Abstract
Exaggerated blood pressure (BP) responses to dynamic exercise predict cardiovascular mortality in patients with peripheral arterial disease (PAD). However, the underlying mechanisms are unclear and no attempt has been made to attenuate this response using antioxidants. Three physiological studies were conducted in patients with PAD and controls. In Protocol 1, subjects underwent 4 min of low-intensity (0.5–2.0 kg), rhythmic plantar flexion in the supine posture. In Protocol 2, patients with PAD received high-dose ascorbic acid intravenously before exercise. In Protocol 3, involuntary exercise was conducted via electrical stimulation of the tibial nerve. The primary outcome measure was Δ mean arterial pressure (MAP) during the first 20 s of exercise (i.e. the onset of sympathoexcitation by muscle afferents). Compared to controls, patients with PAD had significantly greater ΔMAP during plantar flexion, particularly at 0.5 kg with the most affected leg (11 ± 2 vs. 2 ± 1 mmHg) as well as the least affected leg (7 ± 1 vs. 1 ± 1 mmHg). This augmented response occurred before the onset of claudication pain and was attenuated by ∼50% with ascorbic acid. Electrically evoked exercise also elicited larger haemodynamic changes in patients with PAD compared to controls. Further, the ΔMAP during 0.5 kg plantar flexion inversely correlated with the ankle–brachial index, indicating that patients with more severe resting limb ischaemia have a larger BP response to exercise. The BP response to low-intensity exercise was enhanced in PAD. Chronic limb ischaemia may sensitize muscle afferents and potentiate the BP response to muscle contraction in a dose-dependent manner.
Key points
Peripheral arterial disease (PAD) is a common and debilitating condition linked with heightened risk of cardiovascular mortality.
Dynamic exercise elicits augmented blood pressure responses in PAD that could put the patient at risk for adverse event but the underlying mechanisms are unknown.
The exercise pressor reflex is comprised of group III and group IV muscle afferents that increase their discharge in response to mechanical and/or chemical stimulation.
In this study, we demonstrate that mechanically sensitive muscle afferents cause augmented reflex elevations in blood pressure during dynamic plantar flexion exercise in PAD. These responses occur prior to claudication pain, are related to disease severity and can be partly reduced by acute antioxidant infusion.
Introduction
Peripheral arterial disease (PAD) is an atherosclerotic disease that affects ∼10 million Americans (Criqui, 2001; Hirsch et al. 2006). Patients with PAD have a reduced ankle–brachial index (ABI) compared to healthy people. A low ABI (i.e. more severe disease) correlates with an increased risk for cardiovascular events (McKenna et al. 1991). In fact, patients with PAD have the same risk of cardiac death as patients with coronary artery disease (Hiatt, 2001; Golomb et al. 2006). The most common symptom in these patients is intermittent claudication, defined as pain in one or both legs that is aggravated by walking and is relieved by rest. However, less than half of all patients with PAD experience claudication, which makes the disease challenging to diagnose and treat (Meru et al. 2006). Current therapy includes risk factor management, lifestyle modification, antiplatelet therapy and aerobic exercise (Thompson, 2003).
Cardiovascular responses to exercise are mediated by both feed-forward (‘central command’) and feedback (i.e. ‘the exercise pressor reflex’, EPR) mechanisms. When mechanically and chemically sensitive afferent nerves in contracting muscle increase their discharge, the EPR is initiated. The rise in BP is due to sympathetic activation (McCloskey & Mitchell, 1972; Matsukawa, 2012). Previous authors have shown that the pressor response to dynamic exercise (upright treadmill and supine plantar flexion) is augmented in PAD (Lorentsen, 1972; Baccelli et al. 1999; Bakke et al. 2007). However, the precise mechanism(s) that elicits this response is unclear. Considering that an exaggerated BP response to dynamic exercise is a risk factor for cardiovascular morbidity (Kannel et al. 1971; Kurl et al. 2001) and mortality (de Liefde et al. 2008; Weiss et al. 2010), identifying ways to mitigate the augmented pressor response in PAD would certainly be of clinical relevance.
Oxidative stress (OS) plays a major role in the onset and progression of atherosclerosis (Harrison et al. 2003). Endothelial cells and vascular smooth muscle cells produce reactive oxygen species (ROS), which are highly reactive due to their unpaired valence electrons. Reduced endothelial function has been reported in PAD (Brevetti et al. 2003) and oscillatory shear due to plaque build-up can also stimulate superoxide production (Harrison et al. 2003). This state of vascular pathology and increased OS coupled with a reduced antioxidant system in PAD (Langlois et al. 2001) may ultimately lead to cellular structure damage and/or worsening of disease state (Fisher-Wellman et al. 2009). This process may be further exacerbated during exercise during which time ROS production also increases (Karamouzis et al. 2004; Rietjens et al. 2007). In an animal model of heart failure (Koba et al. 2009), the BP response to muscle contraction (i.e. EPR) was enhanced via an OS mechanism. Rodent models of PAD have also shown an exaggerated EPR but OS was not shown to play a major role in this process (McCord et al. 2011). To our knowledge, the effect of ROS on EPR in humans with PAD is unknown.
Based on previous literature, we developed a series of questions related to the EPR in humans with PAD. First, is the EPR augmented in PAD, compared to healthy control subjects? Second, is the pressor response associated with leg pain? Third, is the pressor response associated with disease severity (i.e. ABI)? Fourth, does OS play a role in the pressor response? Fifth, does involuntary exercise in PAD elicit a similar pressor response? We hypothesized that the EPR would be augmented in patients with PAD and we postulated that this effect could be attenuated in the presence of ascorbic acid.
Methods
Study design and subjects
Studies were approved by the IRB and were conducted in the Clinical Research Center at Penn State Milton S. Hershey Medical Center CTSI. Participants (n= 2302) were screened through the Vascular Clinic on campus. To meet inclusion criteria, patients had to be <75 years old, have a BMI <35 kg m−2, have an ABI <0.9 and be free of coronary artery disease, peripheral neuropathy, heart failure, chronic obstructive pulmonary disease, diabetes and renal disease. Approximately 95% of the patients screened did not meet inclusion criteria due to diabetes (n= 563), coronary artery disease/previous myocardial infarction (n= 480), chronic obstructive pulmonary disease (n= 181), amputation/wounds (n= 522), renal disease (n= 190), heart failure (n= 154), being >75 years old (n= 665) and/or not having current claudication (n= 586). A total of 60 patients met inclusion criteria and were contacted by telephone or mailing. Of these, 46 declined participation or did not respond. The remaining 14 patients with PAD (Fontaine Stage I–II) volunteered to participate and signed informed consent. One subject developed premature ventricular contractions during exercise (i.e. >10 per minute) and his data were excluded from analysis. Of the 13 remaining patients, medications included beta-blockers (n= 5), statins (n= 9), antiplatelet agents (n= 10), anticoagulants (n= 1), angiotensin-converting enzyme inhibitors (n= 6), angiotensin receptor antagonists (n= 1), calcium channel antagonists (n= 1) and diuretics (n= 4). Medications were withheld on the morning of the study. One of the patients with PAD was a smoker at the time of the study and five patients had recently quit smoking.
Protocol 1. Effect of peripheral arterial disease on heart rate and blood pressure responses to low-intensity rhythmic plantar flexion exercise
All experiments were conducted in the morning, following an overnight fast. Room temperature was consistently between 22 and 25°C. Upon arrival at the laboratory, height and weight were obtained, a brief physical examination was conducted and measurement of ABI was performed for each leg. Participants (n= 13 PAD and n= 9 controls, Table 1) were supine and instrumented with a standard three-lead electrocardiogram (Cardiocap/ 5, GE Healthcare, Waukesha, WI, USA), a finger cuff (Finometer, FMS, Arnhem, The Netherlands) to monitor BP and a pneumobelt to monitor respiration. Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) from the Finometer were each corrected to match the BP obtained with an automated sphygmomanometer (Philips SureSigns Vs3, Andover, MA, USA). The calf of the supine volunteer was supported by cushions to assure a full range of motion (i.e. active plantar flexion), consistent with previous experiments (Lorentsen, 1972; Isbell et al. 2006). A custom ‘sandal’ was secured to the volunteer's foot with Velcro straps. A nylon cord connected the sandal's toe, via a pulley system, to a suspended weight pan instrumented with a load cell to provide a calibrated workload signal. In addition, one of the pulleys was instrumented with a potentiometer to provide a range of motion signal.
Table 1.
Baseline characteristics between patients with peripheral arterial disease (PAD) and aged-matched healthy controls (CON)
| PAD | CON | |
|---|---|---|
| Sample size | 13 | 9 |
| Men/women | 8/5 | 6/3 |
| Age (years) | 63 ± 2 | 64 ± 2 |
| Height (m) | 1.66 ± 0.03 | 1.73 ± 0.03 |
| Weight (kg) | 77 ± 4 | 78 ± 4 |
| BMI (kg m−2) | 27.8 ± 1.0 | 25.7 ± 0.7 |
| SBP (mmHg) | 139 ± 6 | 134 ± 5 |
| DBP (mmHg) | 75 ± 3 | 78 ± 3 |
| MAP (mmHg) | 95 ± 3 | 95 ± 4 |
| Pulse pressure (mmHg) | 64 ± 6 | 56 ± 4 |
| HR (bpm) | 68 ± 3 | 60 ± 3 |
| ABI most affected leg | 0.57 ± 0.04* | 1.06 ± 0.03 |
| ABI least affected leg | 0.80 ± 0.04* | 1.07 ± 0.04 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; ABI, ankle–brachial index. Data are mean ± SEM. *P < 0.05 between groups.
Following a 3 min baseline, subjects underwent a 4 min bout of one-leg rhythmic plantar flexion as weight was progressively added (0.5 kg during the first minute, 1.0 kg during the second minute, 1.5 kg during the third minute, 2.0 kg during the fourth minute). During exercise, subjects were instructed to ‘point their toe’ in cue to a metronome (30 contractions min-1). In patients with PAD, the most affected leg was always tested first followed by a 20 min rest period and then the same procedures were repeated for the least affected leg. Control subjects performed the right leg and left leg plantar flexion in random order. Data were collected continuously at 200 Hz and analysed offline (PowerLab, ADInstruments, Castle Hill, NSW, Australia). At the end of each minute, subjects verbally rated their perceived exertion and leg pain (Borg, 1998). All subjects completed the entire 4 min protocol.
Protocol 2. Effect of ascorbic acid on heart rate and blood pressure responses to low-intensity rhythmic plantar flexion exercise in patients with peripheral arterial disease
On a separate day, a subset of patients with PAD (n= 8) returned to the laboratory and underwent baseline measurements in the supine position. A 20 min intravenous loading dose of ascorbic acid (vitamin C, Bioniche Pharma, Lake Forest, IL, USA; 45 mg kg−1 in 100 ml saline) was then given over 20 min and was followed by a maintenance dose (15 mg kg−1 in 33 ml saline) for the remainder of the study. This dose was chosen based on past human experiments in which acute intravenous ascorbic acid evoked an antioxidant physiologic effect (Monahan et al. 2004). As the loading dose neared completion, subjects performed plantar flexion with the most affected leg. Following a 20 min rest period, the procedures were repeated for the least affected leg. The control subjects did not undergo ascorbic acid infusions due to the minimal pressor response observed during low-level plantar flexion in Protocol 1.
Protocol 3. Effect of electrically evoked plantar flexion on heart rate and blood pressure
On a separate day, five patients with PAD and five healthy controls underwent tibial nerve stimulation (1 Hz monophasic current, pulse width 50 ms). This was done to activate muscle sensory fibres without activating central command. With the subject supine, external stimulation was applied to the popliteal fossa with a Grass S48 stimulator (Quincy, MA, USA). The voltage was adjusted to find motor threshold (i.e. first visibly noticeable involuntary plantar flexion) and 1.5 × threshold was used for stimulation (4 min duration). This voltage is well below the threshold necessary to directly stimulate thin fibre muscle afferents, thought to be involved in the EPR. After 20 min of rest, this procedure was repeated on the opposite leg. Some participants reported discomfort during minutes 3 and 4, and some acknowledged that it felt different than voluntary exercise.
Data collection and statistical analysis
Statistics were conducted using SPPS 19.0 and graphics were produced using Microsoft Excel and Adobe Illustrator CS5. Exercise data were averaged in 20 s bins (i.e. three per workload) and the first bin of each workload was analysed. Owing to known BP differences in PAD (i.e. wider pulse pressure than healthy people with little change in MAP or DBP) (Safar & London, 1987), SBP, DBP and MAP were analysed separately and each was corrected to match the automated brachial artery BP obtained immediately before the protocol. The overall study used a repeated measures design and therefore a two-way anova (time and either group or treatment) was used to assess each hypothesis and absolute changes from the respective baseline were used. When a significant interaction was found, the Holm adjustment to the Bonferroni correction was used. Planned comparisons were used for the first 20 s of the 0.5 kg stage because of our a priori hypothesis that the muscle mechanoreflex would be augmented in PAD (Lorentsen, 1972; Baccelli et al. 1999; Tsuchimochi et al. 2010). The Mann–Whitney non-parametric test was used to assess group differences in ratings of perceived exertion and leg pain during exercise. Exploratory bivariate correlations were performed between ABI and ΔMAP during the first 20 s of the 0.5 kg stage. Data are presented as mean ± SEM and P < 0.05 was considered statistically significant.
Results
Protocol 1
The groups were well matched for baseline haemodynamic and anthropometric variables (Table 1). As shown in Table 2, the first stage of exercise (i.e. 0.5 kg) was rated as ‘very, very light’ to ‘fairly light’. The control subjects did not perceive pain at any point of the study. For patients with PAD, the median rating for leg pain in the first exercise stage was zero (range 0–3) for the most affected leg and zero (range 0–3) for the least affected leg. During the fourth minute, the median pain rating was 1 (range 0–5) in the most affected leg and 0 (range 0–5) in the least affected leg.
Table 2.
Ratings of perceived exertion
| 0.5 kg | 1.0 kg | 1.5 kg | 2.0 kg | ||
|---|---|---|---|---|---|
| Protocol 1 | |||||
| CON | First leg | 7 (6-10) | 9 (7-12) | 11 (8-13) | 12 (9-13) |
| CON | Second leg | 8 (6-9) | 9 (7-11) | 10 (9-12) | 11 (9-14) |
| PAD | Most affected leg | 7 (6-9) | 9.5 (7-11) | 10.5 (7-13) | 12 (7-13) |
| PAD | Least affected leg | 7 (6-10) | 8.5 (7-11) | 10.5 (7-13) | 11 (7-13) |
| Protocol 2 | |||||
| PAD | Most affected leg | 8 (6-11) | 10 (6-12) | 12 (6-14) | 13 (6-13) |
| PAD | Least affected leg | 8 (6-9) | 9 (6-12) | 11 (6-13) | 12.5 (6-15) |
Patients with peripheral arterial disease (PAD) and age-matched healthy controls (CON) underwent 4 min of one leg, low-intensity rhythmic plantar flexion exercise. Data are presented as median (range). The Borg scale was presented during the last 15 s of each stage (6 = very, very light; 9 = very light; 13 = somewhat hard).
For the most affected leg, SBP, DBP and MAP were greatly augmented in patients with PAD during exercise, compared to age-matched healthy controls, but HR did not show a group difference (Fig. 1A–D). The greater increase in MAP in patients with PAD (P= 0.002) occurred within the first 20 s and these differences were sustained throughout the protocol as workload increased. The least affected leg also demonstrated group differences in SBP, DBP and MAP within the first 20 s but HR was not different between groups (Fig. 1E–H).
Figure 1. Effect of one-leg, low-intensity rhythmic plantar flexion exercise on the change in systolic blood pressure (ΔSBP, A and E), diastolic blood pressure (ΔDBP, B and F), mean arterial pressure (ΔMAP, C and G) and heart rate (ΔHR, D and H) in patients with peripheral arterial disease (PAD; (n= 13, filled diamonds) and age-matched healthy controls (CON; n= 9, open squares).
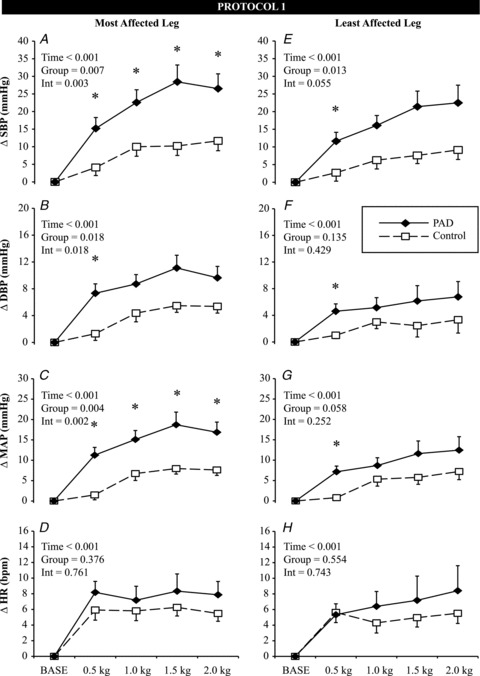
Mean data from the first 20 s of each exercise stage are presented. Data are mean ± SEM, *P < 0.05 between groups at a specific time point. INT = interaction.
Using MAP responses in only patients with PAD, a 2 (most affected leg, least affected leg) by 5 (time) repeated measured anova revealed a main effect for time (P < 0.001) and leg (P= 0.011). As shown in Fig. 1, the most affected leg (continuous lines left panel) had a higher MAP irrespective of time compared to the least affected leg (continuous lines right panel), but this difference was not evident within the first 20 s of exercise (P > 0.05). Thus, we speculate that the muscle mechanoreflex-mediated increments in BP were similar in both legs of patients with PAD.
Protocol 2
Infusion of ascorbic acid (range: 2500–4400 mg) did not change resting MAP (93 ± 3 vs. 95 ± 4 mmHg) or HR (70 ± 3 vs. 68 ± 4) in patients with PAD during Protocol 2. As shown in Fig. 2, ascorbic acid attenuated the MAP response to low-intensity rhythmic plantar flexion in both the most (P= 0.021) and least affected (P= 0.031) legs. Further, in the most affected leg, the HR response was also attenuated (P= 0.046). It is important to note that these attenuated HR and MAP responses in patients with PAD were still of greater magnitude than that of healthy controls. Thus, ascorbic acid did not entirely reverse the abnormal responses in patients with PAD.
Figure 2. Effect of intravenous ascorbic acid (Vit C) on the change in systolic blood pressure (ΔSBP, A and E), diastolic blood pressure (ΔDBP, B and F), mean arterial pressure (ΔMAP, C and G) and heart rate (ΔHR, D and H) in patients with peripheral arterial disease (PAD) during bouts of one-leg, low-intensity rhythmic plantar flexion exercise.
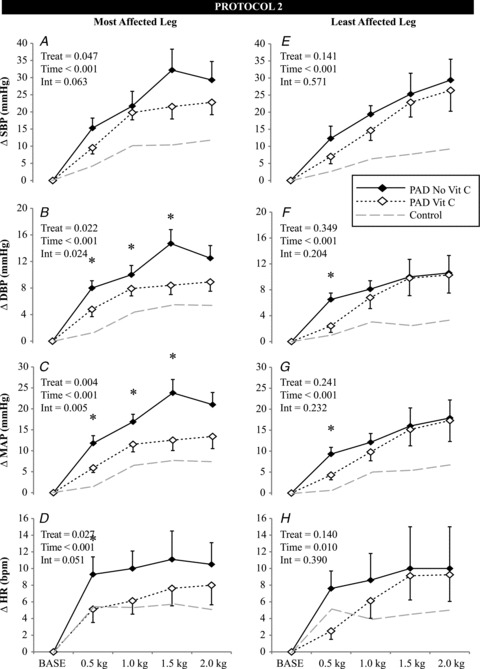
Control data from Protocol 1 (grey dashed line) are included for reference. Mean data from the first 20 s of each exercise stage are presented. Data are mean ± SEM, n= 8, *P < 0.05 between treatments at a specific time point. INT = interaction.
To determine the effect of disease severity on the early pressor response in PAD (i.e. muscle mechanoreflex), an exploratory bivariate correlation was conducted counting each leg separately. As shown in Fig. 3, there was a moderate inverse relationship between ABI and the ΔMAP within the first 20 s of plantar flexion exercise and this relationship was evident in the presence of ascorbic acid. Further subgroup analyses (i.e. with a reduced number of limbs per group) demonstrated that without ascorbic acid, the most affected leg had a stronger coefficient of determination (R2= 0.322, P= 0.043) compared to the least affected leg (R2= 0.179, P= 0.150). After infusion of ascorbic acid, the least affected leg (R2= 0.447, P= 0.070) had a higher value than the most affected leg (R2= 0.112, P= 0.417). Although the data in Fig. 3 contain retrospective analyses of a relatively small sample and cannot confirm a cause and effect relationship, they support the concept that chronic muscle ischaemia evokes OS, which sensitizes muscle mechanoreceptors.
Figure 3.
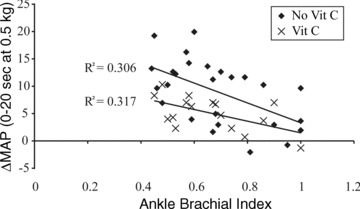
Relationship between disease severity (ankle– brachial index, x-axis) and muscle mechanoreflex (Δ in MAP in the first 20 s of exercise, y-axis) in patients with peripheral arterial disease with (crosses) and without (filled diamonds) ascorbic acid (Vit C) infusion
Protocol 3
All haemodynamic parameters increased over time (main effect P < 0.001, Table 3) with electrically evoked plantar flexion. SBP (P= 0.004) and MAP (P= 0.048) also demonstrated a main effect for group such that patients with PAD had a greater change in BP regardless of time. Within the first 20 s of electrically evoked exercise (i.e. prior to discomfort), ΔSBP, ΔDBP and ΔMAP were significantly higher in patients with PAD.
Table 3.
Effect of electrically evoked plantar flexion on haemodynamics in patients with PAD and age-matched CON
| 1 min | 2 min | 3 min | 4 min | |
|---|---|---|---|---|
| ΔSBP | ||||
| CON | −7 ± 2* | 3 ± 2 | 4 ± 2 | 6 ± 2 |
| PAD | 4 ± 2 | 11 ± 3 | 15 ± 4 | 16 ± 4 |
| ΔDBP | ||||
| CON | −4 ± 1* | 1 ± 1 | 2 ± 1 | 3 ± 1 |
| PAD | 2 ± 1 | 3 ± 2 | 4 ± 2 | 3 ± 2 |
| ΔMAP | ||||
| CON | −4 ± 1* | 2 ± 1 | 3 ± 1 | 5 ± 2 |
| PAD | 3 ± 1 | 5 ± 2 | 8 ± 2 | 7 ± 3 |
| ΔHR | ||||
| CON | 3 ± 1 | 1 ± 1 | 1 ± 1 | 3 ± 1 |
| PAD | 7 ± 2 | 4 ± 3 | 6 ± 3 | 5 ± 3 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; ABI, ankle–brachial index; CON, healthy controls; PAD, peripheral arterial disease. Data are mean ± SEM, *P < 0.05 between groups at a specific time point.
Discussion
The purpose of these studies was to evaluate the EPR in humans with PAD. The data clearly demonstrate that low-intensity, one-leg rhythmic plantar flexion elicited a large, early and sustained BP response in patients with PAD. This augmented response (relative to age-matched healthy controls) occurred in both legs of patients with PAD, occurred before the onset of pain, was related to disease severity and was evident during electrically evoked exercise. Furthermore, ascorbic acid attenuated the augmented BP response in these patients.
Previous research has shown that both treadmill walking (Baccelli et al. 1999; Bakke et al. 2007) and supine rhythmic plantar flexion (Lorentsen, 1972) elicit augmented BP responses in patients with PAD. These cited studies measured BP on a beat-to-beat basis but the lack of a healthy control group (Lorentsen, 1972) and use of two-leg exercise (Baccelli et al. 1999; Bakke et al. 2007) complicates identification of specific reflex mechanisms. We have confirmed these previous reports and further show that the augmented pressor response in PAD (relative to age-matched healthy subjects) is inversely related to ABI and not the presence or absence of claudication per se. Thus, we suspect that patients with reduced ABI will have an augmented pressor response to exercise. This could have important clinical implications as an exaggerated BP response to dynamic exercise is a risk factor for cardiovascular morbidity (Kannel et al. 1971; Kurl et al. 2001) and mortality (de Liefde et al. 2008; Weiss et al. 2010).
There is firm evidence that atherosclerosis is mediated by OS (Harrison et al. 2003; Fisher-Wellman et al. 2009) and patients with PAD often have impaired antioxidant systems (Langlois et al. 2001). Protocol 2 was undertaken to determine if ROS plays a role in the augmented pressor response in PAD. The current data demonstrate that high-dose intravenous ascorbic acid attenuates the pressor response to plantar flexion in PAD. As exercise intensity increased, the effect of ascorbic acid was less impressive but treatment effects were noted nonetheless. A previous experiment in patients with PAD revealed that maximal treadmill walking reduced flow-mediated dilatation (Silvestro et al. 2002). This effect was greatly reduced in a group of patients who received an infusion of ascorbic acid (50 mg min−1 for 20 min) before exercise (Silvestro et al. 2002). In our experiment, ascorbic acid caused a downward shift in the ABI to BP relationship, although the relationship was not very strong (Fig. 3). Taken together, we suggest that endothelial dysfunction, exaggerated sympathoexcitation and other unidentified factors contribute to the poor cardiovascular outcomes seen in patients with PAD. Whether oral supplementation of ascorbic acid (which has a smaller antioxidant effect) provide similar benefits in PAD warrants further study.
In humans, muscle contraction elevates interstitial (Karamouzis et al. 2004) and skeletal muscle (Rietjens et al. 2007) OS. Further, ROS (particularly hydrogen peroxide) are known to directly stimulate carotid sinus baroreceptors (Li et al. 1996), and cardiac sympathetic afferents (Huang et al. 1995) in animals. Delliaux et al. (2009) recently provided evidence in rats that group IV muscle afferents (i.e. metaboreceptors) increase discharge after hydrogen peroxide. However, it is unclear how group III muscle afferents (i.e. mechanoreceptors) respond to OS. TRPA1 receptors, located in the dorsal root ganglion and spinal cord, are sensitive to OS (Andersson et al. 2008) and might mediate the elevated pressor response in PAD. Cyclooxygenase products are known to sensitize mechanoreceptors (Middlekauff & Chiu, 2004) and may be involved. While the specific molecular target is yet to be determined, we suggest that OS plays a physiologically important role in this common and debilitating disease.
Protocol 3 utilized electrically evoked plantar flexion to isolate the muscle mechanoreflex without the confounding influence of central command. Our data (Table 3) are similar to previous experiments that demonstrated small changes in HR, MAP, muscle sympathetic nerve activity and/or renal blood flow in response to involuntary biceps contraction (Mark et al. 1985; McClain et al. 1993; Momen et al. 2003) or plantar flexion (Carrington et al. 2003). Although the time course and low rating of perceived exertion seen in Protocol 1 strongly implicated an augmented mechanoreflex in PAD (Sinoway et al. 1993; Herr et al. 1999), an effect of limb ischaemia on central command (i.e. volitional effort) could not be excluded. By using tibial nerve stimulation to evoke plantar flexion, we demonstrate that patients with PAD have an augmented muscle mechanoreflex.
Recent experiments have investigated the EPR using a decerebrate rat model of PAD. Specifically, a 72 h femoral artery occlusion in one hindlimb was performed and the response to static contraction of this hindlimb revealed an augmented pressor response (Xing et al. 2008; Tsuchimochi et al. 2010, 2011; Leal et al. 2011). Similar to current human data, the first 20 s of muscle contraction in ligated rats showed a large reflex increase in MAP (Tsuchimochi et al. 2010). These animal experiments have clarified that neurotrophic growth factor plays a key role in augmenting the muscle metaboreflex via increasing the expression of acid sensing ion channel 3, transient receptor potential vanilloid type 1 and P2×3 within the dorsal root ganglion neurons (Xing et al. 2008; Liu et al. 2011; Lu et al. 2012). Neurotrophic growth factor, which is upregulated in this animal model of PAD (Xing et al. 2009), is also able to change neuronal phenotype, which may alter afferent signalling (Hunter et al. 2000). It should be noted that this animal model of PAD involves 72 h of ischaemia but not long-term atherosclerosis. Based on these data in experimental animals, it is possible that both the muscle mechanoreflex and muscle metaboreflex are enhanced in PAD due to chronic resting ischaemia. Further work in humans is needed to delineate how muscle ischaemia and/or atherosclerosis impacts mechanisms of circulatory control.
Limitations
Both the muscle mechanoreflex and muscle metaboreflex are activated during voluntary exercise and many of the afferent fibres are polymodal (Adreani & Kaufman, 1998). The mode of exercise in this study was chosen to mimic the type of activity in daily life but other modes of exercise may elicit a different response. It should also be noted that the most affected leg was always tested first and there was not a saline time control during Protocol 2. Furthermore, plasma markers of ascorbic acid and OS were not measured in this study. These markers are likely to differ between groups of patients with cardiovascular disease. However, the ABI is most valuable in the assessment and treatment of patients with PAD, not plasma ROS (McKenna et al. 1991; Feinstein et al. 2002). Ascorbic acid is widely used in human physiology studies to explore OS mechanisms but it is a non-specific scavenger of ROS. In patients with scurvy (ascorbic acid deficiency), the brain is largely unaffected despite widespread systemic symptomology. Indeed, brain levels are fairly stable due to the body's ability to transport dehydroascorbic acid via GLUT1 (Agus et al. 1997). This line of reasoning makes it unlikely that ascorbic acid affected central sympathetic outflow in Protocol 2. Lastly, the relationship between ABI and MAP (Fig. 3) is not strong; this suggests that chronic limb ischaemia is only one of many factors contributing to the augmented pressor response in this disease.
In conclusion, the current data provide strong evidence that the EPR is augmented in patients with PAD. At very low workloads, patients with PAD experience a larger increase in BP compared to healthy subjects. This augmented pressor response in PAD occurred before the onset of pain and may partly explain why PAD is such a strong predictor for cardiac-related morbidity and mortality (Golomb et al. 2006). The augmented pressor response in PAD was correlated to disease severity (i.e. ABI) and was attenuated in the presence of high-dose ascorbic acid. These findings suggest that both reduced resting limb perfusion and OS play a role in the augmented BP responses to exercise in these patients.
Acknowledgments
The authors thank Dr Michael Herr for engineering contribution to this project and to Dr Afsana Momen, Todd Nicklas, Josh Oman, Lauren Estep, Hardikkumar Patel and Jon Pollock for study assistance. We thank Anne Muller for preparing the graphics. Finally, the authors acknowledge the administrative guidance of Kris Gray and Jen Stoner. This work was supported by National Institutes of Health Grants P01 HL096570 (L.I.S.), and UL1 RR033184 (L.I.S.) as well as C06 RR016499. There are no conflicts of interest.
Glossary
- ABI
ankle–brachial index
- BP
blood pressure
- EPR
exercise pressor reflex
- HR
heart rate
- MAP
mean arterial pressure
- OS
oxidative stress
- PAD
peripheral arterial disease
- ROS
reactive oxygen species
- TRPA1
transient receptor potential ankyrin 1
Author contributions
The studies were performed in the Clinical Research Center of the Penn State Milton S. Hershey Medical Center. Conception and design of the experiments: all authors; data collection: M.D.M., R.C.D., C.A.B., J.L.M. and J.C.; analysis of data: M.D.M. and R.C.D.; drafting the manuscript: M.D.M. and J.C. All authors approved the final version of the manuscript.
References
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50:361–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg. 2007;33:20–25. doi: 10.1016/j.ejvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- Carrington CA, Ubolsakka C, White MJ. Interaction between muscle metaboreflex and mechanoreflex modulation of arterial baroreflex sensitivity in exercise. J Appl Physiol. 2003;95:43–48. doi: 10.1152/japplphysiol.00895.2002. [DOI] [PubMed] [Google Scholar]
- Criqui MH. Peripheral arterial disease – epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- de Liefde I, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol. 2008;102:921–926. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch. 2009;459:143–150. doi: 10.1007/s00424-009-0713-8. [DOI] [PubMed] [Google Scholar]
- Feinstein SB, Voci P, Pizzuto F. Noninvasive surrogate markers of atherosclerosis. Am J Cardiol. 2002;89:31C–43C. doi: 10.1016/s0002-9149(02)02226-9. discussion 43C–44C. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol. 1999;86:767–772. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)–summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 1398. [DOI] [PubMed] [Google Scholar]
- Huang HS, Pan HL, Stahl GL, Longhurst JC. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol Heart Circ Physiol. 1995;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47:2289–2295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27:335–346. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- Karamouzis I, Christoulas K, Grekas D, Giannoulis K, Vamvakoudis E, Mandroukas K. The response of muscle interstitial F2-isoprostane (8-ISO-PGF2α) during dynamic muscle contractions in humans. Prostaglandins Leukot Essent Fatty Acids. 2004;71:87–90. doi: 10.1016/j.plefa.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Koba S, Gao Z, Sinoway L. Oxidative stress and the muscle reflex in heart failure. J Physiol. 2009;587:5227–5237. doi: 10.1113/jphysiol.2009.177071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–1868. doi: 10.1161/01.cir.103.14.1863. [DOI] [PubMed] [Google Scholar]
- Leal AK, McCord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;301:H2140–H2146. doi: 10.1152/ajpheart.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res. 1996;79:802–811. doi: 10.1161/01.res.79.4.802. [DOI] [PubMed] [Google Scholar]
- Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;301:H1070–H1079. doi: 10.1152/ajpheart.00188.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation. 1972;46:257–263. doi: 10.1161/01.cir.46.2.257. [DOI] [PubMed] [Google Scholar]
- Lu J, Xing J, Li J. Role for NGF in augmented sympathetic responses to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.00617.2012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol. 2012;97:20–28. doi: 10.1113/expphysiol.2011.057661. [DOI] [PubMed] [Google Scholar]
- McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise: Implications for congestive heart failure. J Clin Invest. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Yamauchi K, Leal AK, Kaufman MP. Tempol attenuates the exercise pressor reflex independently of neutralizing reactive oxygen species in femoral arterial ligated rats. J Appl Physiol. 2011;111:971–979. doi: 10.1152/japplphysiol.00535.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- Meru AV, Mittra S, Thyagarajan B, Chugh A. Intermittent claudication: an overview. Atherosclerosis. 2006;187:221–237. doi: 10.1016/j.atherosclerosis.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- Momen A, Leuenberger UA, Ray CA, Cha S, Sinoway LI. Renal vascular responses to static handgrip: role of the muscle mechanoreflex. Am J Physiol Heart Circ Physiol. 2003;285:H1247–H1253. doi: 10.1152/ajpheart.00214.2003. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol. 2004;286:H2113–H2117. doi: 10.1152/ajpheart.01054.2003. [DOI] [PubMed] [Google Scholar]
- Rietjens SJ, Beelen M, Koopman R, Van Loon LJ, Bast A, Haenen GR. A single session of resistance exercise induces oxidative damage in untrained men. Med Sci Sports Exerc. 2007;39:2145–2151. doi: 10.1249/mss.0b013e318157936d. [DOI] [PubMed] [Google Scholar]
- Safar ME, London GM. Arterial and venous compliance in sustained essential hypertension. Hypertension. 1987;10:133–139. doi: 10.1161/01.hyp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis. 2002;165:277–283. doi: 10.1016/s0021-9150(02)00235-6. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1319–1321. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol. 2011;589:6173–6189. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol. 2008;295:H1262–H1269. doi: 10.1152/ajpheart.00271.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2009;296:H1380–H1387. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


