/j.1440-1681.2007.04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120:625–633. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104–4113. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282:H816–H820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


