Abstract
There is an increasing awareness that impulse control disorders (ICDs), including compulsive gambling, buying, sexual behavior, and eating, can occur as a complication of Parkinson’s disease (PD). In addition, other impulsive or compulsive disorders have been reported to occur, including dopamine dysregulation syndrome (DDS) and punding. Case reporting and prospective studies have reported an association between ICDs and the use of dopamine agonists (DAs), particularly at greater dosages, whereas dopamine dysregulation syndrome has been associated with greater dosages of levodopa or short-acting DAs. Data suggest that risk factors for an ICD may include male sex, younger age or younger age at PD onset, a pre-PD history of ICD symptoms, personal or family history of substance abuse or bipolar disorder, and a personality style characterized by impulsiveness. Although psychiatric medications are used clinically in the treatment of ICDs, there is no empiric evidence supporting their use in PD. Therefore, management for clinically significant ICD symptoms should consist of modifications to dopamine replacement therapy, particularly DAs, and there is emerging evidence that such management is associated with an overall improvement in ICD symptomatology. It is important that PD patients be aware that DA use may lead to the development of an ICD, and that clinicians monitor patients as part of routine clinical care. As empirically validated treatments for ICDs are emerging, it will be important to examine their efficacy and tolerability in individuals with cooccurring PD and ICDs.
As clinicians have become more successful at treating the motor symptoms of Parkinson’s disease (PD), many of the nonmotor symptoms, particularly cognitive and neurobehavioral complications, are increasingly recognized as significant contributors to long-term disability, impaired quality of life, and caregiver distress. Although dementia and depression are the most recognizable and common of the neurobehavioral manifestations of PD, psychosis, apathy, anxiety, impulse control disorders (ICDs), and ICD-related disorders also occur not frequently. ICDs are particularly important to recognize, because they can cause considerable distress to the patient and caregiver, may have disastrous personal and financial consequences, and appear to be underreported. The recent awareness of ICDs in PD has fueled the search for an increased understanding of their epidemiology, the underlying neural mechanisms, and the most effective treatment approaches.
ICDs constitute a group of psychiatric disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),1 and are characterized by a failure to resist an impulse, drive, or temptation to perform a typically pleasurable activity that is ultimately harmful to the person or to others because of its excessive nature. Pathological gambling (PG) is the most common ICD, and other ICDs without formal DSM-IV-TR diagnostic criteria include compulsive sexual behavior and compulsive buying.2,3 Binge eating disorder, classified as an eating disorder in the DSM-IV-TR, shares many of the clinical features of ICDs. Recent observational studies suggest that a range of ICDs may occur4 at a greater frequency in PD than in the general population and may be associated with the use of dopamine agonists (DAs).
Other related disorders reported to occur in PD and characterized by repetitive, or compulsive, behaviors include: (1) dopamine dysregulation syndrome (DDS) or hedonic homeostatic dysregulation,5 an addiction-like state marked by excessive dopaminergic medication usage, particularly L-dopa and short-acting DAs (eg, subcutaneous apomorphine); (2) punding, an intense fascination with meaningless movements or activities (eg, collecting, arranging, or taking apart objects)6; and (3) walkabout, defined as excessive, aimless wandering.5
Epidemiology of Impulse Control Disorders in Parkinson’s Disease
Nomenclature
Multiple terms have been used to describe the clinical presentation of compulsive behaviors in PD. These terms include “dopamine dysregulation syndrome” (DDS),6 “hedonistic homeostatic dysregulation,”5,7 “dopamimetic drug addiction,”8 “compulsive behaviors,”9 “compulsive dopaminergic drug use,”10 and “repetitive behaviors.”11 DDS is defined as compulsive use (eg, escalating daily dosages) of dopamine replacement therapy (DRT), but anecdotally, most PD patients with an ICD do not report compulsive use of DRT.
Other psychiatric disorders or behaviors that share features of ICDs have been reported to occur in PD in the context of DRT treatment. For instance, patients have been reported to develop (hypo)mania,5 a mood disorder that can involve excessive involvement in pleasurable activities that have a high potential for painful consequences, with excessive use of DRT. In addition, obsessive-compulsive disorder, an anxiety disorder characterized by the repetition of nonharmful behaviors to reduce anxiety, may occur at an increased frequency in PD, although it has not been reported in association with DRT.11 As mentioned previously, punding and walkabout are characterized by repetitive behaviors and poor impulse control, but the behaviors are typically nonpleasurable or low in risk-reward characteristics when compared with ICD behaviors. Thus, ICDs may represent the severe end of a spectrum of behavioral disturbances in PD that are characterized by poorly or uncontrolled repetitive behaviors.
For the purposes of this article, the term impulse control disorder (ICD) is used (except when discussing DDS, which is distinct from ICDs in many ways), because PG is considered to be an ICD in the psychiatric nomenclature, and this and the other disorders discussed herein are characterized by a failure of impulse control that is not better explained by another psychiatric condition.
Prevalence
Driver-Dunckley and colleagues12 reviewed 1,884 charts at a PD research center and identified 9 patients (0.5% of the sample) with documentation of PG. In a subsequent prospective study using face-to-face interviews,13 the prevalences of PG in approximately 200 patients was reported to be 7% (12/188). In a recent prospective study, 4.4% (17/388) of patients at 6 movement disorders centers met criteria for PG, and the rate for patients on a DA was 8.0%.14 Regarding other ICDs, a cumulative incidence rate (after starting DRT) of 2.4% was reported for compulsive sexual behavior in PD,15 and in a recent study, 10% of a sample of 50 consecutively evaluated PD patients met criteria for compulsive buying and 10% for compulsive sexual behavior.16 In another recent study,17 a total prevalence rate of 9% for compulsive gambling, buying, and sexual behaviors was reported. Compulsive or binge eating has been reported in PD, but its prevalence in not known.5,18
The results of two large studies that prospectively screened for ICDs in PD were published recently. In the first study,19 297 patients with idiopathic PD at a tertiary clinic were screened for PG with a modified version of the South Oaks Gambling Scale.20 Lifetime and current (past 3 months) frequencies of PG were 3.4% and 1.7%, respectively. In the second study,21 272 patients with idiopathic PD at 2 movement disorders centers were screened for the presence of several ICDs (compulsive gambling, sexual behavior, and buying). Those who screened positive for one or more ICDs during the course of PD were contacted by phone for follow-up and administered a modified Minnesota Impulsive Disorders Interview,22 which includes queries for the presence of clinically significant compulsive gambling, sexual, and buying behaviors. In this study, the frequency rates of at least one active ICD or an ICD anytime during PD was 4.0 and 6.6%, respectively. Among active cases, compulsive sexual behavior was as common as problem gambling (2.6 vs 2.2%, respectively), and the frequency of compulsive buying was 0.4%.
DDS and other ICD-related disorders in PD have not been as well studied as the aforementioned ICDs. A total of 15 DDS cases was reported in the original description of this syndrome in PD,5 but a cross-sectional or cumulative prevalence rate was not reported. Regarding punding, in one series examining PD patients taking greater L-dopa–equivalent daily dosages (LEDDs), 14% met criteria for punding,6 but another, larger study of unselected PD patients reported a prevalence rate of 1.4%.23
Thus, preliminary prevalence (either current or anytime during PD) estimates for ICDs in PD patients overall are approximately 2.0 to 6.0% for pathological or problem gambling, 2.0 to 10.0% for compulsive sexual behavior, and 0.4% to 2.0% for compulsive buying. These studies have also suggested that these ICDs may be more common in PD than in the general population24–30 or in assessed healthy control subjects.31,32 However, the true prevalence of ICDs in PD is not known. First, most studies have focused only on gambling disorders, and other ICDs may be as common as PG in PD. Second, the reported values for all disorders may underestimate the true frequencies, because some studies relied on information documented in charts during routine clinical care, and in one of the aforementioned screening studies,21 active ICD cases were rarely documented in the clinical record. Third, patients may be reluctant to acknowledge ICD behaviors in the context of a research study.
It is not known whether ICDs are more common in PD than in the general population because it is difficult to find an appropriate comparison group. However, in a recent prospective study, PD patients were approximately 25 times more likely (odds ratio, 25.6) to have PG than an age- and sex-matched control group.32 In addition, a recent case–control study found that new-onset “heightened interest in or drive” for gambling, shopping, eating, or sexual activity was reported in 14% of PD patients and in none of the age- and sex-matched healthy control subjects.31
Association with Dopamine Replacement Therapy
Case reports have implicated DAs (eg, pramipexole, ropinirole, and pergolide), and less commonly L-dopa,33 as precipitating PG in PD. In Driver-Dunckley and colleagues’12 case series, all nine patients were treated with a DA, including eight with pramipexole. In another case series, all 11 PD patients identified as having criteria for PG were taking a DA, 9 of whom were taking pramipexole and 2 taking ropinirole.34 In another study,13 all 14 subjects identified as having PG were taking a DA. In the most recent case series, all 17 patients identified as having PG were taking a DA.14
Regarding other ICDs in PD, in a series of 15 patients with compulsive sexual behavior and either PD or multiple system atrophy, DA treatment was implicated in the emergence of the sexual behavior in 14 cases.35 This case reporting suggests an association between DA use and ICDs, because only approximately 50% of PD patients surveyed in specialty care are taking a DA.19,21 In a case–control study of PD patients and matched control subjects,31 longer duration of DA treatment (vs never treated) was associated with the new-onset heightened interest in or drive for gambling, shopping, eating, or sexual activity.
In one of the aforementioned prospective screening studies,19 PG was significantly more common in DA-treated patients than in those receiving L-dopa monotherapy. In the other prospective study,21 on univariate analysis use of a DA or amantadine, each was associated with the presence of an active ICD, and a trend for greater total LEDD was observed. All active ICD cases were currently taking a DA. Entering all the aforementioned variables into a multivariate model, only current DA use remained a significant predictor of an active ICD. No differences were observed between the three DAs examined (pramipexole, ropinirole, and pergolide) in their association with ICDs. Examining only patients who were taking a DA at the time of screening, an active ICD was associated a greater mean daily DA dosage.
Thus, the pharmacotherapy risk associated with ICDs appears to be specific to the DA medication class because no association was found between daily L-dopa dosage or total LEDD and the presence of an ICD.19,21 These results suggest a distinct mechanism of action, as opposed to an additive effect, for DAs in the development of ICDs. In addition, a differential association between specific DAs and ICDs was not supported in two larger studies,19,21 suggesting a class, as opposed to specific medication, effect. Finally, the finding that the greatest risk for development of an ICD with DA treatment may involve doses at the high end of the therapeutic range is consistent with previous case reporting.12,34 By definition, DDS is associated with escalating dosages of PD medications, typically L-dopa or short-acting DA medications (eg, subcutaneous apomorphine).5 In studies of punding, an association with DA treatment23 or greater LEDDs6 has been reported, with the latter study concluding that punding and DDS often co-occur. In a study of PD patients unselected for an ICD diagnosis, greater scores on the self-completed Punding Scale were associated with greater DA dosages.36
Other Clinical Correlates
Among the published case reports, PD patients with ICDs have disproportionately been younger male individuals,34,35,36 suggesting age and sex as potential risk factors for the development of ICDs in this population. These findings are consistent with those from epidemiological and clinical studies that observe high rates of PG and compulsive sexual behaviors in young male individuals in the general population and treatment settings.3
In one of the aforementioned prospective studies,21 on univariate analysis, younger age, longer duration of PD, and a history of ICD symptomatology before PD each were associated with the presence of an active ICD. Entering the aforementioned variables into a multivariate model, only a history of ICD symptomatology before PD remained a significant predictor of an active ICD. In the other prospective study,19 on univariate analysis, PG was associated with earlier PD onset. In addition, 60% of patients with a history of PG had either a premorbid personal or family history of alcohol use disorder, or a family history of bipolar disorder. Finally, in a recent case–control study,31 PD patients with new-onset heightened interest or drive for a range of ICD behaviors had younger age at PD onset and were more likely to be male than unaffected PD patients.
In some instances,12,34,35 substantial time elapsed between initiation of DA treatment and the onset of ICD behaviors in PD patients. This delay could reflect either a priming effect, a threshold in the PD neurodegenerative process that must be crossed before ICD behavior manifests itself, or the importance of escalating dosages over time and the need to cross a necessary dosage threshold before becoming symptomatic.
Although case reports have suggested that younger patients are disproportionately affected, older patients are less likely to be treated with DAs because of concerns about adverse events.21 Considering only patients taking a DA, younger patients in one study21 were found to have been treated with greater dosages. Thus, the previous reporting of younger PD patients being disproportionately affected with ICDs may, in part, reflect prescribing patterns.
There is controversy about whether certain personality traits or psychiatric disorders predispose to ICD development in PD, as cross-sectional studies of patients symptomatic with an ICD may confound “state” effects for “trait” effects. That being said, greater scores on impulsivity and compulsivity inventories were reported in PD ICD patients compared with PD control subjects in one study.16 In a study focusing on psychiatric comorbidity, a range of ICDs were associated with depressed mood, disinhibition, irritability, and appetite disturbance.17
Regarding DDS, in one study, patients with DDS (more than half of whom had one or more comorbid ICDs) had younger age of PD onset and were more likely to have a history of experimental drug use.10 Although there has been comparatively little research on punding, in a study of PD not selected for an ICD diagnosis, greater scores on the Punding scale was associated with younger age at PD onset and greater impulsivity scores.36
Neural Substrate of Impulse Control Disorders
General Population
The pathophysiology of ICDs has been reported to involve alterations in specific neurotransmitter systems, brain regions, and neural circuitry (Figure). Regarding neurotransmitters, dopamine function, particularly within the mesocorticolimbic pathways, is critical in the mediation of reward and reinforcement behaviors.38 The brain regions most implicated in ICDs include the ventromedial39,40 and orbitofrontal regions41 of the prefrontal cortex, the ventral striatum (particularly the nucleus accumbens),38,42 and the amygdala,43,44 all of which are thought to mediate aspects of motivated behaviors underlying engagement in risky, compulsive behaviors. Finally, data suggest that alterations in corticostriato-thalamocortical circuitry contribute to ICD behaviors, with projections coursing through the more ventral component of the striatum (including the nucleus accumbens) more implicated in urges and impulsive aspects, and those involving the dorsal striatum more implicated in motoric habits and compulsive aspects.45,46 This neurocircuitry involves additional brain regions (eg, the amygdala providing affective salience, and the hippocampus contextual memories), with disruptions in one area having the potential to influence function throughout the circuitry.
Fig.
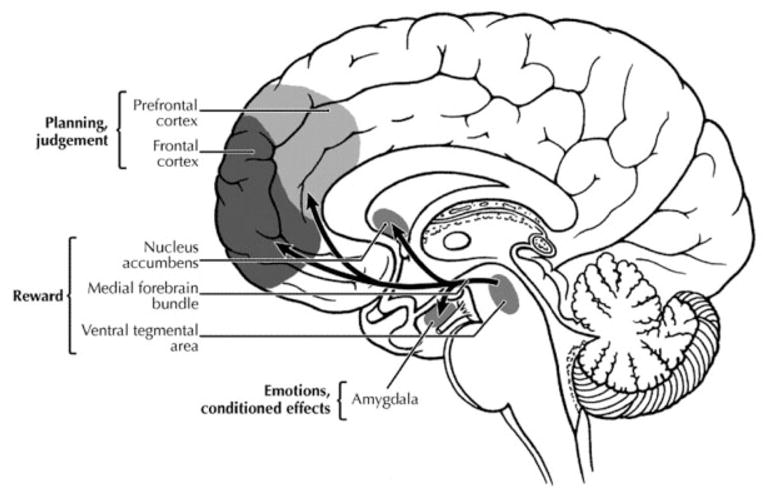
Main brain areas and neurotransmitter pathways implicated in reward processes. (Reproduced from Tomkins and Sellers,71 by permission.)
Parkinson’s Disease
There are several plausible explanations for a possible association between ICDs in PD and treatment with DRT, especially DAs. First, PD leads to a loss of dopaminergic neurons in the substantia nigra, resulting in a pronounced depletion of dopamine in the nigrostriatal pathway.47 This depletion influences dopaminergic cortical-subcortical circuits, leading to cognitive and emotional impairment that may predispose to the development of numerous psychiatric and cognitive disorders, including ICDs.
Second, PD patients, even those without dementia, commonly display a range of impairment in executive abilities,48,49 which has been linked to degeneration in the striatal-frontal tracts secondary to cell loss within the substantia nigra.50,51 On a computerized gambling task of theoretical relevance to ICD behaviors, PD patients were significantly more impaired than control subjects in performance (both number of disadvantageous choices and ability to use negative feedback for a decision shift to an advantageous alternative), and task impairment correlated with impairment on other executive measures.51 In a recent study52 that involved administration of computerized decision-making tasks to PD patients on and off PD medications, the medicated group showed impairment in the ability to learn from negative decision outcomes, a psychological deficit that also may have relevance to the maintenance of ICD behaviors. Regarding differential effects of PD medications, there is also some evidence that DA, but not L-dopa, treatment impairs executive abilities in patients with early or mild PD.53
Third, DAs, compared with L-dopa, have significantly greater D3:D2 and D3:D1 striatal receptor activation ratios.54 D1 and D2 receptors are located in the dorsal striatum, and their activation by different PD pharmacotherapies is associated primarily with the motor effects of the medications. In contrast, the D3 receptor is concentrated in limbic areas of the brain, including the ventral striatum, and may mediate psychiatric manifestations of dopamine receptor stimulation.55
Recent cognitive neuroscience studies in PD have focused on differential neurodegeneration of the striatum in early or mild PD, with the dorsal striatum being more affected than the ventral striatum.56,57 This depletion differentially affects distinct cortical-basal ganglia-thalamo-cortical circuits that subserve multiple processes, including motor, cognitive, and limbic functions. It has been proposed that in mild PD, dopaminergic stimulation of dopamine-deficient dorsal striatal receptors is associated with cognitive enhancement on tasks that require activation of the dorsal striatum, whereas dopaminergic stimulation of the relatively intact ventral striatal receptors is associated with impairment on cognitive tasks that depend on ventral striatal activation (ie, dopaminergic “overdose” hypothesis in PD). Of note, the cognitive tasks that rely on ventral striatal activation include stimulus-outcome tasks, which involve the ability to modify behaviors by outcomes, an ability that is impaired at a clinical level in PD patients with ICDs. Thus, one hypothesis is that excessive, targeted dopamine stimulation of intact ventral striatal receptors in early or mild PD leads to an “overdose” of ventral striatal-cortical circuitry that can manifest itself in the clinical phenomenon of impulsive-compulsive behaviors, including ICDs. These behaviors, similar to addictive disorders, initially may be maintained because of the pleasure feelings induced by the activities, but eventually they are experienced as nonpleasurable, yet uncontrollable.58 The behaviors are maintained by ongoing dopaminergic stimulation of a sensitized ventral striatal system, which is manifested clinically as an increased drive for certain behaviors (ie, limbic system overactivation) and maintained by an inability to learn from negative decision outcomes (ie, prefrontal cortex overactivation).
Regarding the association between ICDs and a history of ICD behavior or substance use disorders, differences in the neural substrate that predispose to the development of ICD-related behaviors before PD onset may make patients more vulnerable to manifest similar behaviors during PD in the context of DA treatment. It has been proposed that addiction is “the product of an imbalance between two separate, but interacting, neural systems that control decision making: an impulsive, amygdala system for signaling pain or pleasure of immediate prospects, and a reflective, prefrontal cortex system for signaling pain or pleasure of future prospects…drugs can trigger bottom-up, involuntary signals originating from the amygdala that modulate, bias or even hijack the goal-driven cognitive resources that are needed for the normal operation of the reflective system.”59 This proposed neural substrate of disorders of addiction may be similar to the neural substrate of ICDs in PD.
In the only published study examining the neurobiology of any of the discussed disorders in PD,58 a small group of patients with and without active DDS underwent positron emission tomography scanning with the D2/D3 receptor ligand 12C-raclopride before and after L-dopa oral challenge. PD patients with DDS exhibited enhanced L-dopa–induced ventral striatal dopamine release compared with non-DDS patients. In addition, the degree of sensitized striatal dopamine release correlated with self-reported drug “wanting” but not “liking.” The authors conclude that that compulsive medication use in PD is associated with sensitization of ventral striatal circuitry.
Clinical Assessment and Management
Screening and Assessment
The apparent underrecognition of ICDs in PD should be addressed initially through a careful history and patient education before initiating DA treatment, and by regular monitoring or screening for ICDs throughout the course of treatment. Once the possible association between DRT (particularly DA treatment) and ICDs in PD has been introduced, clinicians should inquire about ICD behaviors in the context of routine clinical care.
Screening instruments that lend structure to the questioning can assist in making a determination if a clinically significant problem exists. Unfortunately, there is no single instrument that has been developed and validated in PD for the range of ICD and ICD-related disorders that can occur in this population. One existing instrument is the Minnesota Impulsive Disorders Interview,22 which queries for the presence of some of the ICDs reported to occur in PD, including compulsive gambling, buying, and sexual behaviors. The South Oaks Gambling Screen is a more detailed instrument that is widely used to screen for PG specifically.60 The Early Intervention Gambling Health Test is a brief screen that has been validated in primary care settings.61 If a clinician suspects the presence of an ICD and needs assistance in the assessment and management process, then the patient should be promptly referred to a psychiatrist for a comprehensive evaluation and ongoing care.
Treatment
Regarding the neurological management of ICDs in PD, case reporting and anecdotal experience suggest that ICD behaviors often resolve after reducing the dose of the existing DA, switching to a different agonist, discontinuing DA treatment entirely, or perhaps receiving counseling.12,34 In a recent publication that documented the long-term clinical outcomes of 15 ICD patients,62 80% of patients discontinued or significantly decreased DA treatment, all of whom experienced full or partial remission of ICD symptoms by self-report. On average, patients significantly decreased their DA dosage and significantly increased their L-dopa dosage, with no change in total LEDD. In addition, there was no change in UPDRS motor scores over time. These results suggest that many ICD patients can be adequately managed by making changes to their PD pharmacotherapy regimen.
The relationship between deep brain stimulation (DBS) and ICDs appears to be complex. On the one hand, chronic subthalamic nucleus DBS has been associated with improvement in ICD symptoms in a small case series, perhaps secondary to significant reductions in DRT that occurred after surgery.63 However, there is also anecdotal evidence that although ICDs may begin or worsen transiently immediately after subthalamic nucleus DBS.64 In addition a recent cognitive neuroscience study of PD DBS patients without an ICD found that patients were more impulsive in their decision making when their stimulators were turned on.52
A range of psychiatric treatments, most commonly selective serotonin reuptake inhibitors, have been used in the treatment of ICDs in PD, but there is only mixed empirical evidence to support their use for this indication in non-PD subjects,65 and no empirical evidence has been reported in PD patients. There are two case reports of successful treatment of PG in PD with either risperidone34 or quetiapine,66 although the only controlled study of an atypical antipsychotic drug (olanzapine) for PG in non-PD subjects was negative.65 In non-PD patients, recent research suggests that nalmefene and naltrexone, both opioid antagonists, are efficacious in the treatment of PG.67,68
Pharmacotherapy selection for treatment of ICDs in the general population currently is guided, in part, by cooccurring psychiatric disorders.60,69 However, the extent to which the treatment of cooccurring psychiatric disorders (eg, depression or anxiety) in individuals with PD and ICDs influences ICD symptom severity is unknown. Behavioral treatments (eg, cognitive behavioral therapy and motivational interviewing) appear effective in specific groups of patients with PG,70 but their efficacy has not been examined in individuals with PD, and one might predict that certain PD patients (eg, those with executive impairment) might not respond as well to structured behavioral treatments. Similarly, although attendance at Gamblers Anonymous meetings has been associated with better treatment outcome in non-PD samples,70 no studies have examined its effectiveness in individuals with PD and PG.
Conclusions
Mounting data, including from prospective studies, suggest that DA treatment is associated with the development of a variety of ICDs in a subset of PD patients, particularly those with certain demographic or clinical characteristics, whereas greater dosages of L-dopa and short-acting DAs may be associated with DDS and punding. Given the potential substantial impact of ICDs and related disorders on personal, familial, social, and financial well-being, clinicians should educate PD patients about and closely monitor for the occurrence of these disorders. Prevention and treatment strategies involve appropriate patient education, clinical assessment (including questioning regarding personal or family history of ICD or related behaviors), careful DA dosing (using lowest effective dosages), and ICD symptom monitoring throughout treatment.
Existing data suggest that clinical management of clinically significant ICDs should involve serious consideration of a prompt discontinuation or decrease in DA treatment. The risk/benefit ratio of continuing with DA therapy should be evaluated for severity of parkinsonism, severity of ICD behaviors, and available options for the treatment of both PD and ICDs. In addition, empirically validated treatments are emerging for ICDs and should be considered for patients with co-occurring PD and ICDs.
The association between DA treatment and ICDs in PD is understandable given existing knowledge regarding the importance of the dopamine system, executive impairment, and prefrontal cortex-ventral striatal circuitry in the development of ICDs. This association also offers an opportunity to improve our understanding of the neural substrate of a variety of ICDs, because discontinuation of DA treatment often leads to a prompt resolution of ICD behaviors. As a result, PD patients with an ICD can be studied in both symptomatic and asymptomatic states at two proximate time points, allowing determination of “state” versus “trait” neuropsychological and neurobiological correlates. This may ultimately lead to improved recognition and treatment of ICDs not only in PD, but in the population at large.
Acknowledgments
This work was supported by the NIH (National Institute of Mental Health, K23MH067894).
Footnotes
Potential conflicts of interest: This article is part of a supplement sponsored by Boehringer Ingelheim (BI). D.W. has served as a consultant to, has served on advisory boards for, and has received grant support from BI. D.W. has also consulted for and served on advisory boards for Novartis, Merck Serono, Brain Cells, and Osmotica Pharmaceuticals.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162:2184–2188. doi: 10.1176/appi.ajp.162.11.2184. [DOI] [PubMed] [Google Scholar]
- 3.Potenza MN, Hollander E. Pathological gambling and impulse control disorders. In: Coyle JT, Nemeroff CB, Charney DS, et al., editors. Neuropsychopharmacology: the 5th generation of progress. Baltimore: Lippincott Williams & Wilkins; 2002. pp. 1725–1742. [Google Scholar]
- 4.Bonvin C, Horvath J, Christe B, et al. Compulsive singing: Another aspect of punding in Parkinson’s disease. Ann Neurol. 2007;62:525–528. doi: 10.1002/ana.21202. [DOI] [PubMed] [Google Scholar]
- 5.Giovannoni G, O’Sullivan JD, Turner K, et al. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 7.Pezzella FR, Di Rezze S, Chianese M, et al. Hedonistic homeostatic dysregulation in Parkinson’s disease: a short screening questionnaire. Neurol Sci. 2003;24:205–206. doi: 10.1007/s10072-003-0132-0. [DOI] [PubMed] [Google Scholar]
- 8.Serrano-Dueñas M. Chronic dopamimetic drug addiction and pathologic gambling in patients with Parkinson’s disease—presentation of four cases. German J Psychiatry. 2002;5:62–66. [Google Scholar]
- 9.Voon V. Repetition, repetition, and repetition: compulsive and punding behaviors in Parkinson’s disease. Mov Disord. 2004;19:367–370. doi: 10.1002/mds.20046. [DOI] [PubMed] [Google Scholar]
- 10.Evans AH, Lawrence AD, Potts J, et al. Factors influencing susceptibility to compulsive dopaminergic drug use in Parkinson disease. Neurology. 2005;65:1570–1574. doi: 10.1212/01.wnl.0000184487.72289.f0. [DOI] [PubMed] [Google Scholar]
- 11.Kurlan R. Disabling repetitive behaviors in Parkinson’s disease. Mov Disord. 2004;19:433–437. doi: 10.1002/mds.10625. [DOI] [PubMed] [Google Scholar]
- 12.Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61:422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Bharmal A, Suchowersky O. Gambling and Parkinson’s Disease. Arch Neuro. 2006;63:298. doi: 10.1001/archneur.63.2.298-a. [DOI] [PubMed] [Google Scholar]
- 14.Grosset KA, Macphee G, Pal G, et al. Problematic gambling on dopamine agonists: not such a rarity. Mov Disord. 2006;21:2206–2208. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- 15.Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- 16.Isaias IU, Siri C, Cilia R, et al. The relationship between impulsivity and impulse control disorders in Parkinson’s disease. Mov Disord. 2008;23:411–415. doi: 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- 17.Pontone G, Williams JR, Bassett SS, et al. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67:1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- 18.Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–529. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- 19.Voon V, Hassan K, Zurowski M, et al. Prospective prevalence of pathological gambling and medication association in Parkinson disease. Neurology. 2006;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- 20.Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- 21.Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christenson GA, Faber RJ, deZwaan M. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55:5–11. [PubMed] [Google Scholar]
- 23.Miyasaki J, Hassan KL, Lang AE, et al. Punding prevalence in Parkinson’s disease. Mov Disord. 2007;22:1179–1181. doi: 10.1002/mds.21296. [DOI] [PubMed] [Google Scholar]
- 24.Pietrzak RH, Morasco BJ, Blanco C, et al. Gambling level and psychiatric and medical disorders in older adults: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Geriatr Psychiatry. 2007;15:301–313. doi: 10.1097/01.JGP.0000239353.40880.cc. [DOI] [PubMed] [Google Scholar]
- 25.Stucki S, Rihs-Middel M. Prevalence of adult problem and pathological gambling between 2000 and 2005: an update. J Gambl Stud. 2008;23:245–257. doi: 10.1007/s10899-006-9031-7. [DOI] [PubMed] [Google Scholar]
- 26.Volberg RA, Nysse-Carris KL, Gerstein DR. California Problem Gambling Prevalence Survey. Chicago: National Opinion Research Center at the University of Chicago; 2006. [Google Scholar]
- 27.Shaffer HJ, Hall MN, Vander J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89:1369–1376. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6:14–18. [PMC free article] [PubMed] [Google Scholar]
- 29.Lilenfeld LRR, Ringham R, Kalarchian MA, et al. A family history study of binge-eating disorder. Compr Psychiatry. 2008;49:247–254. doi: 10.1016/j.comppsych.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22:320–325. [Google Scholar]
- 31.Giladi N, Weitzman N, Schreiber S, et al. New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson’s disease: the role of dopamine agonist treatment and age at motor symptoms onset. J Psychopharmacol (Oxf) 2007;21:501–506. doi: 10.1177/0269881106073109. [DOI] [PubMed] [Google Scholar]
- 32.Avanzi M, Baratti M, Cabrini S, et al. Prevalence of pathological gambling in patients with Parkinson’s disease. Mov Disord. 2006;21:2068–2072. doi: 10.1002/mds.21072. [DOI] [PubMed] [Google Scholar]
- 33.Tyne HL, Medley G, Ghadiali E, Steigner MJ. Gambling in Parkinson’s disease. Mo Disord. 2004;19(suppl 9):S195. [Google Scholar]
- 34.Dodd ML, Klos KJ, Bower JH, et al. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005;62:1–5. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- 35.Klos KJ, Bower JH, Josephs KA, et al. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11:381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence AJ, Blackwell AD, Barker RA, et al. Predictors of punding in Parkinson’s disease: results from a questionnaire survey. Mov Disord. 2007;22:2339–2345. doi: 10.1002/mds.21702. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher DA, O’Sullivan SS, Evans AH, et al. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007;22:1757–1763. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 38.Hollander E, Evers M. New developments in impulsivity. Lancet. 2001;358:949–950. doi: 10.1016/S0140-6736(01)06114-1. [DOI] [PubMed] [Google Scholar]
- 39.Potenza MN, Leung H-C, Blumberg HP, et al. An fMRI Stroop task study in ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 40.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 41.O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 42.Reuter J, Raedler T, Rose M. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 43.Bechara A, Damasio H, Damasio AR, et al. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. J Gambl Stud. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- 45.Chambers RA, Taylor JR, Potenza MN. Developmental neuro-circuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 47.Bjarkam CR, Sørensen JC. Therapeutic strategies for neurodegenerative disorders: emerging clues from Parkinson’s disease. Biol Psychiatry. 2004;56:213–216. doi: 10.1016/j.biopsych.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. In: Weiner WJ, Lang AE, editors. Behavioral neurology of movement disorders. New York: Raven Press; 1995. pp. 85–95. [PubMed] [Google Scholar]
- 49.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 50.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 51.Brand M, Labudda K, Kalbe E, et al. Decision-making impairments in patients with Parkinson’s disease. Behav Neurol. 2004;15:77–85. doi: 10.1155/2004/578354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank JF, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 53.Brusa L, Bassi A, Stefani A, et al. Pramipexole in comparison to l-dopa: a neuropsychological study. J Neural Transm. 2003;110:373–380. doi: 10.1007/s00702-002-0811-7. [DOI] [PubMed] [Google Scholar]
- 54.Gerlach M, Double K, Arzberger T, et al. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm. 2003;110:1119–1127. doi: 10.1007/s00702-003-0027-5. [DOI] [PubMed] [Google Scholar]
- 55.Sokoloff P, Giros B, Martres MP, et al. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 56.Cools R. Dopaminergic modulation of cognitive function—implications for l-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 58.Evans AH, Pavese N, Lawrence AD, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 59.Bechara A. Decision making, impulse control and loss of will-power to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. 60. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 60.Pathological gambling: a clinical guide to treatment. Arlington, VA: American Psychiatric Publishing; 2004. [Google Scholar]
- 61.Potenza MN, Fiellin DA, Heninger GR, et al. Gambling: an addictive behavior with health and primary care implications. J Gen Intern Med. 2002;17:721–732. doi: 10.1046/j.1525-1497.2002.10812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamikonyan E, Siderowf AD, Duda JE, et al. Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord. 2008;23:75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ardouin C, Voon V, Worbe Y, et al. Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov Disord. 2006;21:1941–1946. doi: 10.1002/mds.21098. [DOI] [PubMed] [Google Scholar]
- 64.Smeding HMM, Goudriaan AE, Foncke EMJ, et al. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry. 2007;78:517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant JE, Potenza MN. Impulse control disorders: clinical characteristics and pharmacological management. Ann Clin Psychiatry. 2004;16:27–34. doi: 10.1080/10401230490281366. [DOI] [PubMed] [Google Scholar]
- 66.Sevincok L, Akoglu A, Akyol A. Quetiapine in a case with Parkinson disease and pathological gambling [letter] J Clin Psychopharmacol. 2007;27:107–108. doi: 10.1097/JCP.0b013e31802e98c3. [DOI] [PubMed] [Google Scholar]
- 67.Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- 68.Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- 69.Potenza MN. Impulse control disorders and co-occurring disorders: dual diagnosis considerations. J Dual Diagn. 2007;3:47–57. [Google Scholar]
- 70.Hodgins DC, Petry NM. Cognitive and behavioral treatments. In: Grant JE, Potenza MN, editors. Pathological gambling: a clinical guide to treatment. Washington, DC: American Psychiatric Press; 2004. pp. 169–188. [Google Scholar]
- 71.Tomkins DM, Sellers EM. Addiction and the brain: the role of neurotransmitters in the cause and treatment of drug dependence. CMAJ. 2001;164:817–821. [PMC free article] [PubMed] [Google Scholar]


