Abstract
Objective
Amygdala habituation, the rapid decrease in amygdala responsiveness to repeated presentation of stimuli, is fundamental to the nervous system. Habituation is important for maintaining adaptive levels of arousal to predictable social stimuli and reduced habituation is associated with heightened anxiety. Input from the ventromedial prefrontal cortex (vmPFC) regulates amygdala activity. Although previous research demonstrated abnormal amygdala function in youth with autism spectrum disorders (ASD), no study had examined amygdala habituation in a young sample or whether habituation related to amygdala connectivity with the vmPFC.
Method
Data were analyzed from 32 children and adolescents with ASD and 56 typically developing controls who underwent functional magnetic resonance imaging (fMRI) scanning while performing a gender identification task for faces that were fearful, happy, sad, or neutral. Habituation was tested by comparing amygdala activation to faces during the first half versus the second half of the session. VmPFC–amygdala connectivity was examined through psychophysiological interaction analysis.
Results
Youth with ASD had decreased amygdala habituation to sad and neutral faces relative to controls. Moreover, reduced amygdala habituation correlated with autism severity as measured by the Social Responsiveness Scale. There was a group difference in vmPFC–amygdala connectivity while viewing sad faces, and connectivity predicted amygdala habituation to sad faces within controls.
Conclusions
Sustained amygdala activation to faces suggests that repeated faces are processed differently in individuals with ASD, which could contribute to social impairments. Abnormal modulation of the amygdala by the vmPFC may play a role in reduced habituation.
Keywords: fMRI, habituation, autism, adolescent, emotion
INTRODUCTION
Autism spectrum disorders (ASD) are characterized by impairments in social function including difficulties with face and emotion recognition and following eye gaze.1 Several studies have suggested that individuals with ASD are over-aroused by social stimuli, which may relate to the social impairments characteristic of these disorders.2-5 In line with this hypothesis, children with ASD demonstrate increased skin conductance response, a measure of physiological arousal, to faces.6 In searching for neural substrates associated with over-arousal, researchers have highlighted the role of the amygdala, which is involved in assigning emotional salience to stimuli.7
The nature of amygdala abnormalities in ASD is unclear and previous research has produced different results. Whereas some studies using functional magnetic resonance imaging (fMRI) showed amygdala hypo-activation in ASD, (e.g.,8) others demonstrated amygdala hyper-activation.2,4,9 One potential explanation for this discrepancy is differences in presentation times across studies. Individuals with ASD attend less to faces compared to controls;10 thus, tasks using long presentation times allow participants to attend away from stimuli, which could account for findings of amygdala hypo-activation. In contrast, studies that have limited group differences in attention to faces, such as using brief stimulus presentation times, have generally shown amygdala hyper-activation in ASD.
Although there is now evidence supporting amygdala hyper-activation in ASD, the mechanism underlying this finding is still unclear. One potential mechanism is reduced amygdala habituation.11 Habituation refers to decreased neural response with repeated presentation of a stimulus.12 In healthy individuals, the amygdala responds to faces at the beginning of a scanning session but then quickly habituates to repeated presentation of faces.13-14 Failure to habituate may lead to sustained amygdala activation and over-arousal. For example, Herry et al.15 demonstrated that both mice and human participants had reduced amygdala habituation for unpredictable compared to predictable stimuli, and that unpredictable stimuli increased anxiety-related behaviors. They suggested that amygdala habituation helps maintain adaptive levels of arousal to predictable stimuli. In line with this, reduced amygdala habituation is related to higher trait anxiety scores in healthy individuals.14
An alternative explanation for previous findings of increased amygdala activation in ASD is that across the entire scanning session the amygdala shows heightened responsiveness to faces. In order to clarify the mechanisms underlying previous findings of amygdala hyper-activity in ASD, we examined how amygdala activity changes over the course of scanning. Kleinhans et al.11 demonstrated that adults 18 to 44 years old with ASD had reduced amygdala habituation to neutral faces compared to controls, but habituation has never been investigated in children or adolescents with ASD. The developmental period from childhood to adolescence is thought to result in a reorganization of the neural circuitry involved in face processing, including changes in amygdala responsiveness to faces.16 Therefore, amygdala habituation in youth with ASD may differ from adults. Moreover, Wright et al.17 demonstrated different rates of habituation in the prefrontal cortex to fearful and happy faces, suggesting that different patterns of habituation may be observed for facial expressions other than the neutral faces used by Kleinhans et al. Finally, Kleinhans et al. used relatively long stimulus presentation times; therefore, it is unknown whether a different pattern of habituation would be obtained with brief presentations.
The ventromedial prefrontal cortex (vmPFC) is thought to be involved in amygdala habituation. Animal models show that the vmPFC regulates amygdala activity by signaling inhibitory interneurons in the amygdala.18 In humans, stronger vmPFC–amygdala connectivity predicts greater amygdala habituation.14 Moreover, the density of serotonin receptors in the medial PFC, part of the pathway providing negative feedback to the amygdala, correlates with amygdala habituation in healthy adults.19 Therefore, abnormal functioning of this circuit may relate to reduced amygdala habituation in ASD.
The objective of the present study was to examine amygdala habituation to faces and its relation to vmPFC–amygdala connectivity in children and adolescents with ASD. To accomplish this, we compared amygdala activation to briefly presented faces viewed during the first half versus the second half of an fMRI acquisition sequence. Habituation was operationalized as a decrease in activation from the first to second half. We included fearful, happy, sad, and neutral faces in order to examine habituation to different expressions. We hypothesized that children and adolescents with ASD would exhibit reduced amygdala habituation to faces compared to typically developing controls. Moreover, we hypothesized that decreased amygdala habituation would predict greater autism severity as measured by the Social Responsiveness Scale. Second, we examined vmPFC–amygdala connectivity to test whether this related to amygdala habituation. We hypothesized that participants with ASD would have decreased connectivity while viewing faces and that connectivity would correlate with habituation.
METHOD
Participants
Fifty-nine children and adolescents with ASD and 64 typically developing controls underwent fMRI scanning. A subset of these participants was included in a previous study.2 Participants with ASD were recruited through a clinic affiliated with the University and controls were recruited from the community through fliers. All procedures were approved by the University’s Institutional Review Board. Participants 18 years and older signed informed consent forms; minor participants gave assent and their parents provided written consent. All participants with ASD were diagnosed with the Autism Diagnostic Observation Schedule (ADOS),20 the Autism Diagnostic Interview–Revised (ADI-R),21 and based on clinical consensus. Twenty-one participants were removed from the sample for not reaching at least 80% accuracy on the behavioral task (7 ASD) or for having movement>3 mm in any direction during scanning (11 ASD and 2 controls) and 1 control was removed due to technical problems, leaving 102 participants with valid fMRI data. In order to ensure that signal dropout did not mask activity, we inspected each participant’s contrast image for the effect of all early faces vs. all late faces and eliminated an additional 5 ASD participants with visible signal dropout in the prefrontal cortex and 7 participants (3 ASD and 4 controls) missing more than 10% of voxels in the bilateral amygdala, defined structurally by the Wake Forest University Pickatlas (WFU Pickatlas),22 applied to each participant’s contrast image. For the remaining participants, registration between the normalized anatomical images and bilateral amygdala region of interest (ROI) were visually inspected to ensure coverage of the amygdala. Finally, one control and 1 participant with ASD were identified as outliers >3 times the interquartile range for mean habituation across both runs or within one run and were removed from subsequent analyses.
After removing these participants, 32 ASD and 56 controls between 8 and 19 years of age remained (Table 1). Pubertal status was measured using the Pubertal Development Scale,23 with a total score ranging from 1 to 4. Details on verbal and nonverbal IQ testing have been reported previously.2 Any participants with IQ<80 were excluded from participating in the study. ASD symptom scores were measured using the Social Responsiveness Scale (SRS).24 Total raw scores ranged from 0–71 in controls and 44–163 in the ASD group. Following prior work examining anxiety in ASD, anxiety symptoms were measured using the Spence Children’s Anxiety Scale (SCAS).25 Because SCAS scores differed between groups, we examined the correlation between anxiety and habituation. Mother’s education was used as a proxy for socioeconomic status; 12% of participants’ mothers had a high school degree, 30% had some college education, and 58% had a 4-year degree or more.
Table 1.
Participant Characteristics.
| ASD (Mean, S.D.) n = 32 |
Control (Mean, S.D.) n = 56 |
Independent-samples t- test |
|
|---|---|---|---|
| Age | 13.57 (2.3) | 14.66 (2.9) | t(86)=−1.83, p>.05 |
| Puberty | 2.38 (.9) | 2.64 (.9) | t(86)=−1.31, p>.05 |
| Verbal IQ | 113.4 (17.6) | 114.0 (12.8) | t(86)=−.16, p>.05 |
| Nonverbal IQ | 108.8 (17.9) | 103.7 (11.4) | t(82)=1.61, p>.05 |
| Gender ratio (M to F) | .81 | .79 | t(86)=.30, p>.05 |
| Proportion left-handed | .13 | .13 | t(81)=.02, p>.05 |
| SRS total raw scores | 94.56 (26.8) | 20.11 (15.5) | t(85)=16.46, p<.001 |
| SCAS total scores | 22.7 (13.5) | 15.6 (8.2) | t(86)=3.07, p<.01 |
| Gender ID accuracy | 94.3% (3.8) | 96.8% (3.3) | t(85)=−3.29, p<.01 |
| Gender ID RT (ms), Correct trials |
789.4 (146.7) | 734.0 (140.9) | t(85)=1.74, p>.05 |
| Gender ID RT (ms), Incorrect trials |
509.5 (278.4) | 551.9 (320.5) | t(74)=−.59, p>.05 |
| Gender ID RT (ms), All trials | 768.9 (123.9) | 727.0 (135.2) | t(85)=1.44, p>.05 |
| ER accuracy | 87.3% (7.2) | 86.8% (10.3) | t(83)=.26, p>.05 |
| ER RT (ms) | 1322.9 (349.1) | 1199.0 (252.9) | t(83)=1.88, p>.05 |
| African American or AA/Caucasian |
6% | 10.5% | |
| Asian or Asian/Caucasian | 0% | 8.5% | |
| Caucasian | 94% | 74% | |
| Hispanic/Latino | 0% | 7% |
Note: Data were missing for the following measures: Nonverbal IQ (3 controls and 1 autism spectrum disorder [ASD]), Handedness (3 controls and 2 ASD), social responsiveness scale (SRS) scores (1 control), Gender identification task (ID) accuracy and reaction time (RT) (1 control), emotion recognition task (ER) accuracy and reaction time (RT) (3 ASD). Bolded t-tests indicate significant group differences. Puberty was measured with the Pubertal Development Scale; SCAS=Spence Children’s Anxiety Scale.
Sixteen of the participants with ASD were on medication; 5 on selective serotonin reuptake inhibitors (SSRIs), 11 on stimulants and 4 on atypical antipsychotics. The participants on medications did not differ in amygdala habituation to faces within the bilateral amygdala (all early faces>all late faces, Medications>No Medications, t(30)=.84, p=.82 and Medications<No Medications, t(30)=1.42, p=.63) and so we included all participants in the analyses.
Procedures
FMRI data acquisition
MRI images were acquired with a 3 Tesla GE Signa. Participants made responses with a button box linked to an integrated functional imaging system (IFIS) system. The task was projected onto a screen and participants wore goggles with mirrors to view the task. Details on fMRI acquisition have been reported elsewhere.2 Briefly, a high-resolution sagittal SPGR image was collected for the structural image and T2*-weighted BOLD images (repetition time [TR]=2,000 ms, echo time [TE]=30 ms) were collected using a reverse spiral sequence for functional images.
Gender identification task
Prior to scanning, participants completed a practice session of the gender identification task. During scanning, participants performed two runs of a gender identification task with a brief rest period between runs. A trial consisted of a 500 ms fixation cross followed by a face presented for 250 ms. After the face, a black screen appeared for 1500 ms, during which participants indicated the gender of the face by pressing the thumb button for male and the index finger button for female. The intertrial interval (ITI) was jittered and ranged from 0 to 6,000 ms (at intervals of 2,000 ms). All faces came from the NimStim set26 and there were equal numbers of males and females. By using a brief stimulus presentation period and requiring a correct behavioral response, the task helped to ensure that participants were looking at the faces.
Within each run, there were a total of 60 trials with 15 of each of the following emotions: fearful, happy, sad, and neutral. Trials were presented in a randomized order for each participant. Each run took approximately 6 minutes to complete. E-prime controlled stimulus presentations and recorded responses and reaction times.
Emotion recognition task
After participants had undergone fMRI scanning, they completed an emotion recognition task outside of the scanner using the same face stimuli that were presented in the gender identification task. The task was administered on a laptop using E-Prime and consisted of 120 trials. Each trial consisted of a 500 ms fixation cross, then a face presented for 250 ms, followed by an instruction screen. Participants were instructed to indicate the emotion on each face by pressing a button corresponding either to fearful, happy, sad or neutral. Response times (RT) and accuracy were recorded.
Analyses
Behavioral data analysis
Mean accuracy and RT to the faces were obtained for the gender identification task performed during scanning. Participants with <80% accuracy on all trials were excluded from the analyses. For the emotion recognition task performed after scanning, a repeated measures analysis of variance (ANOVA) was used to compare emotion recognition accuracy and RT across groups.
Functional MRI data analysis
FMRI data were reconstructed into images using field map correction to reduce distortions. Preprocessing steps have previously been described2 and included slice timing correction, realignment, co-registration of the anatomical to the functional images, normalization of the images to the statistical parametric mapping (SPM) template in Montreal Neurological Institute (MNI) space, and smoothing with an 8 mm full width at half maximum (FWHM) Gaussian kernel. To ensure that movement did not differ across groups, we calculated mean motion and rotation from the initial volume for each participant based on parameters from realignment. The groups did not differ in mean motion, t(86)=−1.25, p>.05, or rotation, t(86)=−1.70, p>.05. We examined volume-to-volume displacement of the translation parameters by calculating the Euclidean distance of translation between volumes. The groups did not differ in mean displacement, t(86)=−.1.30, p>.05 or maximum displacement, t(86)=−.93, p>.05. Condition effects at the individual subject level were examined using the general linear model in SPM. For each participant, conditions were modeled with the SPM canonical hemodynamic response function. To remove the influence of linear drift, a high-pass filter of a period of 128 seconds was applied. Incorrect trials were modeled as a separate condition and excluded from further analyses.
Analyses were conducted using SPM8. Each run was divided in half and a separate regressor was created for each emotion during the first half (early faces) and second half (late faces) of the run, similar to previous research,14 yielding 8 regressors of interest (early and late fearful, happy, sad, and neutral faces) within each run. To test for group differences in habituation to face expressions within runs (i.e., from early to late faces), a repeated measures ANOVA was conducted in SPSS to examine the interaction of group (ASD, Controls) × time (early faces, late faces) × run (run 1, run 2) × emotion (fearful, happy, sad, neutral) × side (left, right amygdala). Group was entered as a between-subjects factor and time, run, emotion, and side were entered as within-subjects factors. Contrast values for each cell were extracted for each participant from the left and right amygdala separately, structurally defined by the WFU Pickatlas; these values were obtained for the contrasts of each regressor vs. baseline (e.g., early fear faces vs. baseline). To test for group differences in habituation across runs (i.e., from run 1 to run 2), we conducted a repeated measures ANOVA for the interaction of group × run × emotion × side, collapsing across early and late faces within each run. Because the length of break between runs varied across participants, we included break length as a covariate in these interactions. We also re-modeled the data with trial RT as a parameter in each participant’s individual model and tested the interactions with these contrast values to control for RT.
Significant interactions were followed up by conducting two-sample t-tests in SPM to examine group differences in habituation to specific emotions. Habituation was examined by performing the following contrast for each emotion: early faces>late faces. The subtraction of late faces from early faces yields activity specific to the timing of faces in the run while controlling for other activity related to the viewing of faces or specific expressions. If habituation occurs, activity should be greater in the first half than the second half. Significance was tested by first displaying whole-brain results at p<.001 uncorrected and then applying a small volume correction with family-wise error (FWE) p<.0125 (Bonferroni corrected for testing across 4 emotions) using the bilateral amygdala defined by WFU Pickatlas as the volume of interest.
Relation between habituation and ASD symptoms
For expressions for which there was a significant group difference in habituation, we examined the relation between habituation and ASD symptoms within the ASD group by entering SRS total raw scores into a regression on the habituation contrast in SPM. Results were small-volume corrected within the structural bilateral amygdala. Based on the results of the first analysis, we conducted this correlation on two emotions (sad and neutral), thus significance was set at p<.025.
Relation between habituation and anxiety
We calculated habituation by extracting the mean contrast value from the structural left or right amygdala ROI for the contrast of early faces>late faces for each emotion. We then examined the correlation between these habituation scores and SCAS scores within each group in SPSS.
Psychophysiological interaction analysis
To test the hypothesis that the ASD group would have reduced vmPFC–amygdala connectivity, we planned to conduct psychophysiological interaction (PPI) analyses in SPM8 for any expressions yielding a group difference in habituation. PPI examines how the interaction between two regions is modulated by psychological context, in this case facial expressions that resulted in habituation vs. facial expressions that did not.27 We planned to select the left or right amygdala as the seed and the facial expressions for the PPI based on the results from our first analysis. Because our first analysis only revealed significant effects in the first run, we modeled the PPI within run 1 only, and collapsed across the early and late halves in order to simplify the interpretation of the results. Because this secondary hypothesis was exploratory and based on the findings of our first, we chose a statistical threshold of p<.05 FWE-corrected when examining differences in PPIs between the ASD and control groups. We selected Brodmann’s area 25 (BA 25) defined structurally by the WFU Pickatlas as the volume of interest for the small-volume correction in the vmPFC as this was used in previous studies of amygdala–vmPFC connectivity and habituation in humans,19 and has been shown in non-human primate tracing studies to have dense output projections to the amygdala.28
To examine how vmPFC–amygdala connectivity related to amygdala habituation, for each participant we extracted contrast values for habituation from a 3 mm sphere around the peak activation of the group difference in amygdala habituation for the habituation contrast (early>late faces). We then entered these habituation values (positive scores representing stronger habituation) as a regressor in the PPI analysis in SPM for each group separately and tested significance using small-volume correction within BA 25.
RESULTS
Behavioral results
Gender identification task performed during scanning
There was no difference between groups in RT to the gender identification task. Although the control group was significantly more accurate than the ASD group (Table 1), both groups performed well on the task (M ASD=94%, M Controls=97%) and only data from correct trials were included in analyses. Accuracy was added as a nuisance covariate for the interaction.
Emotion recognition task
There was no interaction or group difference in emotion recognition accuracy or RT (Table 1). The main effect of emotion was significant, F(3,81)=34.41, p<.001. Participants were most accurate in recognizing happy faces (M=94%), followed by neutral (88%), sad (85%), and fearful (82%).
FMRI results
Group differences in amygdala habituation
Our first hypothesis was that the ASD and control groups would differ in habituation to faces. The 5-way interaction of group × time × emotion × run × side was not significant, F(3,82)=.88, p=.46. The only 4-way interaction that was significant was that of group × time × emotion × run, F(3,82)=3.90, p=.012, d=.44, suggesting the two groups differed in habituation by run and emotion. Follow-up revealed the interaction of group × time × emotion was significant in the first run in the left amygdala, F(3,82)=5.50, p=.002, d=.52 and right, F(3,82)=4.59, p=.005, d=.47, but not in the second run (ps>.05). These results were similar when using contrast values extracted from the individual models with RT as a parameter (run 1, left amygdala: F(3,82)=3.06, p=.03; right amygdala: F(3,82)=3.83, p=.01). The interaction of group × emotion × run examining habituation across runs (from run 1 to run 2) was also not significant (ps>.05). Because the only significant interaction occurred within the first run, the following post-hoc tests and analyses refer to habituation within run 1 only.
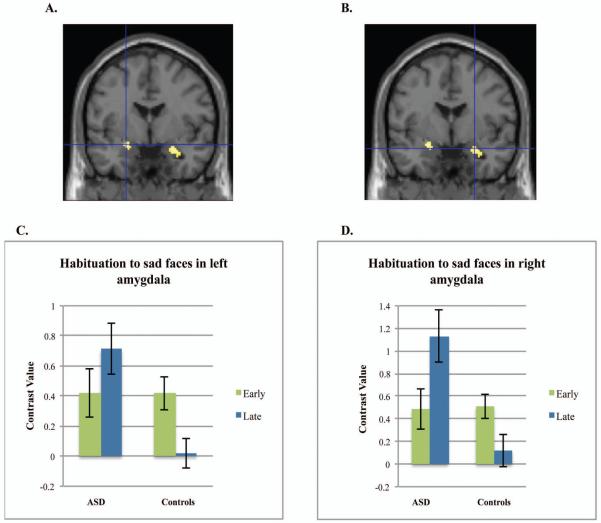
Post-hoc tests conducted in SPM for habituation to specific emotions in the first run indicated that the control and ASD groups differed in amygdala habituation to sad faces, t(86)=3.70, p=.007, d=.80, −24,−2,−14 (left amygdala) and t(86)=3.70, p=.007, d=.80, 26,−2,−18 (right), and neutral faces, t(86)=4.35, p=.001, d=.94, 20, −4, −20 (right), but not to fearful or happy faces (Figures 1 and 2). To characterize habituation within each group, we examined the contrast of early faces>late faces or early faces<late faces for each group separately. Controls alone demonstrated significant habituation to sad faces in the left amygdala, t(86)=3.92, p=.004, d=.85 and for neutral faces in the right amygdala, t(86)=3.59, p=.009, d=.77. For the contrast of early faces<late faces (increases over time), the ASD group demonstrated an increase in right amygdala activation to sad faces, t(86)=3.16, p=.032, d=.68 and neutral faces, t(86)=3.16, p=.030, d=.68. Neither group demonstrated significant habituation or increases in activation to fearful or happy faces, although habituation to happy faces approached significance for controls (p=.06, right amygdala; p=.08, left amygdala).
Figure 1.
Amygdala habituation to sad faces. Note: Controls demonstrate greater habituation to sad faces in left amygdala (A) and right amygdala (B) compared to the group with autism spectrum disorders (ASD). Figure shows the group difference of Controls>ASD for the contrast of sad early faces>sad late faces. Graphs represent the mean contrast values for early and late sad faces extracted from the left (C) or right amygdala (D) structural regions of interest. Error bars represent 1 S.E. All figures are thresholded at p<.001 uncorrected.
Figure 2.
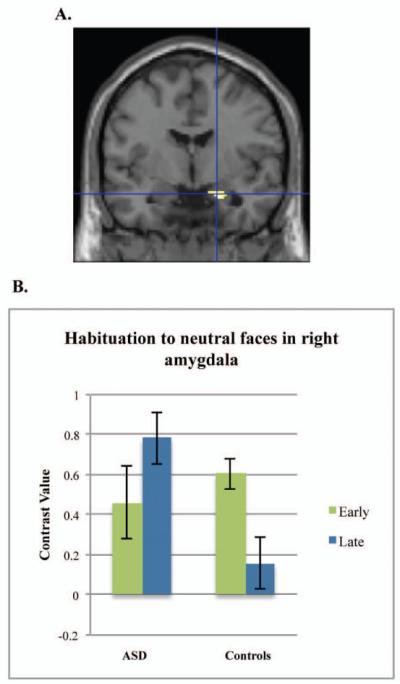
Amygdala habituation to neutral faces. Note: Controls demonstrate greater amygdala habituation to neutral faces compared to the group with autism spectrum disorders for the contrast of neutral early faces > neutral late faces in the right amygdala (A). Graph represents the mean contrast value extracted from the right amygdala region of interest (B).
Relation between amygdala habituation and ASD symptoms
We then tested our hypothesis that weaker amygdala habituation would predict greater autism severity in participants with ASD. There was a negative relation between SRS scores and habituation to neutral faces, t(30)=3.63, p=.019, −26, −2, −14, d=1.33 (left amygdala), indicating that decreased habituation related to greater autism severity.
Relation between amygdala habituation and anxiety
There were no significant correlations with anxiety in either group.
Psychophysiological interaction analysis and correlation with habituation
For the PPI we chose to use sad or neutral versus fearful faces as the psychological comparison because neither group demonstrated significant habituation to fearful faces. We did not include happy faces as a comparison because the controls approached significant habituation to these faces. Therefore, the PPI reflects modulation of amygdala connectivity for faces that resulted in a group difference in habituation (sad or neutral), relative to faces that did not result in habituation (fearful). Based on the results of our first analysis, we conducted the following PPI analyses: sad vs. fearful faces with a left amygdala seed, sad vs. fearful faces with a right amygdala seed, and neutral vs. fearful faces with a right amygdala seed. There was a group difference in left amygdala–vmPFC connectivity for sad vs. fearful faces, with the controls demonstrating increased connectivity during sad faces compared to the ASD group, t(86)=3.34, p=.037, d=.72, 6,30,−16. (Figure 3). Moreover, when left amygdala habituation to sad faces was entered as a regressor in the PPI analysis, there was a significant positive relation between amygdala habituation to sad faces and the PPI within the control group, t(54)=3.69, p=.018, d=1.00, −4, 20, −4, but not within the ASD group. The positive relation indicates that a stronger PPI within the vmPFC related to increased amygdala habituation to sad faces within the control group. There was no group difference in the PPI for right amygdala–vmPFC connectivity during sad or neutral versus fearful faces.
Figure 3.
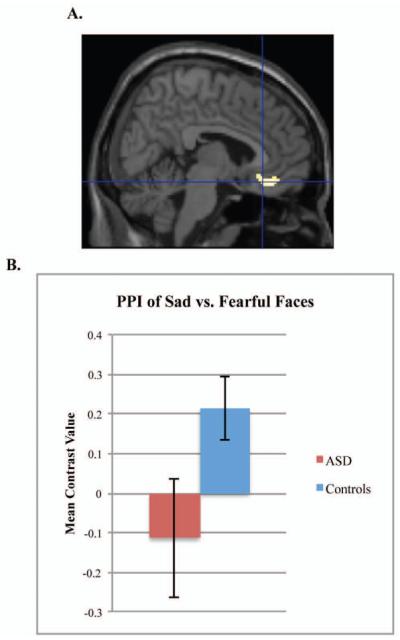
Group differences in psychophysiological interaction (PPI) for sad vs. fearful faces. Note: Participants with autism spectrum disorders (ASD) and controls show different patterns of connectivity. Figure demonstrates the group difference of Controls>ASD for the PPI of left amygdala–ventral prefrontal cortex connectivity for sad vs. fearful faces (A). Graph represents the mean contrast value of the PPI extracted from the Brodmann’s Area 25 structural region of interest (B).
DISCUSSION
We hypothesized that youth with ASD would show reduced habituation to faces compared to typically developing controls. In partial support of the hypothesis, the ASD group habituated less to sad and neutral faces than controls. Moreover, reduced amygdala habituation to neutral faces predicted greater autism severity as measured by the SRS. Additionally, the PPI indicated that the control group had increased amygdala–vmPFC connectivity while viewing sad faces compared to the ASD group and that the strength of this interaction predicted left amygdala habituation to sad faces within controls.
Analysis of the group differences in habituation provided two important insights into the nature of amygdala abnormalities in ASD. First, the participants with ASD did not habituate to sad and neutral faces, and post-hoc analyses demonstrated that amygdala activation increased significantly from the first to the second half of the run. This could indicate a sensitization effect in which the amygdala becomes increasingly responsive with repeated presentations of social stimuli; however, given that we did not hypothesize sensitization, future research will be necessary to confirm this result.
Second, reduced habituation was specific to sad and neutral faces. This emotion-specificity rules out alternative explanations for the results such as non-specific practice effects. In addition, it indicates that individuals with ASD may process sad and neutral faces differently than controls. Heightened amygdala activity could suggest that these expressions are aversive to individuals with ASD. Another possibility is that these faces were more ambiguous to participants with ASD.29 Future research will be necessary to test these possibilities and to address why neither group habituated to fearful or happy faces. It could be that youth are slower to habituate to fearful faces because they signal threat and happy faces since these are salient cues from peers.
Anxiety scores were not related to amygdala habituation in either group, but a potential limitation of the anxiety measure was that it was self-report. Future studies could incorporate measures that do not rely on insight (an area of impairment for those with ASD) such as skin conductance response in order to examine this further.
The PPI suggests that vmPFC-left amygdala connectivity differs in individuals with ASD compared to controls. The control group demonstrated an increase in connectivity while viewing faces to which they habituated (sad) compared to faces for which they did not habituate (fearful). The ASD group, in contrast, did not demonstrate this pattern of modulation. Additionally, there was a correlation between habituation to sad faces and the PPI within a slightly more dorsal region of the vmPFC within controls. These results provide preliminary support for the hypothesis that the vmPFC may be involved in habituation, although it is notable that this pattern was specific to sad faces and not found for neutral faces. It could be that vmPFC–amygdala connectivity is related to a process specific to sad faces, such as disambiguating these faces from other expressions.
There are several important limitations to this study. First, we were unable to detect a significant interaction in habituation for the second run. It is possible that the short rest period between runs did not allow sufficient time for the amygdala response to recover, and thus limited our ability to detect interaction effects for the second run. Nevertheless, the first run was similar in length to previous studies on habituation, and examining the runs separately allowed us to capture habituation that occurred quickly in controls.
Second, attrition in the ASD group was high. Although there were no differences in symptom severity between the included and excluded participants with ASD, the included participants had higher verbal and nonverbal IQ scores compared to the excluded participants, suggesting that these results may only generalize to high-functioning youth with ASD.
Third, although the task included several components to help ensure that participants were attending to faces, such as brief stimulus presentations and requiring a correct behavioral response, we were unable to measure participants’ eye gaze during scanning or assess whether there were group differences in fixation. Future research incorporating eye tracking will be important to test whether these relate to amygdala habituation.
Fourth, although we took steps to ensure that normalization of the images did not introduce bias, normalization could contribute noise to the data. Future studies could include other approaches, such as manual tracing of the amygdala or a functional localizer task, in order to overcome this limitation.
Finally, the PPI results do not indicate directionality or causation. Furthermore, whether connectivity is excitatory or inhibitory is not currently assessable through fMRI. Further research with more advanced techniques that can examine effective connectivity will be necessary in order to fully interpret the mechanisms underlying the group difference in PPI.
The results of this research have implications for clinical practice. The finding of reduced amygdala habituation to sad and neutral faces suggests that these expressions are processed differently in individuals with ASD, and may be perceived as more arousing or ambiguous than in controls. Moreover, reduced habituation to neutral faces related to more severe ASD symptoms. This suggests that potential routes for early intervention could include increasing emotion recognition or reducing over-arousal to faces through training or exposure. Better characterization of the mechanisms involved in abnormal amygdala activation in ASD may lead to improved understanding of how ASD develops and how to intervene to improve functioning.
Acknowledgments
This research was supported by Autism Speaks (C.S.M.) and the National Institutes of Health grants U19 HD035482 and MH066496 (C.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is discussed in an editorial by Dr. Tonya J.H. White on page xxx.
Data from this paper were presented as a poster at the 2012 meeting of the Society of Biological Psychiatry.
The authors thank Jeff Rosen of the University of Michigan for programming assistance. The authors also thank the University of Michigan Functional Magnetic Resonance Imaging Center for technical support and Nicole Cook and Samantha Ashinoff of the University of Michigan for data collection assistance. Finally, the authors thank the families for participation in this study.
Disclosure: Dr. Lord has received royalties from a publisher of diagnostic instruments described in this paper. She gives all profits generated by the University of Michigan Autism and Communication Disorders Center (UMACC) to a charity. Drs. Carrasco and Monk, Ms. Swartz, and Ms. Wiggins report no biomedical financial interests or potential conflicts of interest.
References
- 1.Howard MA, Cowell PE, Boucher J, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11(13):2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- 2.Weng SJ, Carrasco M, Swartz JR, et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corden B, Chilvers R, Skuse D. Avoidance of emotionally arousing stimuli predicts social-perceptual impairment in Asperger’s syndrome. Neuropsychologia. 2008;46(1):137–147. doi: 10.1016/j.neuropsychologia.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kliemann D, Dziobek I, Hatri A, Steimke R, Heekeren HR. Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. J Neurosci. 2010;30(37):12281–12287. doi: 10.1523/JNEUROSCI.0688-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph RM, Ehrman K, Mcnally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. J Int Neuropsychol Soc. 2008;14:947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- 7.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Monk C, Weng SJ, Wiggins JL, et al. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35(2):105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 11.Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 12.Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92(2):135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: A functional MRI study. Brain Res Bull. 2003;59(5):387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 14.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herry C, Bach DR, Esposito F, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27(22):5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherf KS, Behrmann M, Dahl RE. Facing changes and changing faces in adolescence: A new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev Cogn Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19(11):2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 21.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 22.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 23.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 24.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 25.Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36:545–566. doi: 10.1016/s0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 26.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 28.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace GL, Case LK, Harms MB, Silvers JA, Kenworthy L, Martin A. Diminished sensitivity to sad facial expressions in high functioning autism spectrum disorders is associated with symptomatology and adaptive functioning. J Autism Dev Disord. 2011;41:1475–1486. doi: 10.1007/s10803-010-1170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]