Abstract
The circadian system co-ordinates the temporal patterning of behaviour and many underlying biological processes. In some cases, the regulated outputs of the circadian system, such as activity, may be able to feed back to alter core clock processes. In our studies, we used four wheel-access conditions (no access; free access; early night; and late night) to manipulate the duration and timing of activity while under the influence of a light–dark cycle. In wild-type mice, scheduled wheel access was able to increase ambulatory activity, inducing a level of exercise driven at various phases of the light–dark cycle. Scheduled exercise also manipulated the magnitude and phasing of the circadian-regulated outputs of heart rate and body temperature. At a molecular level, the phasing and amplitude of PER2::LUCIFERASE (PER2::LUC) expression rhythms in the SCN and peripheral tissues of Per2::Luc knockin mice were altered by scheduled exercise. We then tested whether scheduled wheel access could improve deficits observed in vasointestinal polypeptide-deficient mice under the influence of a light–dark cycle. We found that scheduled wheel access during the late night improved many of the behavioural, physiological and molecular deficits previously described in vasointestinal polypeptide-deficient mice. Our results raise the possibility that scheduled exercise could be used as a tool to modulate daily rhythms and, when applied, may counteract some of the negative impacts of ageing and disease on the circadian system.
Key points
The circadian system drives rhythms of behaviour, physiology and gene expression in alignment to a light–dark cycle, and misalignment of the internal clock with the external environment can lead to disease.
We sought to determine whether scheduled exercise could alter rhythmic properties in mice while subjected to the strong entrainment effects of light and whether we could improve diurnal deficits observed in the vasointestinal polypeptide (VIP)-deficient mouse.
Scheduled exercise altered daily rhythms of activity, physiology and gene expression in wild-type and VIP-deficient mice.
Scheduled exercise during the late night improved many of the rhythmic deficits observed in VIP-deficient mice, including changes in gene expression within the suprachiasmatic nucleus, the site of circadian rhythm generation.
The results raise the possibility that scheduled exercise could be a tool to drive and improve daily rhythms in humans to mitigate the negative consequences of circadian misalignment.
Introduction
The endogenous circadian system drives co-ordinated rhythms of behaviour, physiology and gene expression and responds to a variety of external cues in order to synchronize to the changing environment. Photic input is the primary driver that resets the circadian clock by directly modulating the function of the central oscillator located in the hypothalamus, called the suprachiasmatic nucleus (SCN; Reppert & Weaver, 2002). Light can alter the molecular feedback loop found in the individual neurons of the SCN, by shifting the timing of expression of its clock gene components (i.e. Period, Cryptochrome and Bmal1; Yamazaki et al. 2000; Davidson et al. 2008). The SCN, through neural, humoral and paracrine mechanisms, drives the molecular clock and rhythms of individual cells in various peripheral tissues that enable the co-ordination of biological processes (Kalsbeek et al. 2006; Dibner et al. 2010). In addition to light, the circadian system is responsive to changes in its environment, resources and conditions. For example, activity is a behavioural output regulated by the circadian system and, at altered intensities or timing, can feed back and modulate circadian regulation of outputs (Yamada et al. 1988; Reebs & Mrosovsky, 1989; Edgar & Dement, 1991; Mrosovsky, 1996).
In rodents, the effects of stimulated activity induced by wheel access on the circadian system have been demonstrated by a variety of studies. The phase of the circadian system shifts in response to wheel access administered at certain times of the day (Reebs & Mrosovsky, 1989). Wheel access scheduled at 24 h intervals during constant dark conditions synchronized rhythms of wild-type (WT) mice (Edgar & Dement, 1991) and helped improve behavioural rhythms in circadian-compromised mouse models (Power et al. 2010). Furthermore, re-entrainment to a shifted light–dark cycle can be accelerated or delayed depending on whether wheel access is allowed in phase or out of phase of the new light–dark cycle, respectively (Mrosovsky & Salmon, 1987; Dallmann & Mrosovsky, 2006; Castillo et al. 2011). Activity during the subjective day has also been shown to result in an acute decrease the expression of clock genes (Period1 and Period2) in the SCN (Maywood et al. 1999; Yannielli et al. 2002), indicating an effect of activity on the central pacemaker. Furthermore, recent in vivo studies have described close correlations between the firing rate of neurons in the SCN and the level of behavioural activity, supporting a direct interaction between the two processes (Schaap & Meijer, 2001; Houben et al. 2009).
In our studies, we measured rhythms of behaviour, physiology and gene expression in WT mice subjected to wheel-access conditions that manipulated the timing and duration of activity, during a 12 h–12 h light–dark cycle. We first examined the effects of free wheel access on activity and then restricted the opportunity to run, by providing scheduled wheel access during the first or second half of the dark phase. Scheduled wheel access better reflects the limited time that humans spend active or exercising. Furthermore, examination of the effects of wheel access during a light–dark cycle takes into account interactions of photic and non-photic input, conditions in which the circadian system has evolved. We also measured the ability of the wheel-access conditions to reorganize daily rhythms of heart rate (HR), body temperature and gene expression. Lastly, to test whether activity alters or rescues a disrupted circadian system, we extended our studies and examined the effects of wheel access on vasointestinal polypeptide (VIP)-deficient mice, a model that exhibits circadian deficits.
Methods
Ethical approval
All experiments were conducted according to the recommendations and guidelines of the University of California Los Angeles Division of Laboratory Animals and the National Institutes of Health.
Experimental animals
We examined the effects of wheel access in Vip−/−;Per2::Luc and age-matched Vip+/+;Per2::Luc littermate control mice. The mPer2Luc knockin line on the C57BL/6J background (a gift from Dr J. S. Takahashi; Yoo et al. 2004) was crossed with the Vip−/−;Phi−/− line, also on the C57BL/6J background (created and maintained in our facilities; Colwell et al. 2003) and backcrossed for five or six generations. Mice from this line were maintained as a homozygous line for the Per2::Luc fusion gene. The peptide histidine isoleucine (Phi) is coded by the same locus as Vip; therefore both peptides are deficient.
Telemetry measurements
Wild-type (n= 7) and VIP-deficient mice (n= 7; 4–5 months old) were anaesthetized with isoflurane (2–3%) vaporized in oxygen, surgically implanted with a wireless radiofrequency transmitter (ETA-F20; Data Sciences International, St Paul, MN, USA) and subsequently housed in individual cages. These cages contained running wheels equipped with an automated wheel-locking device that prohibits/allows wheel running at specified times of the day without human stimulation or arousal. Cages were placed on top of telemetry receivers (Data Sciences International) in a light- and temperature-controlled chamber, where mice were provided with standard rodent chow ad libitum. Data collection began 2 weeks postsurgery, to allow mice to recover in the 12 h–12 h light–dark cycle condition. Throughout the experiment, we recorded 20 s of the ECG waveform every 10 min, which was used to extrapolate HR using the RR interval. Likewise, 20 s recordings of body temperature and ambulatory activity (in arbitrary units; a.u.) were averaged and recorded every 10 min.
In conditions of a 12 h–12 h light–dark cycle, four WT and three VIP-deficient mice were subjected to no wheel access, free wheel access, followed by wheel access between zeitgeber (ZT) 12–18 and 18–24 (h); ZT 0 is defined as lights on. For the remaining mice (three WT and four VIP-deficient mice) the order of the conditions was altered (no access, free access, ZT 18–24 and ZT 12–18) to minimize any order effects of scheduled wheel access. Each mouse was subjected to all of the wheel-access conditions and remained in each condition for a minimum of 16 days.
Behavioural analysis
We produced sample actograms for ambulatory activity, HR and body temperature from WT and VIP-deficient mice subjected to various wheel-access conditions. The y-axes were kept consistent within and between genotypes for all three measurements, namely ambulatory activity (0–30 a.u.), body temperature (35–38°C) and HR (475–675 beats min−1). We produced average waveforms by binning 3 h averages of HR, body temperature and ambulatory activity and aligned 10 consecutive days of data using the time of lights on. Rhythms of HR, body temperature and ambulatory activity were analysed by periodogram analysis combined with a χ2 test (El Temps software, Barcelona, Spain) using the strongest amplitude (i.e. power, calculated as percent of variance, % V) of periodicities (within 20 and 31 h limits) to reflect the robustness of the rhythm. We used ClockLab software (Actimetrics, Wilmette, IL, USA) to calculate the acrophase of daily rhythms, which is the phase in the cycle during which the cycle peaks. Data from the last 10 days in each wheel-access condition were used for all analyses.
Activity monitoring via video-tracking system
Wild-type (n= 4) and VIP-deficient mice (n= 5; 3–6 months old) were individually housed in cages with a 12 h–12 h light–dark cycle while provided with ad libitum access to food and water. Wheel-access conditions included no access and late night access and were locked/unlocked manually. The computer was equipped with video-capture cards (Adlink Technology, Inc., Irvine, CA, USA) connected to surveillance cameras with a visible light filter attached (Gadspot, Inc., City of Industry, CA, USA), with one camera for every two cages viewed from the side. Infrared lighting was mounted above the cages for consistent lighting in changing light–dark conditions. Any-Maze software (Stoelting, Co., Wood Dale, IL, USA) was used to track the animals’ ambulatory activity by background subtracting the live video footage with an image of an empty cage (Fisher et al. 2012). The duration of ambulatory movement was summed into 1 h intervals, and average waveforms were produced using 2 days of data from each mouse.
Bioluminescence
Wild-type and VIP-deficient mice (n= 6–9; 4–6 months old) were subjected to each of the wheel-access conditions for at least 2 weeks before they were deeply anaesthetized with isoflurane and sacrificed by decapitation for PER2-driven bioluminescence recording using a photomultiplier tube photodetector (Lumicycler, Actimetrics, Wilmette, IL, USA). For these studies, wheels were locked manually. Our culture conditions are as described by Yamazaki & Takahashi (2005), and dissections are detailed in Loh et al. (2011). Briefly, mice were killed during ZT 10–12, and the SCN, liver, heart and adrenals were dissected and placed on Millicell membranes (0.4 μm, PICMORG50; Millipore, Bedford, MA, USA) in 35 mm dishes with 1.2 ml of recording media containing 0.1 mm luciferin (sodium salt monohydrate; Biosynth, Staad, Switzerland). Heart explants were taken from atrial tissue using a single cut with a sterile scalpel. The dishes were sealed using high-vacuum grease (Dow Corning, Midland, MI, USA) and placed in the Lumicycler maintained at 37°C. Bioluminescence was recorded for at least 7 days. Using the Lumicycler analysis software (Actimetrics, Wilmette, IL, USA), raw data were normalized by subtraction of the recorded baseline followed by the subtraction of the 24 h running average. Peaks and troughs were identified to calculate amplitude, period and timing of PER2::LUCIFERASE (PER2::LUC) peaks. To calculate the timing of PER2::LUC peaks, we calculated the number of hours from ZT 12 of dissection day, and compared the average timing of the peak that occurred between 36 and 60 h from the time of dissection. Period was calculated by averaging the time interval between four peaks, starting from the first peak that occurred after 36 h of recording. Amplitude was calculated by measuring the bioluminescence value at the peak of the waveform that occurred between 36 and 60 h and subtracting the bioluminescence value of the subsequent trough.
Statistics
For all analyses, we compared free-access and scheduled access against no-wheel-access conditions, separately. For within-genotype analysis, we used Student's paired t test to compare average ambulatory activity, HR and body temperature, as well as rhythmic power and acrophase between no-access and free-wheel-access conditions. We used a one-way repeated measures ANOVA to compare these same parameters between no-access and the two scheduled wheel-access conditions. Average waveform comparisons between no-access and free-access conditions and between no-access and scheduled access conditions were analysed using a two-way repeated measures ANOVA. Comparisons of period, amplitude and timing of PER2:LUC peaks between no-wheel-access and free-wheel-access conditions were analysed using Student's unpaired t test, while comparisons between no wheel access and scheduled access were analysed using a one-way ANOVA.
For genotypic comparisons between WT and VIP-deficient mice, we used a two-way repeated measures ANOVA to compare the power and acrophase of ambulatory activity, HR and body temperature between no access and free access or between no access and scheduled wheel access. A two-way ANOVA was used to compare period, amplitude and timing of PER2:LUC peaks between genotypes.
Statistical analyses were performed using Sigma Plot 10.0 software (San Jose, CA, USA). All data were tested for normal distribution and variance, but in cases where the data did not pass these tests we used non-parametric statistical tests (e.g. Wilcoxon signed rank test or Mann–Whitney rank sum test) to determine significance. Following ANOVA, post hoc tests (Holm–Sidak or Student–Newman–Keuls method) were used to identify significantly different groups. We report the appropriate t, Q, F or H statistics and include the degrees of freedom for each analysis. We report some of the post hoc statistics for comparisons that were not significant.
Results
Ambulatory activity in WT mice provided with free access to the wheel
Using telemetry, we recorded ambulatory activity in freely moving WT mice given either no access or free access to the running wheel (Fig. 1A). Free access increased the 24 h averages of ambulatory activity compared with conditions without access (t6=−4.22, P= 0.006; Supplemental Table 1). In comparing the average waveforms of ambulatory activity (Fig. 1B), we found that ambulatory activity increased selectively during the dark phase of the light–dark cycle (Supplemental Table 2), demonstrating an induction of exercise during the normal active phase of WT mice. Lastly, the power of ambulatory activity rhythms was improved with free access to the wheel (t6=−4.86, P= 0.003; Fig. 1C), while acrophase was not altered (t6= 2.053, P= 0.086; Fig. 1D).
Figure 1. Wheel access increased levels and altered rhythmic properties of ambulatory activity in wild-type (WT) mice.
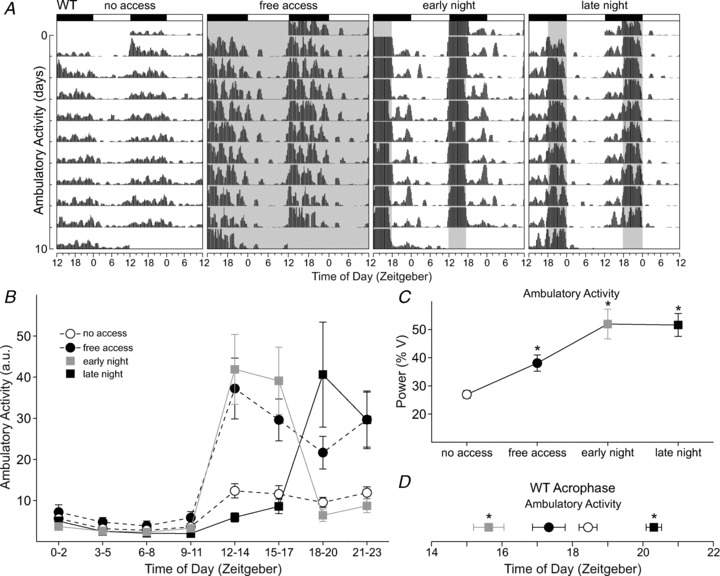
A, double-plotted raster plot using 10 consecutive days of ambulatory activity recording from the same WT mouse subjected to the four wheel-access conditions in the presence of a 12 h–12 h light–dark cycle. In this and subsequent figures, each horizontal row represents a 24 h period plotted twice with each day/row plotted in succession. The light–dark cycle is specified by the white and black bars above each graph. Shaded areas indicate when the wheel was available. The profile of ambulatory activity rhythms is clearly altered by the various wheel-access conditions. B, group mean waveforms using 10 days of ambulatory activity data from WT mice (n= 7) subjected to the various wheel-access conditions. Ambulatory activity data are grouped into 3 h bins. Ambulatory activity levels increased when wheel access was available. For statistical results, see Supplemental Table 2. C, increased power of ambulatory activity rhythms in WT mice subjected to wheel-access conditions. Power measurement was produced by periodogram analysis using 10 days of behavioural data. (% V = percent of variance) D, shifts in the acrophase of ambulatory activity rhythms were induced by scheduled wheel access. Early night access advanced while late night access delayed the acrophase of ambulatory activity. For this and subsequent figures the average acrophase from 10 days of data was calculated using the ClockLab software, average waveform comparisons were analysed using a two-way repeated measures ANOVA, and power and acrophase comparisons were analysed using a one-way repeated measures ANOVA. Error bars represent SEM (*P < 0.05).
Ambulatory activity in WT mice provided with scheduled access to the wheel
We shortened the duration of wheel access to two different phases of the light–dark cycle to test whether the limited opportunity for running would alter the levels and timing of ambulatory activity in WT mice when compared with no-access conditions. Wheel access restricted to 6 h each day, scheduled either during the early night (ZT 12–18) or the late night (ZT 18–24; Fig. 1A), did not change the 24 h averages of activity (Supplemental Table 1). However, we detected significant increases in ambulatory activity that were dependent on the time of day (Fig. 1B and Supplemental Table 2). Compared with no-access conditions, ambulatory activity was significantly increased during the times of wheel availability. The power of ambulatory activity rhythms was significantly improved (F2,20= 21.0, P < 0.001) with access in the early night (t12= 5.65, P < 0.001) and late night (t12= 5.57, P < 0.001; Fig. 1C). Lastly, scheduled wheel access shifted the acrophase of ambulatory activity rhythms (F2,20= 74.0, P < 0.001). Access in the early night advanced the acrophase (t12= 7.27, P < 0.001), whereas access in the late night (t12= 4.82, P < 0.001) delayed the acrophase of activity (Fig. 1D). Video analysis confirmed that the WT mice were more active when the wheel was available in the late night compared with no-access conditions (Supplemental Fig. 1A and Supplemental Table 3). We demonstrate that scheduled wheel access altered levels of activity, improved ambulatory activity rhythms and shifted its acrophase, indicating that manipulation of wheel access can induce exercise at specified times of the light–dark cycle.
Effect of wheel access on HR and body temperature of WT mice
We recorded HR (Fig. 2A) and body temperature (Fig. 3A) from WT mice subjected to the various exercise schedules to determine whether other circadian-regulated physiological outputs were altered. Compared with no-wheel-access conditions, 24 h averages of HR were increased with free access (t6=−3.66, P= 0.011) as well as scheduled wheel access (F2,20= 13.2, P < 0.001), during either the early night (t12= 4.31, P= 0.001) or the late night (t12= 4.59, P < 0.001; Supplemental Table 1). Free access increased HR at all time points of the day except during ZT 12–14 (Fig. 2B and Supplemental Table 2). Wheel access during the early night increased HR at all time points except for the few hours before lights off. Late night access increased HR starting at ZT 18, when the wheel was available and throughout most of the light phase up until ZT 8. Free access did not alter the power of HR rhythms, but scheduled wheel access (F2,20= 4.41, P= 0.037) during the early night (t12= 2.79, P= 0.017) significantly increased power (Fig. 2C). The acrophase of HR rhythms was not shifted by free access, but scheduled access (F2,20= 24.7, P < 0.001) during the late night effectively delayed the acrophase (t12= 5.77, P < 0.001; Fig. 2D).
Figure 2. Wheel access altered daily rhythms of heart rate (HR) in WT mice.
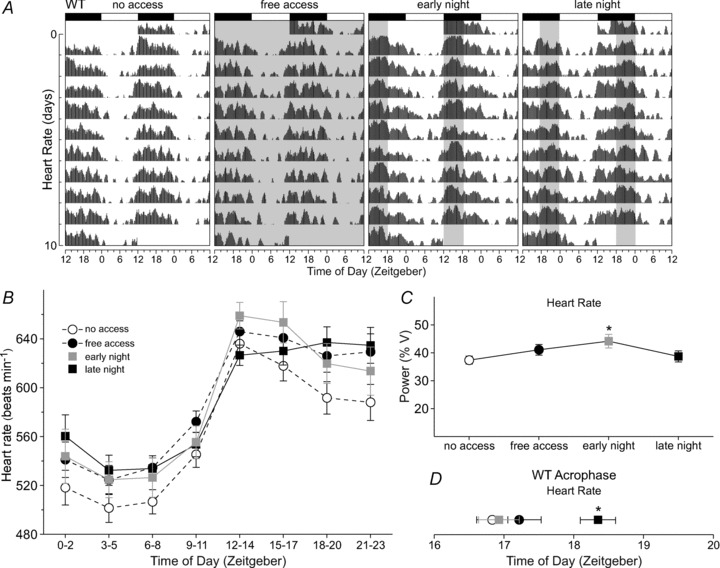
A, double-plotted raster plot of HR using 10 consecutive days of HR recording from the same WT mouse subjected to the four wheel-access conditions in the presence of a 12 h–12 h light–dark cycle. B, group mean waveforms using 10 days of HR recordings from WT mice (n= 7) subjected to the various wheel-access conditions. The HR data were grouped into 3 h bins. Heart rates were increased at most time points of the light–dark cycle, including periods when the wheel was available. For statistical results, see Supplemental Table 2. C, increased power of HR rhythms in WT mice subjected to early night wheel access. Power measurement was produced by periodogram analysis using 10 days of HR data. D, late night access delayed the acrophase of HR rhythms of WT mice. Error bars are SEM (*P < 0.05).
Figure 3. Wheel access altered daily rhythms of body temperature in WT mice.
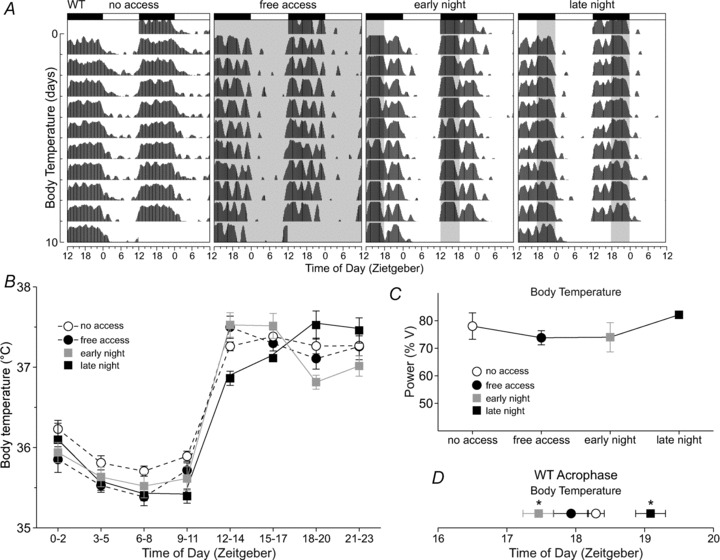
A, double-plotted raster plot of body temperature using 10 consecutive days of temperature recording from the same WT mouse subjected to the four wheel-access conditions in the presence of a 12 h–12 h light–dark cycle. B, group mean waveforms using 10 days of temperature recordings from WT mice (n= 7) subjected to the various wheel-access conditions. Temperature data were grouped into 3 h bins. Wheel access increased temperature when wheel access was available, but also decreased temperatures at certain time points of their rest phase. For statistical results see Supplemental Table 2. C, wheel access did not alter the power of body temperature rhythms in WT mice. Power measurement was produced by periodogram analysis using 10 days of temperature data. D, wheel access altered the acrophase of body temperature rhythms. Early night access advanced while late night access delayed the acrophase of body temperature. The average acrophase from 10 days of data was calculated using the ClockLab software. Error bars are SEM (*P < 0.05).
Average body temperatures of WT mice were decreased by free access (t6= 3.15, P= 0.02), as well as by scheduled access (F2,20= 7.43, P= 0.008) in both the early night (t12= 3.12, P= 0.009) and the late night (t12= 3.52, P= 0.004) when compared with no wheel access (Supplemental Table 1). Free access decreased body temperatures during most of the light phase and increased body temperature during the first few hours of dark (Fig. 3B and Supplemental Table 2). Wheel access in the early night resulted in significantly higher temperatures at the time of wheel access and a decrease in temperature in the hours following the end of the wheel-access period. Access in the late night increased body temperature at the beginning of wheel access, and decreased body temperatures in the late part of the day and early night. The power of body temperature rhythms was not changed with either free or scheduled wheel access (Fig. 3C). Lastly, free wheel access did not change the acrophase of body temperature rhythms, but scheduled access (χ22= 10.3, P= 0.004) during the early night advanced the acrophase (q12= 3.21, P < 0.05), while access in the late night delayed the acrophase (q12= 3.21, P < 0.05; Fig. 3D).
Effect of wheel access on PER2:LUC rhythms in the SCN and peripheral tissues of WT mice
In WT mice, manipulating the timing of wheel access altered properties of behaviour and physiology. We wanted to explore whether wheel access would also change properties of the molecular clock by recording PER2-driven bioluminescence rhythms of SCN (Fig. 4A), heart, liver and adrenals from Per2::Luc mice subjected to the various wheel-access conditions.
Figure 4. Effects of wheel access on PER2::LUC rhythms in the suprachiasmatic nucleus (SCN) and peripheral tissues of WT mice.
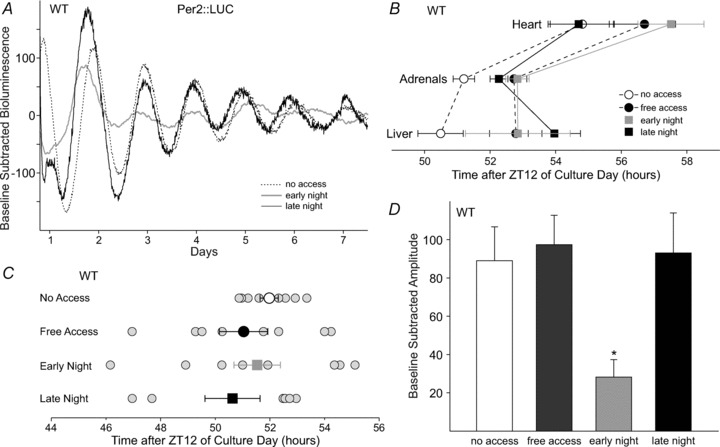
A, example bioluminescence waveforms from the SCN of WT mice subjected to wheel-access conditions. B, average timing of PER2::LUCIFERASE (PER2::LUC) peaks in the peripheral tissues of WT mice. Significant delays in timing were detected in the adrenals and livers. C, average timing of PER2::LUC peaks in the SCN of WT mice (larger symbols with error bars) did not significantly differ between wheel-access groups; however, when examining individual samples (smaller grey circles), the variability of the timing was increased when mice were provided with any form of wheel access. The x-axis represents the number of hours after ZT (Zeitgeber) 12 of culture day. D, effects of wheel access on the amplitude of PER2::LUC rhythms in the SCN of WT mice, where early night wheel access significantly dampened the amplitude of PER2::LUC rhythms compared with no-access conditions. Average PER2::LUC timing and amplitude were analysed using a one-way ANOVA. For statistical results, see main text. Error bars are SEM (*P < 0.05).
We first examined the effect of wheel access on the timing of PER2::LUC peaks, which would reflect the phasing of gene expression in the various tissues. In the adrenals (t14=−2.97, P= 0.01) and livers of WT mice (t14=−2.17, P= 0.047), free access delayed the timing of PER2::LUC peaks (Fig. 4B). Scheduled wheel access also had a significant effect on the timing of PER2::LUC in both the liver (F2,23= 8.05, P= 0.003) and adrenals (F2,23= 6.73, P= 0.006). In the liver, early night (t14= 3.54, P= 0.002) and late night access (t14= 3.41, P= 0.003) delayed the timing of peaks, whereas in the adrenals only early night access (t14= 3.61, P= 0.002) caused a significant delay. In the heart, free access (t13=−1.34, P= 0.20) and scheduled wheel access (F3,31= 2,23, P= 0.092) had no significant effect on timing (Fig. 4B). In the SCN, the average timing of PER2::LUC peaks was not significantly different between mice subjected to the various wheel-access conditions (free access, U= 24, P= 0.44; and scheduled access, F2,23= 0.60, P= 0.56); however, the range of peak times was increased when mice were allowed any amount of running (no access, 2.48 h; free access, 7.28 h; early night, 9.0 h; and late night, 6.0 h; Fig. 4C).
We next examined the effect of wheel access on the period of PER2::LUC rhythms in the various tissues (Supplemental Table 4). Free-access conditions lengthened the period of PER2::LUC in the SCN (t14=−2.22, P= 0.04) as well as in the heart (t12=−2.29, P= 0.04), but there was no effect in the liver or adrenals. Early night (t14= 4.79, P < 0.001) and late night wheel access (t14= 3.07, P= 0.006) lengthened the period of PER2::LUC bioluminescence in the adrenals (F2,23= 11.8, P < 0.001) but not in the SCN, heart or the liver.
Lastly, we examined the effect of wheel access on the amplitude of PER2::LUC rhythms in the SCN and peripheral tissues. In the adrenals, free access had no effect, but scheduled wheel access (H2= 12.35, P= 0.002) during the early (q14= 3.12, P < 0.05) and late night (q14= 4.95, P < 0.05) increased the amplitude of rhythms (Supplemental Table 5). We did not find a significant change in amplitude in any of the other peripheral tissues. In the SCN, free access did not change the amplitude of PER2::LUC rhythms compared with no access (Fig. 4D). Scheduled wheel access, on the contrary, had a significant effect on SCN PER2::LUC amplitude (H2= 9.3, P= 0.01), whereby wheel access in the early night (Q13= 2.73, P < 0.05) decreased the amplitude.
In summary, wheel access delayed the timing of PER2::LUC rhythms in some of the peripheral tissues and produced a variable effect on timing in the SCN of WT mice. There are also effects on amplitude and period in the SCN and some peripheral tissues.
Late night access rescues circadian parameters of behaviour, physiology and gene expression in VIP-deficient mice
We subjected VIP-deficient mice to the four wheel access conditions (Fig. 5A) to explore whether we could alter or rescue circadian parameters, which are disrupted in these mice. Consistent with previous reports, VIP-deficient mice without wheel access displayed an advanced acrophase in ambulatory activity (t12= 7.21, P < 0.001; Fig. 5C), HR (t12= 3.38, P= 0.002; Fig. 5D) and body temperature (t12= 9.22, P < 0.001; Fig. 5E) as well as lower power of HR (t12= 3.93, P= 0.001; Fig. 2C vs. Supplemental Fig. 2B) and body temperature rhythms (t12= 3.41, P= 0.002; Fig. 3C vs. Supplemental Fig. 2C) when compared with WT control mice (Supplemental Table 6; Schroeder et al. 2011). At a molecular level, we previously reported that the timing of PER2::LUC peaks was advanced in the heart (t14= 2.28, P= 0.03) and adrenals (t14= 2.14, P= 0.041), with a measured trend in the liver (t14= 1.89, P= 0.069; Supplemental Fig. 3A and Supplemental Table 7; Loh et al. 2011). In the SCN, there were no significant differences in the timing of PER2::LUC peaks between genotypes (Supplemental Fig. 3B), but the amplitude of the rhythm was significantly dampened in VIP-deficient mice (t14= 3.37, P= 0.002; Fig. 6A and B and Supplemental Table 7).
Figure 5. Wheel access altered daily rhythmic properties of ambulatory activity, HR and body temperature in vasointestinal polypeptide (VIP)-deficient mice.
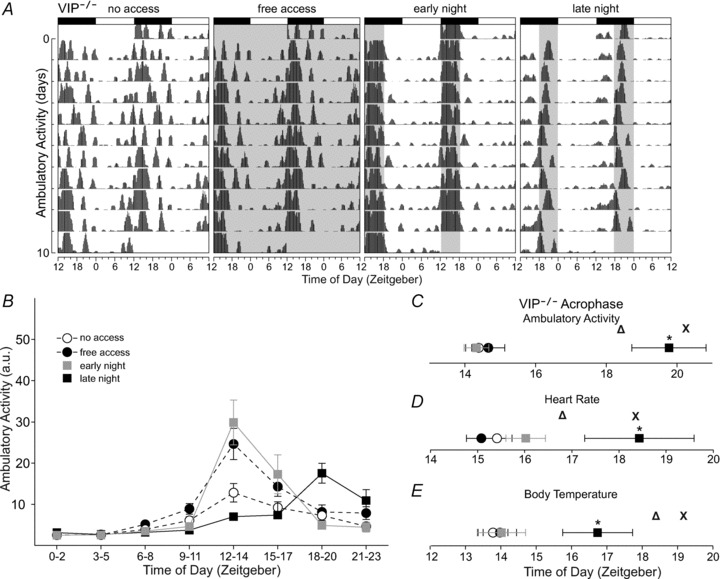
A, double-plotted raster plot using 10 consecutive days of ambulatory activity from the same VIP-deficient mouse subjected to the four wheel-access conditions in the presence of a 12 h–12 h light–dark cycle. The profile of ambulatory activity rhythms is altered by wheel access. B, group mean waveforms using 10 days of ambulatory activity data from VIP-deficient mice (n= 7) subjected to the various wheel-access conditions. Ambulatory activity data were grouped into 3 h bins. Wheel access increased ambulatory activity levels at certain time points of the light–dark cycle, including periods when wheel access was provided. For statistical results see Supplemental Table 8. Late night wheel access delayed and rescued the acrophase of ambulatory activity (C) and HR (D) in VIP-deficient mice. Body temperature acrophase was also delayed but only partly rescued (E; see main text and Supplemental Table 6). Open triangles represent WT acrophase subjected to no wheel access; crosses represent WT acrophase subjected to late night wheel access. Error bars are SEM (*P < 0.05).
Figure 6. Effects of wheel access on amplitude of PER2::LUC rhythms in the SCN of VIP-deficient mice.
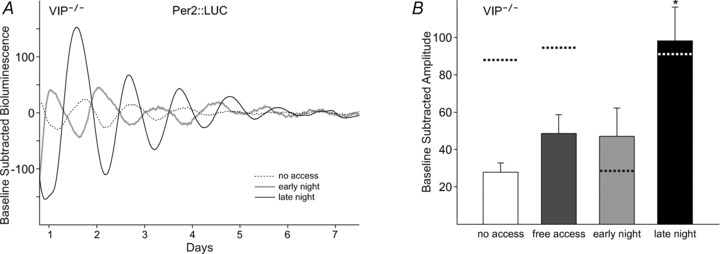
A, example bioluminescence waveforms from VIP-deficient mice subjected to the wheel-access conditions. B, effects of wheel access on the amplitude of PER2::LUCIFERASE (PER2::LUC) rhythms in the SCN of VIP-deficient mice, where late night wheel access significantly improved the amplitude of PER2::LUC rhythms such that the amplitude was no longer significantly different when compared with WT mice (see main text and Supplemental Table 7). Dotted lines represent WT levels. The average PER2::LUC amplitude was analysed using a one-way ANOVA. Genotypic comparisons were analysed using a two-way ANOVA. Error bars are SEM (*P < 0.05).
When VIP-deficient mice were provided with free wheel access, 24 h average ambulatory activity significantly increased (t6=−3.827, P= 0.009; Supplemental Table 1), the power of ambulatory activity rhythm was improved (t6=−3.97, P= 0.007; Supplemental Fig. 2A) and the daily profile of ambulatory activity was altered (Fig. 5A and B and Supplemental Table 8). Scheduled wheel access did not change 24 h average activity in VIP-deficient mice (Supplemental Table 1); however, we did find alterations in ambulatory activity levels dependent on the time of day (Fig. 5A and B and Supplemental Table 8). Only late night wheel access was able to delay the acrophase of ambulatory activity (χ22= 11.1, P= 0.001; q12= 4.54, P < 0.05), effectively rescuing the advanced acrophase when compared with WT mice (Fig. 5C). Video analysis confirmed that the VIP-deficient mice spent more time being active in the late night when the wheel was available (Supplemental Fig. 1B). Scheduled wheel access improved the power of ambulatory activity rhythms (χ22= 8.86, P= 0.008) in the early night (q12= 4.16, P < 0.05) and late night (q12= 3.74, P < 0.05) in VIP-deficient mice, but the increase in power brought about by late night access was significantly lower when compared with WT mice (t12= 3.90, P < 0.001; Fig. 1C, Supplemental Fig. 2A and Supplemental Table 6). Overall, by scheduling wheel access in VIP-deficient mice, we increased levels of ambulatory activity at certain phases of the light–dark cycle when wheel access was provided, improved the power of ambulatory activity rhythms and rescued its timing by providing late night wheel access.
We found that wheel access altered the profile of HR (Supplemental Fig. 4A and B) and body temperature rhythms (Supplemental Fig. 5A and B) in VIP-deficient mice (Supplemental Table 8). Similar to ambulatory activity, late night wheel access delayed the acrophase of HR (χ22= 8.00, P= 0.016; q12= 3.806, P < 0.05; Fig. 5D) and body temperature (F2,20= 6.46, P= 0.012; t12= 3.24, P= 0.007; Fig. 5E) in VIP-deficient mice, which eliminated the differences in acrophase of HR when compared with WT mice, while improving the relative phasing of body temperature (t12= 3.12, P= 0.004; Supplemental Table 6). The acrophase resulting from free wheel access remained advanced in VIP-deficient mice (ambulatory activity, t12= 2.58, P= 0.013; HR, t12= 5.28, P < 0.001; and body temperature, t12= 8.08, P < 0.001), whereas early night running eliminated differences between genotypes because WT mice advanced their acrophase (Supplemental Table 6).
At a molecular level, free (T= 46, P= 0.024) and scheduled wheel access (H2= 8.21, P= 0.017; early night, q14= 4.05, P < 0.05; and late night, q14= 2.90, P < 0.05) significantly delayed the timing of PER2::LUC peaks in the heart, such that the differences in phasing between WT and VIP-deficient mice were eliminated (Supplemental Fig. 3A and Supplemental Table 7). In the liver, we did not detect changes in the timing of peaks in VIP-deficient mice subjected to either free or scheduled wheel access (Supplemental Fig. 3A). When compared with WT, PER2::LUC timing in the livers of VIP-deficient mice was different during free access (t14= 2.76, P= 0.01), but no differences were detected when the mice were subjected to scheduled wheel access (Supplemental Table 7). In the adrenals, only free access delayed PER2::LUC peaks (t15= 3.32, P= 0.005) and eliminated genotypic differences in timing, but PER2::LUC phasing remained advanced in VIP-deficient mice subjected to scheduled access (early night, t14= 4.12, P < 0.001; and late night t14= 2.80, P= 0.008; Supplemental Fig. 3A and Supplemental Table 7). We also detected some changes in period (Supplemental Table 4) and amplitude of PER2::LUC rhythms (Supplemental Table 5) in peripheral tissues of VIP-deficient mice as a result of wheel access (Supplemental Table 7).
In the SCN, free and scheduled access did not change the average timing of PER2::LUC peaks in VIP-deficient mice (Supplemental Fig. 3B), and no differences were detected between genotypes (Supplemental Table 7). However, there was an increase in the range of timing of PER2::LUC peaks in mice subjected to early night access (no access, 5.5 h; and early night, 19.2 h). Lastly, the amplitude of PER2::LUC rhythms in the SCN of VIP-deficient mice was increased when mice were subjected to late night access (q14= 4.15, P < 0.05; Fig. 6B), which eliminated the differences between genotypes and rescued the amplitude in VIP-deficient mice (Supplemental Table 7).
Discussion
The effects of stimulated activity on the circadian system have previously been studied, usually in conditions of controlled constant environments or by examining responses to single bouts of stimulated activity (Reebs & Mrosovsky, 1989; Edgar & Dement, 1991; Marchant & Mistlberger, 1996; Buxton et al. 1997, 2003; Kas & Edgar, 2001; Maywood & Mrosovsky, 2001; Canal & Piggins, 2006; Koletar et al. 2011). However, most of life and the circadian pacemaker have evolved in the presence of the entrainment effects of daily light–dark cycles; therefore, responses to environmental factors may differ when studied during light–dark cycles or in constant conditions (Mrosovsky, 1996; Roenneberg et al. 2010). In our studies, we examined the ability of voluntary activity to modify rhythms while mice were entrained to a light–dark cycle. We altered the duration and timing of wheel access for at least 2 weeks to drive wheel-running activity at different phases within the dark period in WT mice and examined whether we could reorganize rhythms in behaviour, physiology and gene expression. Also, we subjected VIP-deficient mice to the same scheduled wheel-access conditions in an attempt to improve their abnormal entrainment to a light–dark cycle.
Our studies demonstrated that scheduled wheel access increased levels of ambulatory activity, thereby inducing a level of exercise at specified times of the light–dark cycle in WT mice (Aufradet et al. 2012). Consistent with previous studies, we found that free access to the running wheel during a light–dark cycle increased the levels of ambulatory activity throughout the dark phase (de Visser et al. 2005). We also showed that scheduled wheel access, restricted to 6 h during the early or late night, increased activity levels during the period of wheel availability, thereby advancing and delaying the acrophase of ambulatory activity, respectively. Previous studies in hamsters found that arousal with a novel wheel at the end of the dark phase altered the phasing of activity onset in a light–dark cycle (Mistlberger, 1991). This daily stimulation of activity is encoded within the circadian system, whereby the effects on behaviour are sustained for many days after removal of the stimulus (Sugimoto et al. 1994; Reebs & St-Coeur, 1994; Mistlberger & Holmes, 2000). Furthermore, when animals entrained to various phases of stimulated activity are deprived of arousal and placed in constant conditions, the period of activity diverges, which suggests that encoding of activity within the circadian system is dependent on its timing (Mistlberger, 1991; Reebs & St-Coeur, 1994). The observed modulation of circadian properties by stimulated activity during a light–dark cycle suggests that this manipulation could potentially drive and organize rhythms of other behavioural and physiological outputs regulated by the circadian system.
We were able effectively to induce exercise and alter the timing of ambulatory activity relative to the light–dark cycle in WT mice, which subsequently altered the daily profile and timing of HR and body temperature rhythms (Sugimoto et al. 1994), two physiological outputs responsive to changes in activity and whose function is regulated by the circadian system (Abe et al. 1979; Warren et al. 1994). Exercise during the early night advanced the acrophase of body temperature rhythms, whereas late night exercise delayed the acrophase of both body temperature and HR rhythms. Shifts in the timing of physiological rhythms may be driven by the metabolic cycles induced by scheduled exercise (Kohsaka et al. 2012). Our results demonstrated that even in the presence of the strong entrainment effects of a light–dark cycle, scheduled exercise shifted the timing of physiological rhythms.
In addition to the effects on physiology, scheduled exercise altered properties of the molecular clock in the SCN and peripheral tissues of WT mice, suggesting that daily exercise feeds back to the clock to modulate tissue and cell function and that the changes in behavioural and physiological rhythms are not merely acute responses. Within the SCN, the average timing of PER2::LUC peaks was not significantly different between the four conditions; however, the variability in the timing of PER2::LUC peaks was increased, indicating that scheduled exercise, even in the presence of a light–dark cycle, altered molecular phasing in the SCN of WT mice. Other studies have demonstrated that single bouts of arousal during the middle of the day can also alter the molecular clock of the SCN (Maywood et al. 1999; Yannielli et al. 2002). In addition to changes in phasing, we also detected a significant blunting of PER2::LUC amplitude caused by early night running, which could be a result of reduced PER2::LUC protein levels, a loss of synchrony among individual neurons or a combination of both. This result was fairly surprising, considering the robust rhythms of activity in mice subjected to early night running. Furthermore, mice subjected to free-access conditions, which run during the first 6 h of darkness, retained robust rhythms in PER2::LUC in the SCN. Therefore, running late at night may be providing cues that maintain robust molecular rhythms.
In peripheral tissues, wheel access delayed the timing of the PER2::LUC peak in the liver and adrenals, but no significant effects were measured in the heart of WT mice. We also detected a boost in PER2::LUC amplitude in the adrenals of WT mice. Changes in amplitude and timing of the molecular clock were also documented in zebrafish subjected to exercise during the day (Egg et al. 2012). The adrenals may be responding directly to the level of exercise (Otawa et al. 2007; Cagampang et al. 2011), whereas the molecular phasing in the liver may shift in response to reorganization in the timing of feeding due to scheduled exercise (Hirao et al. 2010). Studies have shown that other non-photic inputs, such as feeding, can uncouple the central and peripheral oscillators during a light–dark cycle, effectively altering the molecular clock in the peripheral tissues but leaving the SCN unaffected (Damiola et al. 2000; Honma & Honma, 2009). Our studies demonstrated a clear effect of non-photic manipulation on properties of the central oscillator in rhythmically robust mice, suggesting that exercise-mediated changes of daily rhythms involve alterations in SCN function. Furthermore, this implies that exercise could be used as a tool to drive rhythms within the SCN even under the influence of a light–dark cycle.
Vasointestinal polypeptide is localized to a subset of neurons within the SCN and is thought to promote coupling among single-cell oscillators to produce coherent output signals (Vosko et al. 2007). During a light–dark cycle and without wheel access, the loss of VIP or its receptor (VIPR2) leads to reduced power of behavioural and physiological rhythms, as well as an advancement in their onset (Bechtold et al. 2008; Sheward et al. 2010; Schroeder et al. 2011; Hannibal et al. 2011). The phasing of the molecular clock in peripheral tissues parallels behaviour in VIP-deficient mice, such that Period2 mRNA or PER2::LUC protein peaks a few hours before that in WT mice (Loh et al. 2011). Within the SCN, although the average phasing of PER2::LUC is not altered, the amplitude of rhythms is significantly depressed in VIP-deficient mice (Loh et al. 2011). Scheduled wheel access during the late night was able to rescue many of the observed deficits of VIP-deficient mice during a light–dark cycle. Exercise in the late night increased ambulatory activity levels and improved the power of its rhythm. Late night exercise also delayed the acrophase of ambulatory activity rhythms such that the phase was no longer different when compared with WT mice. This manipulation was also able to delay the acrophase of both HR and body temperature rhythms significantly in VIP-deficient mice, rescuing or improving the phase when compared with WT mice, respectively. This manipulation, however, did not rescue the power of HR and body temperature rhythms. At a molecular level, the average phasing of PER2::LUC peaks in the SCN were not different between genotypes. Notably, late night wheel access increased the amplitude of PER2::LUC rhythms in the SCN of VIP-deficient mice to WT levels, suggesting an improvement of cell-to-cell synchrony in the SCN despite the absence of VIP. Lastly, wheel access at night delayed the timing of PER2::LUC in the heart and liver of VIP-deficient mice such that they were no longer advanced compared with WT mice, which also improved the phase relationship of peripheral tissues with the SCN. Overall, we demonstrated an improvement in several properties of diurnal rhythms in circadian-compromised VIP-deficient mice. These mice display deficits in the light response pathway, and therefore have poor entrainment when exposed to a light–dark cycle. Exercise specifically in the late night may be altering SCN properties either by promoting better synchrony among neurons and/or altering clock gene expression that optimizes the phase at which light hits the molecular clock, enhancing the ability of light to drive molecular rhythms in the SCN. Future work will need to ascertain the mechanisms by which late night exercise impacts the SCN oscillator.
Exercise itself has many beneficial effects on the body (Warburton et al. 2006; Mercken et al. 2011), but our studies demonstrated that the timing of exercise is important in altering physiology as well as gene expression in various tissues. Although the ability to exercise differs between species, timed exercise could be a tool to manipulate and/or stabilize daily rhythms in humans. Co-ordinated timing of behaviour and physiology, as well as their alignment to the light–dark cycle, is important because misalignments are correlated with the development of disease (Navara & Nelson, 2007; Stevens, 2009; Thorpy, 2011; Kivimäki et al. 2011). During conditions in which entrainment to the light–dark cycle is disrupted, whether as a result of genetics, age or disease (Maywood et al. 2006; Takahashi et al. 2008; Colwell, 2011), timing of exercise could help shift rhythms to realign better with the external environment, which could potentially delay or prevent development of disease.
Acknowledgments
We would like to thank John Parker for engineering the automated wheel-locking equipment, Donna Crandall for technical expertise with figure designs and TGB ‘team glowing balls’ for help with lumicycler experiments. This work was supported by NRSA award F31NS070529 and Neurobiology Training Grant 5T32NS7101-33 from the University of California, Los Angeles to A.M.S. as well as CHDI Foundation Grant A-4339 and American Heart Association Grant 10GRNT430078 to C.S.C.
Glossary
- HR
heart rate
- Per2::Luc
Per2::Luciferase
- SCN
suprachiasmatic nucleus
- VIP
vasointestinal polypeptide
- WT
wild-type
- ZT
zeitgeber
Author contributions
A.M.S. and C.S.C. conceived and designed the research; A.M.S., D.T., D.H.L. and M.C.J. performed the experiments; A.M.S. analysed data, prepared figures and drafted the manuscript; A.M.S., D.T., D.H.L., M.C.J., K.P.R. and C.S.C. edited and revised the manuscript; and all authors approved the final version of the manuscript.
Supplementary material
Supplemental Figure 1
Supplemental Figure 2
Supplemental Figure 3
Supplemental Figure 4
Supplemental Figure 5
Supplemental Table 1
Supplemental Table 2
Supplemental Table 3
Supplemental Table 4
Supplemental Table 5
Supplemental Table 6
Supplemental Table 7
Supplemental Table 8
References
- Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29:119–131. doi: 10.1159/000122913. [DOI] [PubMed] [Google Scholar]
- Aufradet E, Bessaad A, Alsaid H, Schäfer F, Sigovan M, De Souza G, Chirico E, Martin C, Canet-Soulas E. In vivo cardiac anatomical and functional effects of wheel running in mice by magnetic resonance imaging. Exp Biol Med (Maywood) 2012;237:263–270. doi: 10.1258/ebm.2011.011034. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R344–R351. doi: 10.1152/ajpregu.00667.2007. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Frank SA, L’Hermite-Balériaux M, Leproult R, Turek FW, Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol. 1997;273:E536–E542. doi: 10.1152/ajpendo.1997.273.3.E536. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Poore KR, Hanson MA. Developmental origins of the metabolic syndrome: body clocks and stress responses. Brain Behav Immun. 2011;25:214–220. doi: 10.1016/j.bbi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Canal MM, Piggins HD. Resetting of the hamster circadian system by dark pulses. Am J Physiol Regul Integr Comp Physiol. 2006;290:R785–R792. doi: 10.1152/ajpregu.00548.2005. [DOI] [PubMed] [Google Scholar]
- Castillo C, Molyneux P, Carlson R, Harrington ME. Restricted wheel access following a light cycle inversion slows re-entrainment without internal desynchrony as measured in Per2Luc mice. Neuroscience. 2011;182:169–176. doi: 10.1016/j.neuroscience.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nature Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Mrosovsky N. Scheduled wheel access during daytime: a method for studying conflicting zeitgebers. Physiol Behav. 2006;88:459–465. doi: 10.1016/j.physbeh.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Spruijt BM. Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behav Brain Res. 2005;160:382–388. doi: 10.1016/j.bbr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- Egg M, Tischler A, Schwerte T, Sandbichler A, Folterbauer C, Pelster B. Endurance exercise modifies the circadian clock in zebrafish (Danio rerio) temperature independently. Acta Physiol (Oxf) 2012;205:167–176. doi: 10.1111/j.1748-1716.2011.02382.x. [DOI] [PubMed] [Google Scholar]
- Fisher SP, Godinho SIH, Pothecary CA, Hankins MW, Foster RG, Peirson SN. Rapid assessment of sleep-wake behavior in mice. J Biol Rhythms. 2012;27:48–58. doi: 10.1177/0748730411431550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2011;300:R519–R530. doi: 10.1152/ajpregu.00599.2010. [DOI] [PubMed] [Google Scholar]
- Hirao A, Nagahama H, Tsuboi T, Hirao M, Tahara Y, Shibata S. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1045–G1053. doi: 10.1152/ajpgi.00330.2010. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30:1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J Biol Rhythms. 2009;24:477–487. doi: 10.1177/0748730409349895. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kas MJH, Edgar DM. Scheduled voluntary wheel running activity modulates free-running circadian body temperature rhythms in Octodon degus. J Biol Rhythms. 2001;16:66–75. doi: 10.1177/074873040101600108. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Batty GD, Hublin C. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. PLoS Med. 2011;8:e1001138. doi: 10.1371/journal.pmed.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Waki H, Cui H, Gouraud SS, Maeda M. Integration of metabolic and cardiovascular diurnal rhythms by circadian clock. Endocr J. 2012;59:447–456. doi: 10.1507/endocrj.ej12-0057. [DOI] [PubMed] [Google Scholar]
- Koletar MM, Cheng H-YM, Penninger JM, Ralph MR. Loss of dexras1 alters nonphotic circadian phase shifts and reveals a role for the intergeniculate leaflet (IGL) in gene-targeted mice. Chronobiol Int. 2011;28:553–562. doi: 10.3109/07420528.2011.592235. [DOI] [PubMed] [Google Scholar]
- Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011;26:200–209. doi: 10.1177/0748730411401740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav. 1996;60:657–663. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N. A molecular explanation of interactions between photic and non-photic circadian clock-resetting stimuli. Brain Res Gene Expr Patterns. 2001;1:27–31. doi: 10.1016/s1567-133x(01)00005-9. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, O’Neill J, Wong GKY, Reddy AB, Hastings MH. Hypothalamic Integration of Energy Metabolism, Proceedings of the 24th International Summer School of Brain Research, held at the Royal Netherlands Academy of Arts and Sciences. Elsevier; 2006. Circadian timing in health and disease; pp. 253–269. Available at: http://www.sciencedirect.com/science/article/pii/S0079612306530158 [Accessed 15 March 2012] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2011;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Scheduled daily exercise or feeding alters the phase of photic entrainment in Syrian hamsters. Physiol Behav. 1991;50:1257–1260. doi: 10.1016/0031-9384(91)90592-c. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Holmes MM. Behavioral feedback regulation of circadian rhythm phase angle in light-dark entrained mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R813–R821. doi: 10.1152/ajpregu.2000.279.3.R813. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Salmon PA. A behavioural method for accelerating re-entrainment of rhythms to new light–dark cycles. Nature. 1987;330:372–373. doi: 10.1038/330372a0. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 2007;43:215–224. doi: 10.1111/j.1600-079X.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- Otawa M, Arai H, Atomi Y. Molecular aspects of adrenal regulation for circadian glucocorticoid synthesis by chronic voluntary exercise. Life Sci. 2007;80:725–731. doi: 10.1016/j.lfs.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Power A, Hughes ATL, Samuels RE, Piggins HD. Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J Biol Rhythms. 2010;25:235–246. doi: 10.1177/0748730410374446. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- Reebs SG, St-Coeur J. Aftereffects of scheduled daily exercise on free-running circadian period in Syrian hamsters. Physiol Behav. 1994;55:1113–1117. doi: 10.1016/0031-9384(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Hut R, Daan S, Merrow M. Entrainment concepts revisited. J Biol Rhythms. 2010;25:329–339. doi: 10.1177/0748730410379082. [DOI] [PubMed] [Google Scholar]
- Schaap J, Meijer JH. Opposing effects of behavioural activity and light on neurons of the suprachiasmatic nucleus. Eur J Neurosci. 2001;13:1955–1962. doi: 10.1046/j.0953-816x.2001.01561.x. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Loh DH, Jordan MC, Roos KP, Colwell CS. Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol. 2011;300:H241–H250. doi: 10.1152/ajpheart.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, Turek FW, Holmes MC, Zee PC, Harmar AJ. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One. 2010;5:e9783. doi: 10.1371/journal.pone.0009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–970. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Shido O, Sakurada S, Nagasaka T. Persisting changes in the 24-hour profile of locomotor activity by daily activity restriction in rats. Jpn J Physiol. 1994;44:735–742. doi: 10.2170/jjphysiol.44.735. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong H-K, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpy M. Understanding and diagnosing shift work disorder. Postgrad Med. 2011;123:96–105. doi: 10.3810/pgm.2011.09.2464. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WS, Champney TH, Cassone VM. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol Behav. 1994;55:1091–1099. doi: 10.1016/0031-9384(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Ohi K, Takahashi S, Takahashi K. Free-access to a running wheel shortens the period of free-running rhythm in blinded rats. Physiol Behav. 1988;42:87–91. doi: 10.1016/0031-9384(88)90265-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R-I, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannielli PC, McKinley Brewer J, Harrington ME. Is novel wheel inhibition of Per1 and Per2 expression linked to phase shift occurrence. Neuroscience. 2002;112:677–685. doi: 10.1016/s0306-4522(02)00100-8. [DOI] [PubMed] [Google Scholar]
- Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


