Abstract
Background and Objectives
Kappa opioid receptor (κ-OR) activation is known to play a role in analgesia and central sedation. The purpose of the present study was to examine the effect of the κ-OR agonist, U-50488 (an arylacetamide), on Ca2+ channel currents and the signaling proteins involved in acutely isolated rat dorsal root ganglia (DRG) neurons expressing the putative promoter region of the tetrodotoxin (TTX)-resistant Na+ channel (NaV 1.8) that is known to be involved in pain transmission.
Methods
Acutely isolated rat DRG neurons were transfected with cDNA coding for enhanced green fluorescent protein (EGFP), whose expression is driven by the Nav 1.8 promoter region. Thereafter, the whole-cell variant of the patch-clamp technique was employed to record Ca2+ channel currents in neurons expressing EGFP.
Results
Exposure of EGFP-expressing DRG neurons to U-50488 (0.3 to 40 μM) led to voltage-independent inhibition of the Ca2+ channel currents. The modulation of the Ca2+ currents did not appear to be mediated by the Gα protein subfamilies: Gαi/o, Gαs, Gαq/11, Gα14 and Gαz. Furthermore, dialysis of the hydrolysis-resistant GDP analog, GDP-β-S (1 mM), did not affect the U-50488-mediated blocking effect, ruling out involvement of other G protein subunits. Finally, U-50488 (20 μM) blocked Ca2+ channels heterologously expressed in HeLa cells that do not express κ-OR.
Conclusion
These results suggest that the antinociceptive actions mediated by U-50488 are likely due to both a direct block of Ca2+ channels in sensory neurons as well as G protein modulation of Ca2+ currents via κ-OR-expressing neurons.
Introduction
Opioid drugs are generally used as effective broad-spectrum analgesics, yet they exert many adverse effects that restrict their clinical use.1 The 3 “classical” opioid receptor subtypes, mu (μ), kappa (κ), and delta (δ), are expressed within the central nervous system and sensory neurons. The stimulation of κ opioid receptors (κ-OR) has been shown to cause analgesia, central sedation, dysphoria, respiratory depression, and dyspnea.2,3 In general, opioid receptor stimulation results in coupling of heterotrimeric G proteins of the pertussis toxin-sensitive Gαi/o family with effector proteins, such as ion channels. This leads to inhibition of voltage-gated Ca2+ channels and activation of G protein-gated inwardly rectifying K+ channels that, in turn, reduces neuronal excitability and neurotransmitter release to produce analgesia.
In excitable tissues, such as neurons, voltage-gated Na+ channels play a crucial role in generating and propagating action potentials.4 The sodium channel subtype, NaV1.8, is tetrodotoxin (TTX)-resistant and is known to be expressed primarily in small and medium diameter nociceptive sensory neurons that receive variable noxious stimuli, including mechanically-, chemically-, and thermally-induced pain. 5–7 Recently, the putative promoter region of the NaV1.8 channel was identified, and the authors inserted this region in a cDNA construct upstream of the enhanced green fluorescent protein (EGFP) such that EGFP expression in sensory neurons resulted from native transcription factors binding to the promoter site.8 Thus, the use of this clone containing the promoter region and EGFP coding region can be employed as a tool to identify a subpopulation of nociceptive neurons. The purpose of the present study was to examine the G protein-mediated modulation of Ca2+ channel currents following κ-OR activation with the receptor agonist U-50488 in rat sensory neurons transfected with EGFP cDNA, whose expression is driven by the NaV1.8 promoter region.
Methods
Dorsal root ganglion (DRG) isolation and transfection
The Penn State College of Medicine Institutional Animal Care and Use Committee (IACUC) approved the experiments performed. Male Sprague-Dawley rats were first anesthetized with CO2 and then decapitated with a laboratory guillotine. The lumbar (L4–L6) DRG were removed and placed in ice-cold Hank’s balanced salt solution (Sigma-Aldrich Corporation, St Louis, Missouri). Thereafter, the ganglia were incubated in a tissue culture flask with modified Earle’s balanced salt solution containing 0.6 mg/mL collagenase Type D (Roche Applied Science, Indianapolis, Indiana), 0.4 mg/mL trypsin (Worthington Biochemical Corp., Lakewood, New Jersey) and 0.1 mg/mL DNase (Sigma-Aldrich). The flask was placed in a shaking water bath (35°C) for 40 minutes. Afterward, the cells were dissociated by vigorous shaking of the flask and then centrifuged twice for 6 minutes at 50-× g. The dispersed neurons were re-suspended in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (MIDSCI, St. Louis, Missouri), 1% glutamine, and 1% penicillin-streptomycin (both from Life Technologies, Carlsbad, California). The neurons were next plated onto polystyrene culture dishes coated with poly-L-lysine and stored in a humidified atmosphere containing 5% CO2/95% air at 35°C.
Following a 3-hour incubation period, the DRG neurons were microinjected with cDNA plasmids employing an Eppendorf (Brinkmann Instruments Inc., Westbury, New York) 5246 microinjector and 5171 micromanipulator. The pEGFP cDNA construct containing the putative NaV1.8 channel promoter region (a kind gift from Dr. Henry L. Puhl III, NIAAA, Bethesda, Maryland) was injected at a final concentration of 0.4 μg/μl, either alone or in combination with PLC-β1 construct or Gα14 or Gαz cDNA plasmids at a concentration of 0.1 μg/μl. After microinjection, the cells were stored in MEM and supplemented with nerve growth factor (15 ng/mL), ciliary derived growth factor (15 ng/mL), and glial-derived neurotrophic factor (6 ng/mL), and incubated overnight at 35°C.
Electrophysiology and data analysis
Ca2+ channel currents were recorded with an Axopatch 200B amplifier (Molecular Devices, LLC, Sunnyvale, California) and acquired with custom-designed software (S5, written by Dr. Stephen R. Ikeda, NIAAA, Bethesda, MD) on a Macintosh G4 computer (Apple Inc., Cupertino, California) equipped with an ITC-18 data acquisition interface (HEKA Instruments Inc., Bellmore, New York). The ‘double-pulse’ voltage protocol9 was employed to record Ca2+ current modulation. The protocol consists of a holding potential of −80 mV, test pulse to +10mV (prepulse) followed by a strong depolarization step to +80 mV, then a brief return to −80 mV, and finally another test pulse to +10mV (postpulse). The recording electrodes were fabricated from borosilicate glass capillaries (Garner Glass Co., Claremont, California), pulled with a Flaming/Brown P-97 micropipette puller (Sutter Instrument Co., Novato, California), coated with Sylgard (Dow Corning, New York) and fire-polished with a microforge. The pipette solution consisted of (in mM): N-methyl-D-glucamine 80, TEA-OH 20, CsCl 20, CsOH 40, creatine-PO4 14, HEPES 10, CaCl2 1, Mg-ATP 4, Na2GTP 0.3, and EGTA 11. The pH was adjusted to 7.2 with methanesulfonic acid, and the osmolality was 293–302 mOsmol/L. The external solution consisted of (in mM): CH3SO3H 140, TEA-OH 145, HEPES 10, glucose 15, CaCl2 10, and TTX 0.0003. The pH was adjusted to 7.4 with TEA-OH, and the osmolality was 320–330 mOsmol/L. The pipette solution containing GDP-β-S (Sigma-Aldrich) was prepared by directly adding the hydrolysis-resistant guanine nucleotide analogue to the internal solution at a final concentration of 1 mM and omitting Na2GTP. It should be noted that the U-50488 concentration-response relationship (Fig. 1A–C) was determined with 2.5 mM external Ca2+, and the appropriate amount of TEACl was added to keep the osmolality constant.
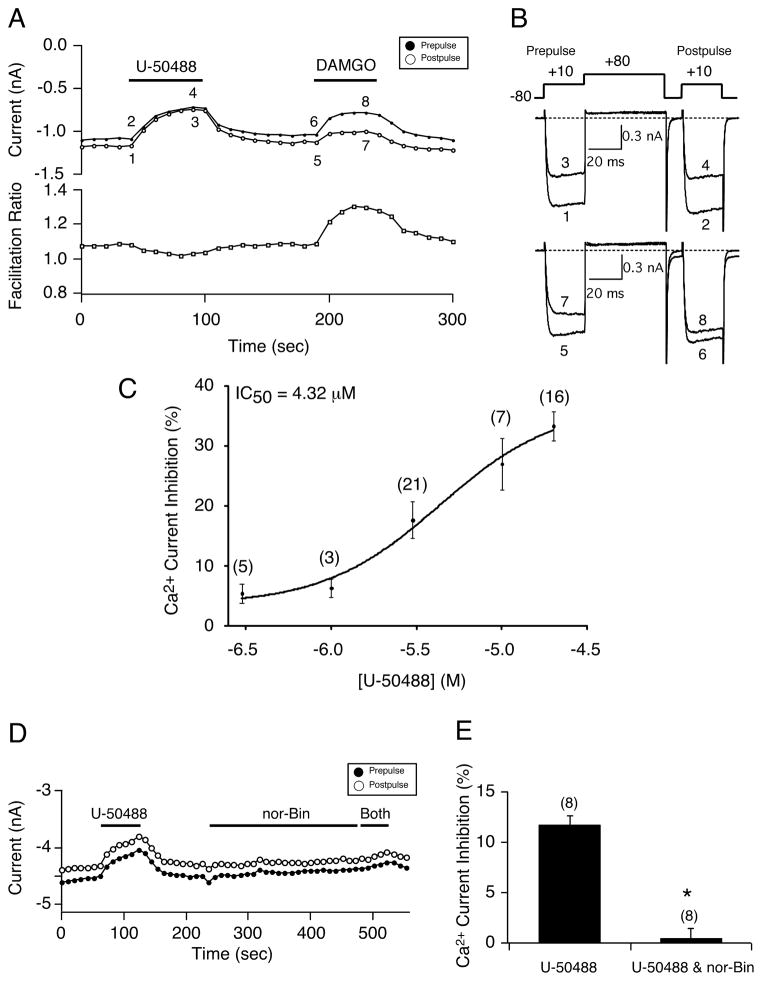
Figure 1.
The κ-OR agonist U-50488 modulates Ca2+ currents in EGFP-expressing rat sensory neurons. A) Time course of Ca2+ current inhibition acquired from the sequential application of U-50488 (20 μM) and DAMGO (10 μM). Filled and empty circles represent prepulse and postpulse Ca2+ currents, respectively, that were evoked every 10 seconds, with the protocol shown top of 1B. The facilitation (postpulse/prepulse) ratio is indicated below, empty squares. B) The numbered current traces indicated in 1A are shown, before (1, 2, 5, 6) and during (3, 4, 7, 8) agonist exposure. C) U-50488 concentration-response relationship in neurons exposed to U-50488. Each point represents the mean (± SE) Ca2+ current inhibition. The smooth curve was obtained by fitting the points to the Hill equation. D) Time course of Ca2+ current amplitude for prepulse (●) and postpulse (○) acquired during the application of U-504488 (3 μM), nor-Bin (15 μM) and both. Filled bars indicate the application of the agents. E) Summary bar graph showing the mean (± SE) Ca2+ current inhibition produced by U-50488 alone, or U-50488 and nor-Bin following the 4-minute application with nor-Bin alone. * indicates P < 0.05. Numbers in parenthesis indicate the number of neurons tested.
In one set of experiments, the Ca2+ channel (CaV 2.2) was heterologously expressed in HeLa cells employing the clones for α (Ca2+ channel pore),β and α2δ (both auxillary subunits) at a ratio of 1:1:2. The cells were cultured in Dulbecco’s Modified Eagle Medium, supplemented as above.
Stock solutions of U-50488, nor-Binaltorphimine dihydrochloride (nor-Bin, both from Tocris Bioscience, Minneapolis, Minnesota), 5′-guanidinonaltrindole (5′-GNTI), [d-Ala2-N-Me-Phe4-Glycol5]-enkephalin (DAMGO, both from Sigma-Aldrich) were prepared in water, while U-69593 (Sigma-Aldrich) was prepared in dimethyl sulfoxide (DMSO). All drugs were diluted in the external solution to their final concentration just prior to use. In some experiments, the neurons were pretreated overnight with pertussis toxin (PTX, List Biological Lab, Campbell, California) or cholera toxin (CTX, Sigma-Aldrich) in the supplemented MEM containing 500 ng/mL of either toxin.
Data and statistical analysis were performed with Igor Pro 6.0 (WaveMetrics, Lake Oswego, Oregon) and Prism (GraphPad Software, Inc., La Jolla, California), respectively, with P < 0.05 considered statistically significant. Graph and current traces were obtained with Igor Pro and Canvas 8.0 (Deneba Software, Miami, Florida) software packages.
Results
In the first set of experiments, the concentration-response relationship for the κ-OR agonist (U-50488) was determined in EGFP-expressing DRG neurons. Ca2+ currents were evoked every 10 seconds with the ‘double-pulse’ voltage protocol shown at the top of Figure 1B. The peak Ca2+ current amplitude was measured isochronally 10 msec following the initiation of the pre-and postpulse. Figure 1A shows the time course of the peak Ca2+ current amplitude of the prepulse (●) and postpulse (○) before and during the application of U-50488 (20 μM) or DAMGO (10 μM), a high-affinity mu opioid receptor agonist. The corresponding numbered traces (1–8) are shown in Figure 1B. Exposure of the neuron to U-50488 resulted in Ca2+ channel block of approximately 30%. The time course in Figure 1A also illustrates the facilitation ratio (postpulse/prepulse amplitude) that is used to indicate whether Ca2+ currents are blocked in a voltage-dependent or voltage-independent manner. Another hallmark of voltage-dependent inhibition is the kinetic slowing of the prepulse current in the presence of an agonist. The plotted ratio shows that the U-50488-mediated Ca2+ channel block was voltage-independent (ie, the magnitude of block for both pre-, trace 3, and postpulse, trace 4, currents was similar and facilitation ratio is ~ 1.0). Following the recovery period, application of DAMGO led to a stronger inhibition of the prepulse current (trace 7) compared to block of the postpulse current (trace 8), indicative of a voltage-dependent block (facilitation ratio ~ 1.3 bottom of Fig. 1A). Also illustrated is that the prepulse current displayed kinetic slowing in the presence of DAMGO (cf. trace 3 vs trace 7). Figure 1C shows the U-50488 concentration response curve with the indicated IC50 of 4.32 μM, the mean (± SE) maximum current inhibition of 33.4± 2.4%, and a Hill coefficient value of 1.28. Note that at concentrations lower than 1 μM, the % inhibition is less than 5%. All data was subsequently acquired in 10 mM external Ca2+. Figure 1D depicts the effect of the κ-OR blocker, nor-Binaltorphimine (nor-Bin) on the U-50488-mediated Ca2+ current inhibition. Application of U-50488 (3 μM) led to the voltage-independent Ca2+ current inhibition (ie, comparable block magnitude of pre- and postpulse currents). After the recovery period, the neuron was exposed to nor-Bin (15 μM) for approximately 4 minutes and thereafter to both agents. It can be seen that nor-Bin abolished the U-50488-mediated inhibition of Ca2+ currents. The results, summarized in Figure 1E, show that nor-Bin significantly (P < 0.05) abolished the U-50488-mediated Ca2+ channel inhibition (11.7 ± 0.9% vs. 0.4 ± 1.0%) in EGFP-expressing neurons. However, in another set of experiments we employed another κ-OR antagonist, 5′-guanidinonaltrindole (5′-GNTI). At concentrations between 30 and 50 μM, 5′-GNTI failed to exert any significant block on the modulatory actions of U-50488 (data not shown). The differential pharmacology exhibited by nor-BIN and 5′-GNTI suggests that the actions of U- 50488 may be independent of κ-OR stimulation (discussed below).
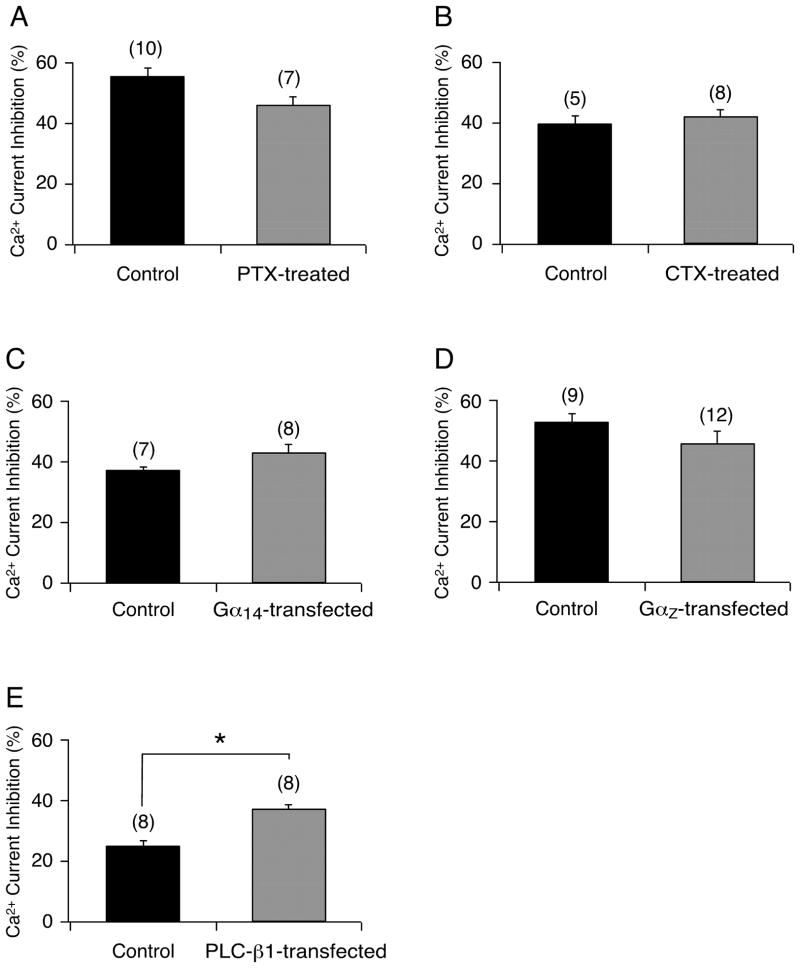
Opioid receptors are known to preferentially couple to effectors via PTX-sensitive Gαi/o subunits.10 The activation of this Gα protein subfamily has been well documented to result in voltage-dependent inhibition of Ca2+ channels (see Figs. 1A and B, DAMGO). Given that the κOR agonist U-50488 inhibited Ca2+ currents in a voltage-independent manner, the next series of experiments was designed to identify the Gα protein subfamily that couples κ-OR to Ca2+ channels in EGFP-expressing neurons. One group of isolated neurons was pretreated overnight with PTX (0.5 μg/mL) to remove Gαi/o signaling, and thereafter U-50488 (40 μM) was applied to the cells. Figure 2A shows that pretreatment of cells with PTX did not significantly affect the mean U-50488-mediated Ca2+ current inhibition. Similarly, when the cells were pretreated with CTX (0.5 μg/ml) overnight in order to eliminate the Gαs-mediated signaling pathway, the effect of U-50488 on Ca2+ currents was unaltered (Fig. 2B). We next microinjected cDNA coding for either Gα14 or GαZ to determine whether their expression in EGFP-expressing neurons would attenuate the U-50488-mediated Ca2+ current inhibition, presumably due to the overexpressed Gα subunits sequestering the free Gβγ protein subunits. The plots shown in Figure 2C and 2D indicate that the Ca2+ channel inhibitory pathway was not altered by the excess Gα14 or GαZ subunits, respectively. The lack of Gαi/o, Gα s, Gα14, and GαZ subunit involvement in the U-50488-mediated inhibitory pathway suggested that Gαq/11 protein subunits were mediating this response.11 Thus, in another group of neurons we microinjected cDNA coding for a phospholipase C-β1 construct (PLC-β1 ct), which we have previously reported to selectively bind Gαq/11-GTP.12 Figure 2E shows that neurons expressing both EGFP and PLC-β1 ct exhibited a significantly (P < 0.05) greater Ca2+ current inhibition following U-50488 application. These results suggest that ‘buffering’ of endogenous Gαq/11 by the construct did not abolish the Ca2+ current inhibition mediated by U-50488. Overall, these data suggest that the Gα protein subunits (Gαi/o, Gαs, Gα14, GαZ, and Gαq/11) do not play a significant role in the U- 50488-mediated voltage-independent Ca2+ current inhibition.
Figure 2.
Bar graphs summarizing the mean (± SE) Ca2+ current inhibition in EGFP-expressing neurons produced by U-50488 (40 μM) alone (Control), or in cells pretreated with 0.5 μg/mL pertussis toxin (PTX) (A), 0.5μg/mL cholera toxin (CTX); (B) or cells transfected with Gα14 (C), GαZ (D) or PLC-β1 construct (E). The current inhibition was determined as that described for Figure 1. Numbers in parenthesis indicate the number of experiments. * indicates P < 0.05 compared to control.
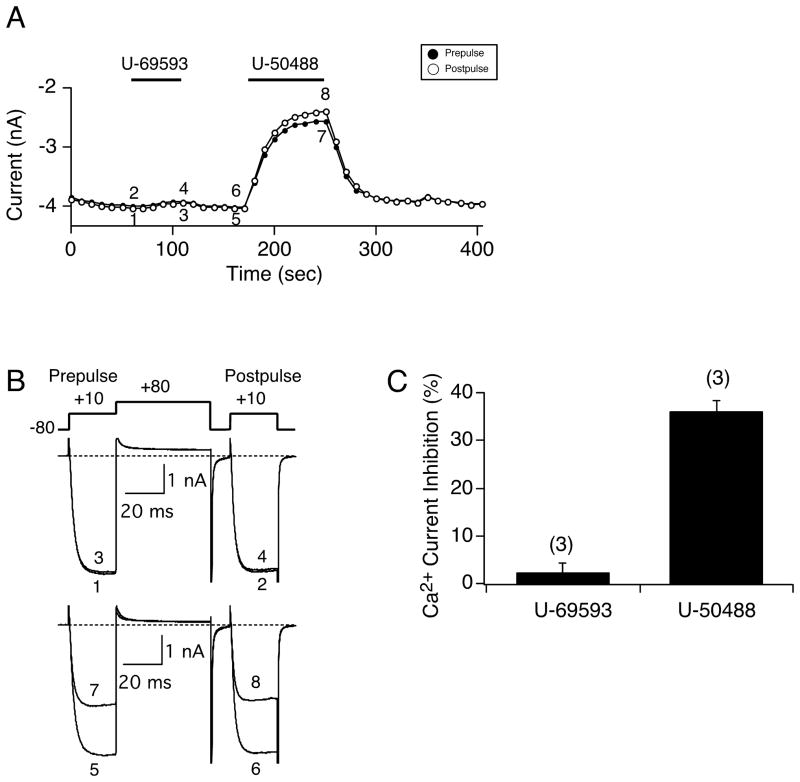
In the next set of experiments, we examined whether other native G protein subunits were involved in the modulation of Ca2+ currents during U-50488 application. In a group of EGFP-expressing neurons we dialyzed a non-hydrolyzable GDP analog, GDP-β-S, via the recording pipet (GTP was excluded) in order to block any agonist-mediated G protein-coupled receptor activation of G proteins. Figure 3A is a time course that shows the effect of U-50488 (20 μM) and U-69593 (10 μM), another κ-OR agonist, on the Ca2+ currents in a cell dialyzed with GDP-β-S. In control experiments (3 of 20 neurons), U-69593 exerted a voltage-dependent block of Ca2+ currents (data not shown), indicative of a receptor-mediated response. Exposure of the cell to U-69593 failed to modulate the Ca2+ currents (traces 1–4), while U-50488 inhibited the Ca2+ currents in both a G protein- and voltage-independent manner (traces 5–8). These data indicate that the U-50488-mediated block does not employ G proteins.
Figure 3.
Dialysis with GDP-β-S in EGFP-expressing neurons does not prevent the U-50488-mediated Ca2+ current inhibition, but the U-69593-mediated Ca2+ current inhibition is absent. A) Time course of Ca2+ current inhibition for prepulse (●) and postpulse (○) acquired from the sequential applications of U-69593 (10 μM) and U- 50488 (20 μM) where GDP-β-S (1 mM) was substituted for Na2GTP in the pipette solution. B) Current traces and voltage paradigm employed to elicit Ca2+ currents for the cell in A. C) Summary bar graph showing the mean (± SE) Ca2+ current inhibition produced by application of U-69593 and U-50488 following dialysis with GDP-β-S. Numbers in parenthesis indicate the number of neurons tested.
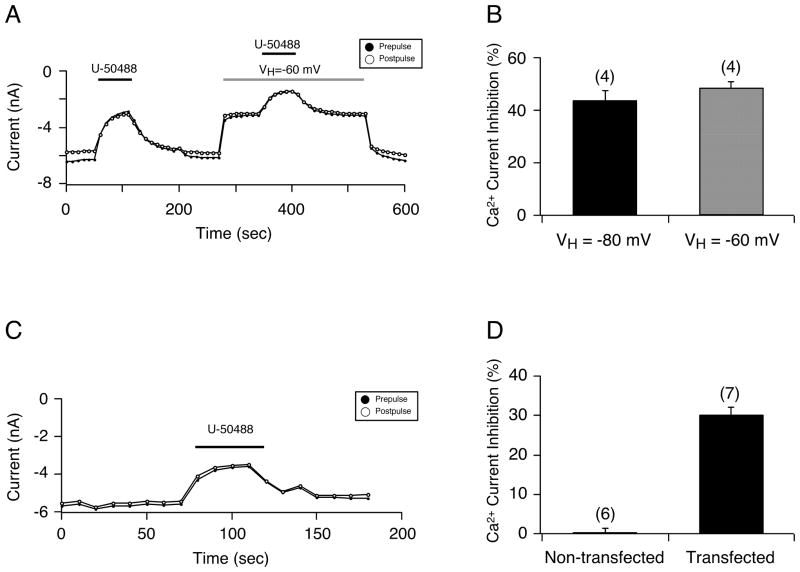
Thus far, these results suggested that the effects mediated by U-50488 resulted from a direct action on Ca2+ channels, reminiscent of previous observations with TTX-resistant Na+ channels in sensory neurons.13–15 In the next set of experiments, we examined whether the Ca2+ channel block would be affected by changing the holding potential, since it has been shown that the block of TTX-resistant Na+ channels by U-50488 is altered by changing this parameter.14 Figure 4A shows the time course of an EGFP-expressing neuron before and during exposure to 20 μM U-50488, followed by switching of the holding potential (VH) to a more depolarized level and re-exposure to 20 μM U-50488. The initial exposure of U-50488 (at VH -80 mV) led to the typical voltage-independent block of Ca2+ currents. Following the recovery period, VH was switched to - 60 mV and peak Ca2+ current decreased. Thereafter, U-50488 was applied to the neuron and Ca2+ currents were also blocked in a voltage-independent manner. The summary plot in Figure 4B illustrates that the U-50488-mediated Ca2+ current inhibition (mean± SE) for both VH of −80 mV and −60 mV is similar.
Figure 4.
A) Time course of Ca2+ current inhibition for prepulse (●) and postpulse (●) current acquired from the sequential application of 20μM U-50488, using the double-pulse voltage protocol (as shown in Figure 1B) and a holding potential (VH) of either −80 mV (1st application) or −60 mV (grey bar). B) Summary plot showing the U-50488-mediated Ca2+ current inhibition (mean ± SE) at VH of −80 mV and −60 mV. C) Time course of peak Ca2+ current for prepulse (●) and postpulse (○) acquired from a HeLa cell heterologously expressing the CaV 2.2 channel before and during U-50488 (20 μM) application. D) Bar graph showing the U-50488 (20 μM) Ca2+ current inhibition (mean ± SE) in HeLa cells non-transfected or transfected with the N-type Ca2+ channel (CaV 2.2). Numbers in parenthesis indicate the number of cells tested.
In the final set of experiments, we heterologously expressed the CaV 2.2 Ca2+ channel (N- type) in HeLa cells (which do not normally express these channels orκ-OR) to determine whether the U-50488-mediated inhibition was a result of a direct block of the Ca2+ channel. Figure 4C is a time course of the peak Ca2+ current recorded from a HeLa cell expressing Ca2+ channels before and during U-50488 (20 μM) exposure. In this cell, U-50488 inhibited the Ca2+ currents approximately 35% in a receptor- and voltage-independent manner. Figure 4D is a summary plot demonstrating that application of 20 μM U-50488 inhibited Ca2+ currents (mean ± SE) in HeLa cells expressing N-type Ca2+ channels (30.1 ± 2.1%, n=7) while it exerted no effect in non-transfected HeLa cells (0 ± 1.4%, n=6).
Discussion
A major challenge associated with developing analgesic drugs that target κ-OR is to limit their ability to cross the blood brain barrier in order to restrict their side-effects.3,16 Thus, there is a need to better understand κ-OR physiology and pharmacology, especially in sensory neurons involved in pain transmission. The arylacetamide derivatives fedotozine and asimadoline had been reported to be effective visceral pain analgesics, especially in patients with irritable bowel syndrome.17–19 Fedotozine, however, has been discontinued,19 while asimadoline appears promising because of its limited CNS penetration.17 In the present study, we employed the arylacetamide prototype, U-50488, κ-OR agonist, to examine its effect on Ca2+ channel currents in acutely isolated rat DRG neurons. The neurons were transfected with the recently described8 clone containing EGFP cDNA and the promoter region of the TTX-resistant Na+ channel, allowing us to study a defined subpopulation (ie, nociceptive) of sensory neurons. The results show that U-50488 exhibits non-receptor-mediated Ca2+ channel block in EGFP-expressing neurons. The Ca2+ current inhibition is voltage-independent and non-G protein-mediated. These findings extend previous observations that have found that this κ-OR agonist can also directly block other ion channels, including TTX-resistant Na+ channels in rat sensory neurons.13–15
The U-50488 pharmacological profile of EGFP-expressing neurons obtained in this study (IC50 ~ 4 μM) exhibited a slightly lower potency than previous studies that examined the effect of U-50488 on other ion channels. In rat colon sensory15 and DRG14 neurons, the observed IC50 values for Na+ channel currents were 8.4 μM for the former, and ranged from 8 μM (with a VH of −40 mV) to 49 μM (with a VH of −100 mV) for the latter. In isolated rat cardiac myocytes, U- 50488 blocked Na+ and K+ currents with an IC50 of 15 μM and 40 to 50 μM, respectively.20 A pharmacokinetic study in mice found that oral administration of a U-50488 enantiomer (150 mg/kg) reached peak-free blood levels of 800 ng/mL (~ 2 μM) within 1 hour and a brain to blood ratio of 8.2.21 It should also be noted that the U-50488 IC50 reported for intracellular Ca2+ changes in cultured lumbosacral DRG22 and forskolin-stimulated cAMP accumulation in PC12 cells23 ranged from 1 to 5.8 nM. The apparent disparity of potency is likely a result of different experimental conditions between electrophysiological recordings and the above-mentioned reports22,23 (ie, steady-state vs. equilibrium conditions).
In some experiments, the U-50488- (3 μM) mediated Ca2+ current inhibition was blocked by nor-Bin (15 μM). Despite the fact that this agonist:blocker ratio indicated block at the receptor level, we found rather inconsistent block employing lower ratios (ie, 1:10 or 1:15, data not shown). Thus, a much higher concentration of nor-Bin was required to inhibit the U-50488-mediated block of Ca2+ channels. Indeed, in rat colon sensory neurons it was reported that 1 μM nor-Bin blocked the 1 μM U-50488-mediated Ca2+ current inhibition.24 However, unlike our findings, this report showed the U-50488-mediated Ca2+ channel inhibition was mediated by Gαi/o proteins because PTX pretreatment abolished the effect of the agonist. The U-50488 concentration-response relationship and whether the receptor agonist inhibited Ca2+ currents in a voltage-dependent or –independent manner were not determined. Therefore, it is hard to reconcile the observed differences. Finally, we observed that another κ-OR blocker (5′-GNTI) failed to inhibit the U-50488 modulatory effects at relatively high concentrations. The pharmacological variability exhibited by both blockers was indicative of non-κ OR mediated effects by U-50488.
Stimulation of G protein-coupled receptors, including opioid receptors, that couple to Gαi/o protein subunits leads to voltage-dependent inhibition of Ca2+ channel currents.11 Pretreatment with PTX should, therefore, attenuate this signal transduction pathway. For example, it has been reported that PTX blocked the U-50488-mediated Ca2+ channel inhibition in rat colon DRG neurons24 and PC-12 cells.23 Our results, however, show that application of U-50488 to EGFP-expressing DRG neurons blocked the voltage-gated Ca2+ channels in a voltage-independent manner and exhibited resistance to PTX exposure. Thus, our initial efforts were aimed at determining which G protein subfamily mediated this response. Pretreatment of the EGFP-expressing neurons with CTX also did not result in loss of coupling, indicating that Gαs protein subunits were not involved in this response. Furthermore, Gα14 and GαZ subunits were overexpressed in order to expose the neurons to a Gβγ ‘sink’ and disrupt the U-50488-mediated Ca2+ current inhibition. Neither subunit exerted a significant effect on U-50488 actions. On the other hand, when the GDP-bound Gαq/11 binding construct, PLCβ1, was overexpressed, the U-50488-mediated Ca2+ current inhibition showed a modest yet significant increase. We further probed the involvement of other G proteins in this pathway by dialyzing the hydrolysis-resistant GDP-β-S to block the GTP-dependent activation of G proteins and the effect of U-50488 was unaltered. Accordingly, the observed block of Ca2+ channels with the κ receptor agonist does not appear to be mediated by the aforementioned Gα proteins or other native G protein subunits.
Unlike previous studies13,14 in sensory neurons, which showed the direct block of TTX-resistant Na+ channels by U-50488 was modulated by the holding potential (VH), our findings indicate that Ca2+ channel inhibition mediated by the κ-OR agonist was not significantly altered when VH was changed from −80 to −60 mV. To determine whether U-50488 acted independent of receptor stimulation, we expressed the N-type Ca2+ channel in HeLa cells and found that the κ-OR agonist inhibited Ca2+ currents in a similar fashion as that observed in EGFP-expressing neurons. Overall, these data support the conclusion that exposure of sensory neurons to U-50488 can lead to the direct block of Ca2+ channels in a voltage-independent manner without κ-OR activation. Given the heterogenous nature of sensory neurons, caution should be observed when employing U-50488 as the selective agonist to effect κ-OR actions.
Acknowledgments
Supported by NIH Grant: AR-059397.
We thank Dr. Gregory Weller (Department of Anesthesiology) for critical reading of an earlier version of the manuscript.
Footnotes
Previously presented at the 41st Society for Neuroscience Meeting 2011 (A341.14).
References
- 1.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–S153. [PubMed] [Google Scholar]
- 2.Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κagonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Sun J, Tao Y, Chi Z, Liu J. The role of κ-opioid receptor activation in mediating antinociception addiction. Acta Pharmacologica Sinica. 2010;31:1065–1070. doi: 10.1038/aps.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 6.Sangamaswaran L, Delgado SG, Fish LM, Koch RD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 7.Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18(6):2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puhl HL, 3rd, Ikeda SR. Identification of the sensory neuron specific regulatory region for the mouse gene encoding the voltage-gated sodium channel NaV1.8. J Neurochem. 2008;106(3):1209–1224. doi: 10.1111/j.1471-4159.2008.05466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurons. J Physiol. 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altier C, Zamponi GW. Signaling complexes of voltage-gated calcium channels and G protein-coupled receptors. J Recept Signal Transduct Res. 2008;28:71–81. doi: 10.1080/10799890801941947. [DOI] [PubMed] [Google Scholar]
- 11.Hille B. Modulation of ion channel function by G-protein coupled receptors. Trends Neurosci. 1994;17:531–535. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 12.Kammermeier PJ, Ruiz-Velasco V, Ikeda SR. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Gαq/11 and Gβγ. J Neurosci. 2000;20:5623–5629. doi: 10.1523/JNEUROSCI.20-15-05623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su X, Joshi SK, Kardos S, Gebhart GF. Sodium channel blocking actions of the k-opioid receptor agonist U50,488 contribute to its visceral antinociceptive effects. J Neurophysiol. 2002;87:1271–1279. doi: 10.1152/jn.00624.2001. [DOI] [PubMed] [Google Scholar]
- 14.Su X, Castle NA, Antonio B, Roeloffs R, Thomas JB, Krafte DS, Chapman ML. The effect of k-opioid receptor agonists on tetrodotoxin-resistant sodium channels in primary sensory neurons. Anesth Analg. 2009;109:632–640. doi: 10.1213/ane.0b013e3181a909a4. [DOI] [PubMed] [Google Scholar]
- 15.Joshi SK, Lamb K, Bielfeldt K, Gebhart GF. Arylacetamide k-opioid receptor agonists produce a tonic- and use-dependent block of tetrodotoxin-sensitive and – resistant sodium currents in colon sensory neurons. J Pharm Exp Ther. 2003;307:367–372. doi: 10.1124/jpet.103.052829. [DOI] [PubMed] [Google Scholar]
- 16.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26:S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 17.Mangel AW, Hicks GA. Asimadoline and its potential for the treatment of diarrhea- predominant irritable bowel syndrome: a review. Clin Exp Gastroenterol. 2012;5:1–10. doi: 10.2147/CEG.S23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig Liver Dis. 2009;41:854–862. doi: 10.1016/j.dld.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradesi S, Herman J, Mayer EA. Visceral analgesics: drugs with a great potential in functional disorders? Curr Opin Pharmacol. 2008;8:697–703. doi: 10.1016/j.coph.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugsley MK, Saint DA, Walker MJ. An electrophysiological basis for the antiarrhythmic actions of the kappa-opioid receptor agonist U-50488H. Eur J Pharmacol. 1994;261:303–309. doi: 10.1016/0014-2999(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 21.Jones DC, Hallyburton I, Stojanovski L, Read KD, Frearson JA, Fairlamb AH. Identification of a k-opioid agonist as a potent and selective lead for drug development against human African trypanosomiasis. Biochem Pharmacol. 2010;80:1478–1486. doi: 10.1016/j.bcp.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gschossmann JM, Chaban VV, McRoberts JA, Raybould HE, Young SH, Ennes HS, Lembo T, Mayer EA. Mechanical activation of dorsal root ganglion cells in vitro: comparison with capsaicin and modulation by κ-opioids. Brain Res. 2000;856:1010–1110. doi: 10.1016/s0006-8993(99)02353-7. [DOI] [PubMed] [Google Scholar]
- 23.Tallent M, Dichter MA, Bell GI, Reisine T. The cloned opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells. Neurosci. 1994;63:1033–1040. doi: 10.1016/0306-4522(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 24.Su x, Wachtel RE, Gebhart GF. Inhibition of calcium currents in rat colon sensory neurons by κ- but not μ- or δ-opioids. J Neurophysiol. 1998;80(6):3112–3119. doi: 10.1152/jn.1998.80.6.3112. [DOI] [PubMed] [Google Scholar]