Abstract
Objective
To investigate the direct effects of prenatal cocaine exposure (PCE) on adolescent drug use, while controlling for other predictors of adolescent use.
Method
Data are from a longitudinal study of PCE in which women and their offspring were assessed throughout childhood. Adolescents were interviewed at 15 years about their age at initiation of alcohol, marijuana, and tobacco. The sample consisted of 214 adolescents and their caregivers. Fifty percent of the sample was Caucasian, 50% African American.
Results
First trimester cocaine exposure significantly predicted earlier adolescent marijuana and alcohol initiation. The hazard of marijuana and alcohol initiation among exposed adolescents was almost two times higher than among non-exposed adolescents, adjusting for other significant factors. There were no differences in tobacco initiation. Other significant predictors of adolescent drug use were family history of alcohol problems, exposure to violence, and childhood maltreatment.
Conclusions
Cocaine exposure during early pregnancy was associated with initiation of marijuana and alcohol use. Exposure to violence, childhood maltreatment, and familial factors also predicted adolescent initiation, but did not mitigate the effects of PCE. The combination of these risk factors has significant implications for the development of later substance use, social, and psychiatric problems.
Keywords: prenatal cocaine, adolescent, alcohol, marijuana
Substance use is prevalent among adolescents, with national surveys reporting rates of alcohol and marijuana use ranging from 29 to 50%.1,2 This is concerning because early onset of substance use is a risk factor for substance use disorder (SUD),3,4 transition from onset of use to SUD is more rapid for those who initiate use earlier,5,6 and early substance use is associated with later delinquency, school dropout, and criminal behaviors.7,8 An important predictor of the early onset of substance use is prenatal drug exposure.9–11 Prenatal cocaine exposure (PCE) has been reported to occur in from 1 to 18% of pregnancies,12 but the effects of PCE on adolescent substance use have not been well-studied and the results are conflicting. Two studies of the effects of PCE on adolescent drug use found no relation between PCE and onset of drug use by 1113 or 12½14 years of age. By contrast, Frank et al.15 found that PCE was associated with initiation of any substance by age 16 and Delaney-Black et al.16 reported that PCE was associated with cocaine use by 14-year-old offspring.
There may be several mechanisms by which prenatal drug exposure affects adolescent substance use. The effects may result from changes in brain circuitry or prefrontal cortex-controlled processes such as learning/memory, attention, or executive function.17,18 In addition, the effects of prenatal drug exposure on adolescent substance use may be due to intervening familial, parenting, or environmental characteristics.9 For example, risk factors for adolescent substance use include childhood conduct disorder,7 attention deficit/hyperactivity,19,20 and aggression,21,22 parental substance use,23,24 unstructured home environment,25 poor quality parenting practices,9 low academic achievement,26 pubertal development,27 peer alcohol and tobacco use,28,29 depression,30 and childhood abuse and violence exposure.31 These factors may be part of the pathway between prenatal exposure and adolescent use.
The aim of this report was to explore pathways between PCE and adolescent drug use. First, we hypothesized that PCE would be directly associated with an increased risk of adolescent drug use, controlling for the effects of other risk factors for adolescent drug use, including other prenatal exposures. However, in earlier analyses, we found that PCE was associated with behavior and mood changes at 10 years of age32 and with childhood maltreatment.33 These characteristics are associated with adolescent substance use and therefore we also investigated whether they would serve as a pathway between PCE and adolescent substance use.
METHOD
Study Design
The women and their offspring are participants in a longitudinal investigation of the effects of PCE. The Research Review and Human Experimentation Committee of Magee-Womens Hospital (MWH) and the University of Pittsburgh’s Institutional Review Board approved this research. Written informed consent was obtained. A Department of Health and Human Services’ Confidentiality Certificate assured that participants’ responses could not be subpoenaed.
Women ≥ 18 years old were approached in the MWH prenatal clinic during their fourth or fifth prenatal month. They were asked about cocaine/crack, alcohol, tobacco, marijuana, and other illicit drug use in the year prior to pregnancy and the first trimester. All women who reported any cocaine/crack use during the first trimester were enrolled, along with the next woman interviewed who reported no cocaine/crack use during both the year prior to pregnancy and the first trimester. Those selected were interviewed during the 7th prenatal month and at 24 hours post-delivery about substance use during the second and third trimesters, respectively. All newborns were examined by research nurses unaware of exposure status. Follow-up assessments occurred at 1, 3, 7, 10, and 15 years postpartum.
The outcome for the current analysis is the 15-year-olds’ report about their substance use, including alcohol (beer, wine, liquor), marijuana, and tobacco. Although we asked about glue sniffing, LSD, and cocaine/crack, we did not analyze these due to low frequencies. Lifetime use, use in the past year, and age of initiation of use were assessed. The questions were from the Health Behavior Questionnaire34 and have been shown to have good construct validity.35 The form was completed by the adolescents in a private setting. Their responses were kept confidential from their parents.
Other adolescent measures included the Pubertal Development Scale (PDS),36 which assesses the adolescents’ perception of their pubertal development relative to their peers, and the Childhood Trauma Questionnaire (CTQ),37 which measures physical and emotional abuse and neglect and sexual abuse. This was scored as the cumulative total of all subscales for which the score was above the cut-point for “moderate to severe abuse”. The Screen for Adolescent Violence Exposure (SAVE)38 was used to assess direct exposure to personal violence such as having been shot, beaten, hurt by a knife; the variable was a total count of incidents. “My Parents”39 has two subscales; the involvement subscale reflects the adolescent’s view of the parent as loving, responsive, and involved, and the supervision subscale assesses their view of parental monitoring and limit setting. The Wechsler Intelligence Scale for Children Third Edition (WISC-III)40 vocabulary and block design short form was used to estimate IQ.41
Measures at 10 years were used to determine whether earlier child characteristics mediated the relationship between PCE and 15-year substance use. These included the Child Behavior Checklist/4–18 (CBCL),42 completed by the caregivers. The internalizing, externalizing, and total behavior problem scales were used in the analyses. The Stanford-Binet Intelligence Scale Fourth Edition43 composite score was used as the measure of intelligence and academic achievement was measured with the Wide Range Achievement Test Third Edition (WRAT-3)44. The total score from the Children’s Depression Inventory (CDI),45 a self-report measure of depressive symptoms, was also included.
At the 15-year follow-up, interviewers asked the mothers structured questions about their sociodemographic and psychosocial characteristics, substance use during the last year, and family history of substance use problems. The Center for Epidemiological Studies–Depression Scale (CES-D)46 was used to assess depression and the Spielberger State-Trait Anxiety Inventory (STAI)47 was used to measure hostility. The interview version of the HOME48 assessed the home environment including availability of reading materials, frequency of television viewing, and types of discipline tactics.
Sample Characteristics
Recruitment occurred between 1988 and 1992; 90% of the women approached agreed to participate, and 320 women met the inclusion criteria and were enrolled into the study. Between enrollment and delivery, 20 subjects were eliminated because of home delivery, miscarriage/abortion/fetal death, moved, lost to follow-up, and refused. Four pairs of twins and one child with Trisomy 21 were excluded from additional follow-up, resulting in a birth cohort of 295 mothers and infants.
By 15 years, 76 subjects were eliminated: 6 children died, 3 mothers lost custody and the children could not be traced, 17 families moved out of the area, 19 mothers refused to participate, and 31 were lost-to-follow-up. These 219 subjects represented 74% of the birth cohort. For the 18% of adolescents not in maternal custody at 15 years, the current caretaker was interviewed.
Five subjects who did not complete the adolescent drug use questionnaire were excluded from the analyses, resulting in an analysis cohort of 214 mothers and adolescents. The women not in this analysis (n=81) had less education (11.7 vs. 12.0 years, p = .02), were less likely to work (33 vs. 46%, p = .05), used more marijuana first trimester (0.49 vs. 0.16 joints/day, p = .02), and were less likely to use cocaine second trimester (1 vs. 10%, p = .02) than those who were included (n=214). There were no other significant differences in maternal sociodemographic, prenatal substance use, or infant birth characteristics between those who were and were not included in this analysis.
At the 15-year phase, the women were, on average, 42.4 years old (range = 33–75), 50% were Caucasian, and 50% were African American. Women had a median family income of $2,000 per month (range = $0–$12,083) and an average education of 13.1 years (range = 9–20). Forty-two percent were married, 52% had a man living in the household, and 71% worked and/or attended school. The mean CES-D score was 40 (range = 21–65) and the mean levels of current alcohol, marijuana, and tobacco use were 0.73 drinks/day (range = 0–9), 0.1 joints/day (range = 0–6.8), and 6.6 cigarettes/day (range = 0–40), respectively.
Fifty-two percent of the offspring were males. The median age at assessment was 15.3 years (range = 14.7–18.0) and the median grade in school was 9th grade. At this phase, 37% (n = 79) of the adolescents had initiated tobacco use, 27% (n = 57) marijuana use, and 42% (n = 91) alcohol use. Mean ages of onset for these drugs were 12, 13, and 13.5 years, respectively. Use during the past year was reported by 21% of the adolescents for tobacco, 21% for marijuana, and 36% for alcohol.
Data Analysis
First trimester cocaine use was defined as use/no use and as frequent use (≥ 1 line/day) versus non-frequent use (< 1 line/day). Cocaine use was dichotomized into use/no use for the second and third trimesters and at 15 years postpartum because of low frequencies during those times. Cocaine and crack use were reported in lines, rocks, or grams.
The maternal alcohol and marijuana variables were average number of drinks or joints per day, respectively. These were log transformed to reduce skewness. Tobacco use was number of cigarettes per day. Alcohol, marijuana, and tobacco use were ascertained for each trimester of pregnancy and at 15 years postpartum. They were used as continuous variables in the analyses, with the exception of 15-year maternal marijuana use, which was dichotomized because of low frequency. Details about calculation of first trimester substance use variables have been published.49 The adolescent outcomes were age of initiation of alcohol, marijuana, and tobacco, and use (yes/no) for each of the three drugs over the past year. Adolescents’ reports of peer substance use were highly correlated with their own use and were not included in these analyses.
The relations between PCE and adolescent substance use variables were first examined bivariately. Variables were considered for inclusion in the models based on the literature and prior analyses of these data, and on their associations with PCE or the outcomes (Table 1). Cox proportional hazards regression analysis was then used to assess the effects of PCE on adolescent age of substance use initiation, controlling for other significant predictors. Covariates were examined hierarchically to avoid saturation of the model. Within each block, the covariates were selected in a stepwise manner to avoid multicollinearity. The trimester-specific PCE variable was entered in the model in the last step. Logistic regression models were used for “use in past year” to adjust for differences between the exposed and non-exposed groups.
Table 1.
Identification of significant covariates associated with age of initiation of marijuana, alcohol, and tobacco using Cox proportional hazard regression.
| Hazard Ratiosa (Confidence intervals) | |||
|---|---|---|---|
| Marijuana | Alcohol | Tobacco | |
| Block 1: Prenatal substance use | |||
| 1st trimester alcoholb (drinks/day) | 1.06 (.95–1.18) | 1.06 (.97–1.16) | 0.92 (.79–1.06) |
| 1st trimester marijuanab (joints/day) | 1.23 (.95–1.61) | 1.05 (.74–1.48) | 1.00 (.69–1.44) |
| 1st trimester tobaccob (cigarettes/day) | 1.03* (1.01–1.06) | 1.02† (1.00–1.04) | 1.02* (1.01–1.04) |
| 2nd trimester alcoholb (drinks/day) | 0.99 (.76–1.29) | 1.07 (.90–1.26) | 0.89 (.64–1.23) |
| 2nd trimester marijuanab (joints/day) | 3.26 (.89–12.00) | 1.00 (.21–4.71) | .71 (.11–4.75) |
| 2nd trimester tobaccob (cigarettes/day) | 1.02 (.99–1.05) | 1.02 (1.00–1.04) | 1.02 (1.00–1.04) |
| 3rd trimester alcoholb (drinks/day) | 1.21 (.98–1.48) | 0.95 (.74–1.23) | 0.80 (.53–1.22) |
| 3rd trimester marijuanab (joints/day) | 2.26* (1.29–3.97) | 2.59* (1.16–5.78) | 2.63* (1.12–6.15) |
| 3rd trimester tobaccob (cigarettes/day) | 1.02† (1.00–1.05) | 1.02† (1.00–1.04) | 1.01 (.99–1.03) |
| Block 2: Family history of: | |||
| Alcohol problemsb (yes=1, no=0) | 2.03* (1.12–3.65) | 2.02** (1.28–3.20) | 1.40 (.88–2.23) |
| Drug problemsb (yes=1, no=0) | 1.80* (1.07–3.04) | 1.56* (1.03–2.36) | 1.61* (1.04–2.51) |
| Block 3: 10-year child characteristics | |||
| Stanford-Binet Composite Score | 1.01 (.99–1.03) | 1.02** (1.01–1.03) | 1.00 (.98–1.01) |
| WRAT-3 Reading | 0.99 (.97–1.01) | 1.01* (1.001–1.03) | 1.00 (.99–1.02) |
| WRAT-3 Spelling | 1.00 (.98–1.02) | 1.03*** (1.01–1.04) | 1.00 (.99–1.02) |
| WRAT-3 Math | 1.01 (.99–1.03) | 1.02** (1.01–1.03) | 1.00 (.98–1.01) |
| Children’s Depression Inventoryb | 1.02 (.98–1.05) | 1.03* (1.01–1.05) | 1.05*** (1.02–1.07) |
| CBCL total behavior problems | 1.01 (.99–1.04) | 1.00 (.98–1.02) | 1.03** (1.01–1.05) |
| CBCL externalizing problemsb | 1.02 (.99–1.05) | 1.00 (.98–1.02) | 1.04** (1.01–1.06) |
| CBCL internalizing problems | 0.99 (.97–1.02) | 1.01 (.99–1.03) | 1.00 (.98–1.03) |
| Block 4: Traumatic events | |||
| Childhood Trauma Questionnaireb | 1.68*** (1.29–2.18) | 1.36** (1.09–1.70) | 1.38* (1.08–1.77) |
| Exposure to violence (SAVE)b | 1.60*** (1.37–1.87) | 1.16† (.99–1.36) | 1.21* (1.02–1.43) |
| Block 5: 15-year characteristics | |||
| Maternal sociodemographics | |||
| Age | 1.02 (.98–1.06) | 1.00 (.97–1.03) | 0.99 (.95–1.02) |
| Education | 1.06 (.90–1.24) | 1.02 (.89–1.16) | 1.02 (.89–1.17) |
| Work/school status (yes=1; no=0) | 0.99 (.56–1.75) | 1.31 (.82–2.10) | 1.04 (.64–1.70) |
| Family incomeb | 0.98** (.96–.99) | 1.00 (.99–1.01) | 0.99 (.98–1.01) |
| Raceb (white=1, African American=0) | 0.65 (.39–1.11) | 1.62* (1.06–2.48) | 1.48† (.94–2.32) |
| Marital statusb (married=1, all other=0) | 0.36*** (.20–.68) | 0.83 (.54–1.28) | 0.67† (.42–1.06) |
| Maternal psychosocial characteristics | |||
| Depression (CES-D) | 1.02† (1.00–1.05) | 1.01 (.99–1.03) | 1.01 (.99–1.03) |
| Hostility (STAI) | 1.04 (.99–1.09) | 1.02 (.98–1.06) | 1.04 (1.00–1.08) |
| Life eventsb | 1.10* (1.02–1.19) | 1.05 (.98–1.11) | 1.00 (.93–1.08) |
| Support from relatives and friends | 0.98 (.87–1.11) | 0.95 (.86–1.04) | 0.98 (.88–1.08) |
| Coping ability | 0.75 (.52–1.07) | 0.85 (.63–1.15) | 0.84 (.61–1.15) |
| Home environmentb | 0.84*** (.76–.92) | 0.94 (.87–1.01) | 0.94 (.87–1.02) |
| Maternal substance use | |||
| Cocaine useb (yes=1; no=0) | 3.37** (1.65–6.89) | 1.49 (.68–3.24) | 0.72 (.26–1.97) |
| Alcoholb (drinks/day) | 1.09 (.94–1.26) | 1.08 (.96–1.21) | 0.97 (.83–1.13) |
| Marijuana useb (yes=1; no=0) | 1.88† (.97–3.63) | 1.03 (.55–1.95) | 1.22 (.65–2.32) |
| Tobaccob (cigarettes/day) | 1.04* (1.01–1.07) | 1.02* (1.01–1.05) | 1.03** (1.01–1.06) |
| Child characteristics | |||
| Genderb (male=1; female=0) | 0.79 (.47–1.33) | 0.69† (.46–1.04) | 0.77 (.50–1.20) |
| Pubertal developmentb (early=1, not=0) | 0.74† (.53–1.03) | 0.64** (.50–.84) | 0.87 (.66–1.16) |
| Maternal custodyb (no=1, yes=0) | 2.29** (1.30–4.03) | 1.50 (.92–2.44) | 1.76* (1.05–2.95) |
| Parental involvement | 0.97 (.91–1.03) | 0.96 (.92–1.01) | 0.96 (.92–1.01) |
| Parental supervisionb | 0.89*** (.83–.95) | 0.92** (.88–.97) | 0.96 (.91–1.02) |
| WISC-III short form | 0.99 (.98–1.01) | 1.01 (1.00–1.03) | 1.00 (.99–1.01) |
| Block 6: Prenatal cocaine exposure | |||
| 1st trimester cocaineb (yes=1; no=0) | 2.67*** (1.56–4.59) | 1.66* (1.09–2.53) | 1.42 (.91–2.21) |
| 2nd trimester cocaineb (yes=1; no=0) | 2.25* (1.05–4.82) | 1.57 (.78–3.15) | 0.50 (.18–1.37) |
| 3rd trimester cocaineb (yes=1; no=0) | 2.37* (1.24–4.50) | 0.84 (.40–1.74) | 1.27 (.65–2.47) |
Note: CBCL = Child Behavior Checklist; CES-D = Center for Epidemiological Studies–Depression Scale; SAVE = Screen for Adolescent Violence Exposure; STAI = State-Trait Anxiety Inventory; WRAT-3 = Wide Range Achievement Test Third Edition.
Hazard ratio: > 1 represents a positive relation and < 1 indicates a negative relation
Variable retained in each of the 3 final, reduced models
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.10
We also evaluated whether there were mediating effects of 10-year depressive symptoms or behavior problems, or of childhood maltreatment or exposure to violence on the 15-year outcomes. In logistic regression, mediation is evaluated through path analysis using the product of coefficients.50 For Cox proportional hazards models, the joint distribution of the coefficients is unknown and tests of mediation may diverge.51 Therefore, for initiation of substance use, mediation was tested by comparing two models, one with and one without the mediator in the model, and by examining the significance of the direct effect after inclusion of the potential mediator.
RESULTS
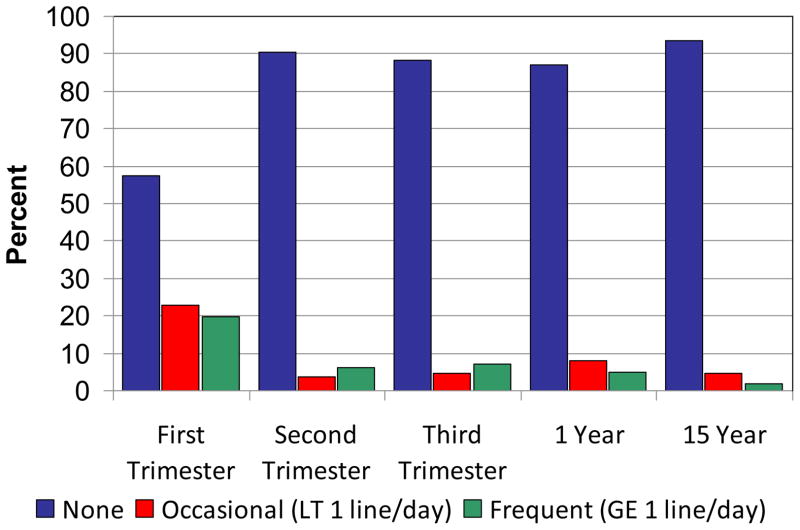
During the first trimester, 19.6% of the women were frequent users (≥ 1 line of powder cocaine per day, or the equivalent in crack) (Figure 1). By the third trimester, 7.1% of the women were frequent users. Only 7% of the women who used in the first trimester also used second and third trimesters. Mean cocaine use for the women who used first trimester was 0.26 g/day (~9 lines/day; range = < 1 line/month–4 g/day). The mean levels of use for the second and third trimester users were 0.18 g/day (~6 lines/day; range = < 1 line/month–1 g/day) and 0.17 g/day (~6 lines/day; range = < 1 line/month–2 g/day), respectively. At the 15-year follow-up, 1.9% of the women were frequent users. The mean level of cocaine among the users was 0.1 g/day (~3 lines/day; range = < 1 line/month–1 g/day).
Figure 1.
Maternal cocaine use by categories of use across phases. Note: GE = greater than or equal to; LT = less than.
Women who used cocaine during the first trimester were significantly older (26 vs. 24 years, p < .01), more likely to be African American (58 vs. 43%, p < .05) and single (89 vs. 71%, p < .01), and less likely to work or attend school (36 vs. 53%, p < .02) at the first prenatal assessment than those who did not use first trimester. First trimester cocaine users also used more alcohol (1.9 vs. 0.3 drinks/day, p < .001), tobacco (10 vs. 6 cigarettes/day, p < .001), and marijuana (0.3 vs. 0.04 joints/day, p < .01) during the first trimester than did first trimester non-cocaine users. First trimester cocaine users were also more likely to use drugs at the 15-year phase compared to first trimester non-users (1 vs. 0.5 drinks/day, p < .05; 18 vs. 8% used marijuana, p < .05; 13 vs. 2% used cocaine, p < .01). The offspring of first trimester cocaine users were more likely to be in non-maternal custody at 15 years than offspring who were not exposed (28 vs. 11%, p < .01). There were no differences in current parenting characteristics, education, work, or coping abilities.
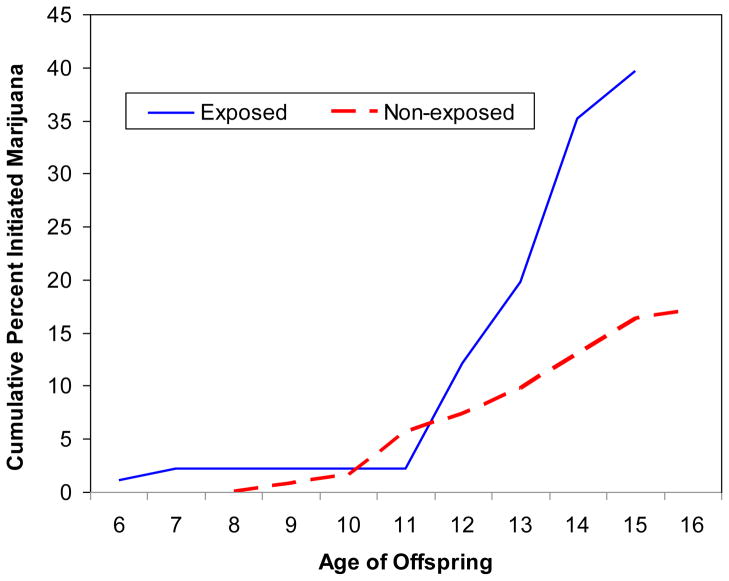
The cumulative percents of marijuana initiation for offspring exposed and not exposed to first trimester cocaine are shown in Figure 2. The rates of initiation for these two groups began to diverge at about age 12 and the gap between the two groups widened over time. At 13 years, marijuana initiation among adolescents prenatally exposed to cocaine was double that of their non-exposed peers (19.8 vs. 9.7%). By 15 years, 39.6% of the exposed adolescents had initiated marijuana use compared to 16.3% of non-exposed adolescents.
Figure 2.
Age of marijuana initiation among offspring exposed and not exposed to cocaine first trimester.
Frequent use did not predict any of the outcomes, so only use/no use results are reported. First trimester cocaine use was significantly related to age of marijuana initiation, controlling for the significant covariates in Table 1. The hazard of marijuana initiation among adolescents who were exposed was almost two times higher than those who were not exposed, adjusting for other significant factors (Table 2). Family history of alcohol problems, exposure to violence, and less adequate home environment were also significantly related to marijuana initiation.
Table 2.
Effects of prenatal cocaine exposure on age of initiation of marijuana, alcohol, and tobacco using the Cox Proportional Hazards Model and controlling for covariates.
| Coefficient | Hazard ratio [95% CI] | Significance (p) | |
|---|---|---|---|
| Age of marijuana initiation | |||
| Family history of alcohol problems | 0.7 | 2.02[1.08–3.8] | 0.02 |
| Exposure to violence | 0.48 | 1.61[1.35–1.93] | 0.000 |
| Home environment | −0.16 | 0.85[0.77–0.94] | 0.002 |
| First trimester cocaine (yes/no) | 0.59 | 1.80[1.02–3.2] | 0.04 |
| Age of alcohol initiation | |||
| Family history of alcohol problems | 0.63 | 1.88[1.15–3.08] | 0.009 |
| Race (white=1, African American=0) | 0.65 | 1.92[1.22–3.05] | 0.004 |
| Pubertal development (early=1, not=0) | −0.41 | 0.66[0.51–0.87] | 0.003 |
| Parental supervision | −0.08 | 0.93[0.87–0.98] | 0.01 |
| First trimester cocaine (yes/no) | 0.59 | 1.80[1.14–2.84] | 0.01 |
| Age of tobacco initiation | |||
| 10-year CDI | 0.03 | 1.03[1.01–1.06] | 0.008 |
| 10-year CBCL externalizing | 0.03 | 1.03[1.01–1.05] | 0.02 |
| Current maternal smoking | 0.04 | 1.04[1.01–1.05] | 0.005 |
| First trimester cocaine (yes/no) | 0.28 | 1.33[0.86–2.06] | 0.21 |
Note: CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory.
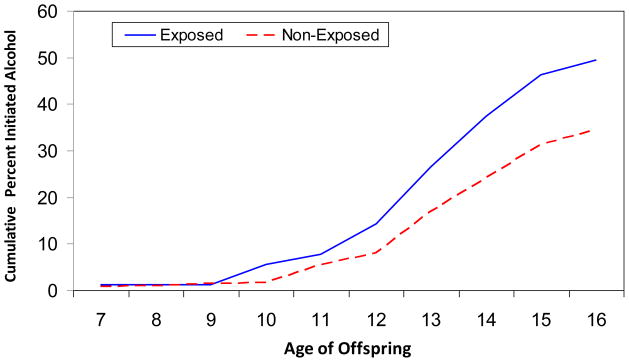
The divergence in alcohol initiation trajectories between first trimester cocaine exposed and non-exposed offspring occurred by age 10 (Figure 3). Alcohol initiation by 13 years among those who were exposed was 1.5 times that of their non-exposed peers (26.4 vs. 16.9%) and by 15 years, 46.2% of the exposed adolescents compared to 31.4% of non-exposed adolescents had initiated alcohol use. First trimester cocaine exposure was a significant predictor of alcohol initiation in the multivariate analysis with a hazard ratio of 1.8 (Table 2). Other predictors of alcohol initiation were family history of alcohol problems, Caucasian race, early pubertal development, and lower levels of parental supervision.
Figure 3.
Age of alcohol initiation among offspring exposed and not exposed to cocaine first trimester.
The rates of tobacco initiation by ages 13 and 15 among adolescents who were cocaine-exposed were 33% and 41.8%, respectively, compared to rates of 23.6% and 31.7% among those who were unexposed. However, there was no significant relation between first trimester cocaine exposure and age of tobacco initiation (Table 2). Child depressive symptoms and externalizing behavior problems at 10 years and current maternal smoking significantly predicted tobacco initiation.
First trimester cocaine use was significantly related to adolescent marijuana use in the past year (Table 3). Thirty-one percent of the exposed adolescents used marijuana in the past year compared to 14% of the non-exposed adolescents. Current adolescent marijuana use was also predicted by childhood maltreatment, exposure to violence, and home environment. First trimester PCE was not related to adolescents’ current alcohol or tobacco use. Current alcohol use was predicted by 10-year depressive symptoms, Caucasian race, and lower parental supervision. Current tobacco use was predicted by childhood maltreatment, female gender, and current maternal tobacco use.
Table 3.
Effects of prenatal cocaine exposure on past year use of marijuana, alcohol, and tobacco using the logistic regression model and controlling for covariates.
| Coefficient | Adjusted odds ratio [95% CI] | Significance (p) | |
|---|---|---|---|
| Marijuana use past year | |||
| Childhood maltreatment | 0.84 | 2.3 [1.35–3.97] | 0.001 |
| Exposure to violence | 0.61 | 1.85 [1.28–2.66] | 0.000 |
| Home environment | 0.25 | 0.78 [0.67–0.92] | 0.001 |
| First trimester cocaine (yes/no) | 1.0 | 2.71 [1.18–6.21] | 0.009 |
| Alcohol use past year | |||
| 10-year CDI | 0.04 | 1.05 [1.01–1.09] | 0.02 |
| Race (white=1, African American=0) | 0.97 | 2.65 [1.4–5.0] | 0.003 |
| Parental supervision | 0.12 | 0.89 [0.82–0.96] | 0.005 |
| First trimester cocaine (yes/no) | 0.18 | 1.20 [0.64–2.25] | 0.58 |
| Tobacco use past year | |||
| Childhood maltreatment | 0.46 | 1.58 [1.02–2.44] | 0.04 |
| Gender (male=1; female=0) | 1.02 | 0.36 [0.17–0.78] | 0.01 |
| Current maternal tobacco use | 0.06 | 1.06 [1.02–1.10] | 0.008 |
| First trimester cocaine (yes/no) | 0.26 | 1.30 [0.62–2.72] | 0.49 |
Note: CBCL = Child Behavior Checklist; CDI = Child Depression Inventory.
There were no significant effects of second or third trimester PCE on age of initiation of marijuana, alcohol, or tobacco, or on use during the past year.
In the mediation analyses, 10-year CDI and CBCL externalizing problem scores were not significantly related to 15-year substance use and therefore could not mediate the effects of PCE on substance use. Exposure to violence was a partial mediator of marijuana initiation: Chi-square fit dropped by 5.5 points (significant compared to χ21, α=0.05) when exposure to violence was added to the model. Mediation based on product of coefficients was also significant (coefficient=0.2, p=0.01). However, first trimester cocaine exposure remained a significant predictor of marijuana initiation with violence exposure in the model. In the logistic regression model, there was a significant mediation of first trimester cocaine exposure on adolescent marijuana use through exposure to violence (coefficient of indirect effect= 0.16, p < 0.05), but the direct effect of PCE remained significant (Table 3). Childhood maltreatment was not a significant mediator of the relation between PCE and adolescent use (estimated coefficient of indirect effect=0.07, p =0.2); the direct effect of PCE remained significant.
DISCUSSION
We investigated the effects of prenatal cocaine exposure on adolescent substance use in a prospective, longitudinal study. Consistent with our hypothesis, adolescents prenatally exposed to cocaine were about twice as likely to initiate marijuana and alcohol use by 15 years compared to their non-exposed peers. The odds of exposed adolescents using marijuana in the past year were about three times larger than those of non-exposed adolescents. These findings are consistent with those of Frank et al.15 who found that PCE was related to substance use initiation in a similar age population. Delaney-Black et al.16 investigated offspring cocaine use only: We did not have enough adolescents who used cocaine so the results cannot be compared.
We investigated potential mediators of the relation between PCE and adolescent substance use and found that child behavior problems and depressive symptoms at 10 years did not mediate the relation between PCE and drug use. Exposure to violence partially mediated the association between PCE and adolescent substance use. However, the direct effect of PCE on adolescent use remained significant in our analyses, as well as in those of Frank et al.15
Adolescent marijuana and alcohol initiation and use were predicted by factors such as family history of alcohol problems, less adequate home environment and parental supervision, early pubertal development, childhood maltreatment, and exposure to violence. Each of these factors has previously been reported to be associated with adolescent substance use, highlighting the consistency of our findings. Glantz and Chambers9 suggested that the effects of prenatal exposure on adolescent substance use may be due to intervening familial (substance use, psychopathology), parenting, child maltreatment, and/or environmental characteristics. However, we have shown that PCE predicted adolescent substance use over and above these factors. Thus, while these factors are important when they are considered in the analysis, the effects of PCE remain significant. Basic research in animal samples will be necessary to further elucidate the exact mechanisms by which PCE affects adolescent substance use.17
There are several potential limitations of the study. First trimester cocaine exposure predicted adolescent substance use, whereas second and third trimester use did not. It is possible that we did not have adequate power to detect the effects of second and third trimester use. A second limitation is that there may have been unidentified factors that impact adolescent drug use. For example, we did not consider peer use because it was too highly correlated with adolescent use, nor did we include drug availability in the school or neighborhood, as that was not assessed. We also did not include maternal psychiatric diagnoses, although we did include measures of maternal psychological symptoms. Another potential limitation is that biological measures were not used to document drug use. It is possible that some women denied drug use and were misclassified. However, this would attenuate any differences between groups and would not affect the significant findings. Cocaine use is often not identified by biological screening because of the short time period for detection.52 Moreover, our interviews identified a higher percentage of users than did urine screening,53 a finding also reported by others.54 Detailed, confidential interviewing is an effective way to identify users and is the only cost-effective way to characterize the timing and pattern of use across pregnancy.52
Although most studies have used populations with exposure to high levels of cocaine, often throughout pregnancy, we studied offspring exposed to lower levels of cocaine, mostly during early pregnancy. These levels and patterns of exposure represent the modal use of cocaine during pregnancy and thus our findings have significant public health implications. Consistency across developmental time points,32,49,55 and with other human and animal studies, validates the results we found with lower ranges of exposure. Additional strengths of our study include a balanced racial composition, detailed trimester-specific measures of substance use, good follow-up rates, and careful assessment of and control for other predictors of adolescent drug use.
By 15 years, almost 40% of the youths who were prenatally exposed to cocaine had initiated marijuana use and 46% had initiated alcohol use. Early onset of use was also associated with family history of substance use problems, violence exposure, and childhood maltreatment. The combination of these risk factors has significant implications for the development of later problems. These exposed adolescents are at significant risk for substance use problems, and behavioral, social, and psychiatric difficulties as they mature.7,8,56 As our sample reaches adulthood, we will be able to study whether offspring who were exposed to cocaine prenatally were more likely to continue or escalate use after adolescence compared to non-exposed offspring and to investigate heavier levels of use and problematic drug use to determine if different factors affect that developmental period.9
Clinical Guidance.
Substance use is prevalent in adolescence and is a risk factor for later behavioral and psychiatric problems.
Prenatal cocaine exposure is an important predictor of adolescent substance use, even in a sample of offspring who were exposed to lower levels of cocaine use early in pregnancy.
At 15 years, 40% of those offspring who were prenatally exposed to cocaine had initiated marijuana use and 46% had initiated alcohol use compared to 16% and 31% of non-exposed offspring, respectively.
Prenatal cocaine exposure predicted adolescent substance use over and above factors such as family substance use, parental supervision, childhood maltreatment, and exposure to violence, suggesting a direct effect of prenatal exposure on adolescent substance use.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA05460, DA12401, DA08916 (G.A.R., PI).
Dr. Goldschmidt served as the statistical expert for this research.
Footnotes
Clinical guidance is available at the end of this article.
Disclosure: Drs. Richardson, Larkby, Goldschmidt, and Day report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Gale A. Richardson, University of Pittsburgh School of Medicine
Dr. Cynthia Larkby, University of Pittsburgh School of Medicine
Dr. Lidush Goldschmidt, University of Pittsburgh Medical Center
Dr. Nancy L. Day, University of Pittsburgh School of Medicine
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. I. Ann Arbor MI: Institute for Social Research, University of Michigan; 2011. Monitoring the Future national survey results on drug use, 1975–2010. [Google Scholar]
- 2.Wu L-T, Woody GE, Yang C, Pan JJ, Blazer DG. Racial/ethnic variations in substance-related disorders among adolescents in the United States. Arch Gen Psychiatry. 2011;68:1176–1185. doi: 10.1001/archgenpsychiatry.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C-Y, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiologic evidence from the United States: 2000–2001. Drug Alcohol Depend. 2005;79:11–22. doi: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 4.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 5.Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Lynsky MT, Heath AC, Bucholz KK, et al. Escalation of drug use in early-onset cannabis users vs. co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DM, Boden JM, Horwood JL. The developmental antecedents of illicit drug use: evidence from a 25-year longitudinal study. Drug Alcohol Depend. 2008;96:165–177. doi: 10.1016/j.drugalcdep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Green KM, Doherty EE, Stuart EA, Ensminger ME. Does heavy adolescent marijuana use lead to criminal involvement in adulthood? Evidence from a multiwave longitudinal study of urban African Americans. Drug Alcohol Depend. 2010;112:117–125. doi: 10.1016/j.drugalcdep.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glantz M, Chambers J. Prenatal drug exposure effects on subsequent vulnerability to drug use. Dev Psychopathol. 2006;18:893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- 10.Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- 11.Day N, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–22. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- 12.Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicol Teratol. 2009;31:325–333. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaGasse L, Hammond J, Liu J, et al. Violence and delinquency, early onset drug use, and psychopathology in drug-exposed youth at 11 years. Ann NY Acad Sci. 2006;1094:313–318. doi: 10.1196/annals.1376.041. [DOI] [PubMed] [Google Scholar]
- 14.Warner T, Behnke M, Eyler F, Szabo N. Early adolescent cocaine use as determined by hair analysis in a prenatal cocaine exposure cohort. Neurotoxicol Teratol. 2011;33:88–99. doi: 10.1016/j.ntt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank D, Rose-Jacobs R, Crooks D, et al. Adolescent initiation of licit and illicit substance use: impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33:100–109. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney-Black V, Chiodo L, Hannigan J, et al. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Dev Brain Res. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Perry J, Joseph J, Jiang Y, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–129. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke JD, Loeber R, White HR, Stouthamer-Loeber M, Pardini DA. Inattention as a key predictor of tobacco use in adolescence. J Abnorm Psychol. 2007;116:249–259. doi: 10.1037/0021-843X.116.2.249. [DOI] [PubMed] [Google Scholar]
- 20.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 21.Ernst M, Luckenbaugh D, Moolchan E, et al. Behavioral predictors of substance-use initiation in adolescents with and without attention–deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- 22.Timmermans M, van Lier PAC, Koot HM. Which forms of child/adolescent externalizing behaviors account for late adolescent risky sexual behavior and substance use? J Child Psychol Psychiatry. 2008;49:386–394. doi: 10.1111/j.1469-7610.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 23.Trim R, Schuckit M, Smith T. Predicting drinking onset with discrete-time survival analysis in offspring from the San Diego prospective study. Drug Alcohol Depend. 2010;107:215–220. doi: 10.1016/j.drugalcdep.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg N, Dielman T, Mandel W, Shope J. Parental drinking and gender factors in the prediction of early adolescent alcohol use. Int J Addict. 1994;29:89–104. doi: 10.3109/10826089409047370. [DOI] [PubMed] [Google Scholar]
- 25.Block J, Block JH, Keyes S. Longitudinally foretelling drug usage in adolescence: early childhood and personality and environmental precursors. Child Dev. 1998;59:336–355. doi: 10.1111/j.1467-8624.1988.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 26.Donovan J, Jessor R, Costa F. Adolescent health behavior and conventionality and unconventionality: an extension of the Problem Behavior Theory. J Health Psychol. 1991;10:52–61. [PubMed] [Google Scholar]
- 27.Patton G, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:e300–e306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelius M, Leech SL, Goldschmidt L, Lebow H, Day NL. Prenatal tobacco exposure: is it a risk factor for preadolescent tobacco use? Nicotine Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman JP. The effects of family structure and family relations on adolescent marijuana use. Int J Addict. 1995;30:1207–1241. doi: 10.3109/10826089509105131. [DOI] [PubMed] [Google Scholar]
- 30.Henry B, Feehan M, McGee R, Stanton W, Moffitt T, Silva P. The importance of conduct problems and depressive symptoms in predicting adolescent substance use. J Abnorm Psychol. 1993;21:469–480. doi: 10.1007/BF00916314. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence. Data from a national sample. J Consult Clin Psychol. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Richardson GA, Day NL. Prenatal cocaine exposure: effects on school-age children’s physical, cognitive, and behavioral development. Presented at the Society for Research in Child Development; Tampa, FL. April 2003. [Google Scholar]
- 33.Larkby C, Leech SL, Cornelius M, Richardson GA. Prenatal cocaine exposure, childhood maltreatment, and adolescent marijuana use. Presented at the College on Problems of Drug Dependence; San Juan, Puerto Rico. June 2008. [Google Scholar]
- 34.Jessor R, Donovan J, Costa F. Health Behavior Questionnaire. Boulder, CO: University of Colorado; 1989. [Google Scholar]
- 35.Donovan J, Jessor R, Costa F. Adolescent problem drinking: stability of psychosocial and behavioral correlates across a generation. J Stud Alcohol. 1999;60:352–361. doi: 10.15288/jsa.1999.60.352. [DOI] [PubMed] [Google Scholar]
- 36.Petersen A, Crockett L, Richards M, Boxer A. A self-report of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein D, Fink L. Childhood trauma questionnaire: a retrospective self-report. San Antonio, TX: Harcourt Brace and Company; 1998. [Google Scholar]
- 38.Hastings T, Kelly M. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) J Abnorm Child Psychol. 1997;25:511–250. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg L, Lamborn S, Dornbusch S, Darling N. Impact of parenting practices on adolescent achievement: authoritative parenting, school-involvement, and encouragement to success. Child Dev. 1992;63:1266–1281. doi: 10.1111/j.1467-8624.1992.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio TX: Psychological Corporation; 1991. [Google Scholar]
- 41.Sattler JM. Assessment of Children – Cognitive Applications - Fourth Edition. San Diego, CA: Jerome M. Sattler, Publisher, Inc; 2001. [Google Scholar]
- 42.Achenbach T. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 43.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. 4. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- 44.Wilkinson GS. The Wide Range Achievement Test Administration Manual 1993 Edition. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 45.Kovacs M. The Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- 46.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 47.Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- 48.Baker PC, Mott FL. National Longitudinal Study of Youth Child Handbook. Center for Human Resource Research: Ohio State University; 1989. [Google Scholar]
- 49.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthén LK, Muthén BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthén and Muthén; 1998–2007. [Google Scholar]
- 51.Tein J-Y, MacKinnon DP. Estimating mediated effects with survival data. In: Yanai H, Rikkyo AO, Shigemasu K, Kano Y, Meulman JJ, editors. New Developments in Psychometrics. Tokyo, Japan: Springer-Verlag Tokyo Inc; 2003. pp. 405–412. [Google Scholar]
- 52.Richardson GA, Huestis M, Day N. Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC, editor. Human Developmental Neurotoxicology. New York, NY: Taylor and Francis Group; 2006. pp. 287–302. [Google Scholar]
- 53.Richardson GA, Hamel S, Goldschmidt L, Day N. Growth of infants prenatally exposed to cocaine/crack: a comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- 54.Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, Shankaran S, Bada H, Walls H, Huestis M, Finnegan L, Maza P. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 55.Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: effects on mother- and teacher-related behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thoma RJ, Monnig MA, Lysne PA, et al. Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcohol Clin Exp Res. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]