Abstract
Objective
Bipolar disorder (BD) is a debilitating psychiatric condition that commonly begins in adolescence, a developmental period that has been associated with increased reward-seeking. Because youth with bipolar disorder are especially vulnerable to negative risk-taking behaviors, understanding the neural mechanisms by which dysregulated affect interacts with neurobehavioral processing of reward is clearly important. One way to clarify how manic symptoms evolve in BD is to “prime” affect before presenting rewarding stimuli. The objective of this study was to investigate the neural effects of an affective priming task designed to positively induce mood prior to reward processing in adolescents with and without BD.
Method
Neural activity and behaviors during the anticipation of and response to monetary reward and loss following an affective prime were compared using functional magnetic resonance imaging (fMRI) in 13- to 18-year-old adolescents with a recent onset of bipolar I disorder (“BD,” n=24) and demographically matched healthy comparison youth (“HC,” n=24).
Results
Relative to HC, youth with BD had speeded reaction times and showed decreased activation in the thalamus and inferior temporal gyrus while anticipating gains after priming but increased activations in the middle frontal gyrus and parietal cortices while anticipating losses after priming. Youth with BD also showed less activation in the inferior parietal lobule, thalamus, and superior frontal gyrus while receiving losses after priming.
Conclusions
Aberrant prefrontal and subcortical activations during reward processing suggest mechanisms that may underlie disordered self-awareness during goal pursuit and motivation in BD. Longitudinal studies are needed to examine whether this pattern of neural activation predicts a poorer long-term outcome.
Keywords: reward processing, fMRI, adolescent, bipolar disorder, affective prime
INTRODUCTION
Bipolar disorder (BD) is a debilitating, recurrent psychiatric condition with an onset typically during adolescence,1 a developmental period that has been associated with increased risk-taking and reward-seeking.2 Clinically, BD is characterized by aberrations in emotion and motivation that may lead to risk-taking behaviors that have maladaptive consequences. Specifically, individuals with BD experience hyperhedonia (e.g., excessive pleasure-seeking and goal-directed activity) during manic states and anhedonia (i.e., reduced pleasure in response to hedonic stimuli or experiences) during depressive episodes.3 These disturbances in core emotional and motivational functions may provide a basis for understanding the origins of symptom manifestations associated with BD. Surprisingly, however, few studies have examined the precise nature of, and neural aspects associated with, these aberrations, particularly in adolescents with BD who are temporally close to the onset of illness and, consequently, are likely to be symptomatic at the time of assessment and to have minimal lifetime exposure to either psychotropic medications or many previous mood episodes.
Studies to date have examined different aspects of reward processing in individuals with BD, including responses to various types of positive stimuli (e.g. money, faces), affective response to rewards, and aspects of decision making (e.g., reversal learning), or judgments, which target similar regions of the brain. These studies have found that adults4 and youth5,6 with BD have decreased reward learning, even when they are euthymic.4 However, happy mood states can be induced in euthymic adults with BD while they are engaging in a reward paradigm, suggesting potentially important interactions between cognitive and emotional responses to rewarding stimuli.7 Other investigators have found that youth with BD, compared with typically developing controls, report increased reward reactivity and greater arousal in reward conditions8, and greater satisfaction with winning.9 The first of four neuroimaging studies examining reward processing in BD used a monetary incentive delay (MID) task in acutely manic and medicated adults with BD.10 In this study, adults with BD did not activate the ventral tegmentum as did healthy and schizophrenia comparison adults while anticipating high versus no reward outcomes, and had a lower differential signal in the nucleus accumbens (NAcc) upon receipt of rewards compared to the healthy control subjects. In the second study, adults with mania did not differ from healthy controls in ventral striatal response to cued incentives.11 However, adults with mania did show significant increases in activation in the left lateral orbitofrontal cortex (OFC; Brodmann Area [BA] 11 and 47) while anticipating increasing gains and decreased activation in this region during expectation of increasing loss, whereas healthy subjects tended to show the opposite effect. In a third study that used a card-guessing paradigm, whole brain analyses found adults with BD to have increased left sided lateral orbitofrontal activation during reward anticipation. Region-of-interest (ROI) analyses in this study revealed that adults with BD, compared with healthy controls, exhibited greater ventral striatal and right-sided orbitofrontal (BA 11) activity during anticipation, but not during outcome, of monetary reward.12 Finally, a recent study related increased activity in the amygdala and the orbitofrontal cortex to heightened sensitivity in response to reward and reward reversal and to deficient prediction error signaling in individuals with BD and their relatives.13 These studies highlight aberrant neural functioning in BD that may be related to enhanced motivation for seeking rewards and to an underestimation of associated risks and potential punishments.
It is not clear, however, whether these patterns of behavioral and neural functioning reflect a developmental process typical of adolescents versus adults, play an etiological role in BD, or are a consequence of chronic exposure to multiple mood episodes or psychotropic medications. Moreover, neither the specific neural features that are associated with aberrant reward processing in adolescents nor the mechanisms by which reward processing interacts with mood state are currently known. In addition, findings across studies are inconsistent, showing different regions and opposite directions of activations during gain and loss conditions, possibly due to type I error or small sample sizes. Therefore, to gain a better understanding of the precise nature and origins of aberrant emotion and motivation associated with BD, it is important to compare neural correlates of reward processing in a large sample of typically developing adolescents and youth who are close to the onset of their first manic episode, to experimentally manipulate mood state while participants are processing rewards by priming their affect prior to the presentation of rewarding stimuli, and to model analyses in order to explore the regions and directions of neural activations associated with reward (gain) versus risk (loss). Such an approach would advance our understanding of the neural basis of how dysregulated mood might lead to the intensification of goal pursuit and motivation in BD.
The first aim of the present study was to examine neural activations associated with reward processing following an affective prime by scanning adolescents with BD I who have experienced only a single episode of mania in their lifetime. Because we were interested in the interaction of cognitive and emotional processes in BD, we predicted that compared with healthy control (HC) youth, youth with BD would demonstrate significant aberrations in frontostriatal activation associated with the MID task that would be more pronounced when the task was preceded by an affective prime, which we posited would intensify emotional and neural responses to rewarding stimuli (similar to the effects of a positive mood induction,7) than when it was preceded by a nonaffective control task. Drawing on previous reports showing differential brain function and behavior during anticipation and receipt of rewards versus punishments in adults with BD10,11,12,13, our second aim was to explore neural activations as a function of the interaction of group and prime within gain and loss conditions separately. In these secondary analyses, we predicted that compared with healthy control (HC) participants, adolescents with BD would exhibit increased activation in the ventral striatum (i.e., NAcc) and OFC (inclusive of BA 10, 11, or 47) while anticipating gains on the MID task and increased activation in the amygdala while receiving gains. We also predicted that while anticipating and receiving losses, youth with BD would show decreased activation in the ventral striatum and OFC but not in the amygdala. Finally, given the likely relation between symptoms of dysregulated motivation in mania and reward processing, we predicted that manic mood state would intensify emotional and neural responses such that those participants with higher levels of mania on the day of scan would show relatively greater activations in orbitofrontal and striatal regions during reward anticipation and receipt, and lower orbitofrontal and striatal activations during the anticipation and receipt of losses.
METHOD
Participants
The university panel of medical research in human subjects approved this research protocol. After hearing a complete description of the study, parents and youth under the age of 18 years gave written informed consent and assent, respectively. Adolescents (ages 13–18 years) with bipolar I disorder (n=24) diagnosed as having their first manic episode within the previous 12 months (mean interval between onset of mania and scan=5 months) were recruited either by referral to a pediatric bipolar disorders clinic or from the surrounding community. HC adolescents (n=24) without any personal or family history of psychiatric diagnoses or psychotropic medication exposure were recruited through local community advertisements. A telephone screening with a parent established that all participants had English fluency and did not have any metal in their body, history of head injury (with loss of consciousness over 5 minutes), seizures, or developmental disorders. Youth with BD who were prescribed stimulants did not take them 24 hours prior to neuroimaging, and were required to not have used recreational drugs for at least 30 days prior to the MRI scan. To avoid risk of mood destabilization, BD subjects were allowed to continue any other psychotropic medications including mood stabilizers, atypical antipsychotics, or antidepressants.
Diagnostic and Clinical Assessments
All participants were evaluated for current and lifetime psychiatric disorders, using the Washington University in St. Louis Kiddie–Schedule for Affective Disorders and Schizophrenia (WASH-U KSADS)14 for affective disorders and the Kiddie–Schedule for Affective Disorders and Schizophrenia Present and Lifetime version (KSADS-PL)15 for other psychopathology, administered separately to parents and children by interviewers with established symptom and diagnostic inter-rater reliability (kappa>0.9). A manic episode was defined by Diagnostic and Statistical Manual–4th Edition–Text Revision (DSM-IV-TR) criteria that lasted at least one week and could not have been precipitated by exposure to recreational drugs, antidepressants, psychostimulants, or other medications or medical conditions. Symptom severity was assessed on the day of scan using the Young Mania Rating Scale (YMRS),16 the Children’s Depression Rating Scale-Revised Version (CDRS-R),17 and the Childhood Global Assessment Scale (CGAS)18 by raters with established reliabilities (all intra-rater ICCs>0.9). Active symptoms on the day of scan were summarized for the purposes of imaging analyses using the following cutoffs: YMRS score >20 for active manic symptoms, CDRS score > 40 for active depression. All participants completed the Barratt Impulsiveness Scale (BIS-11), which yielded subscale scores on the dimensions of attentional, motor, and non-planning trait impulsivity.19 Age, sex, socioeconomic status (Hollingshead Four Factor Index),20 pubertal stage (Pubertal Development Scale),21 IQ (Wechsler Abbreviated Scale of Intelligence) (WASI),22 and handedness (Crovitz Handedness Questionnaire)23 were also assessed.
Monetary Incentive Delay (MID) Task
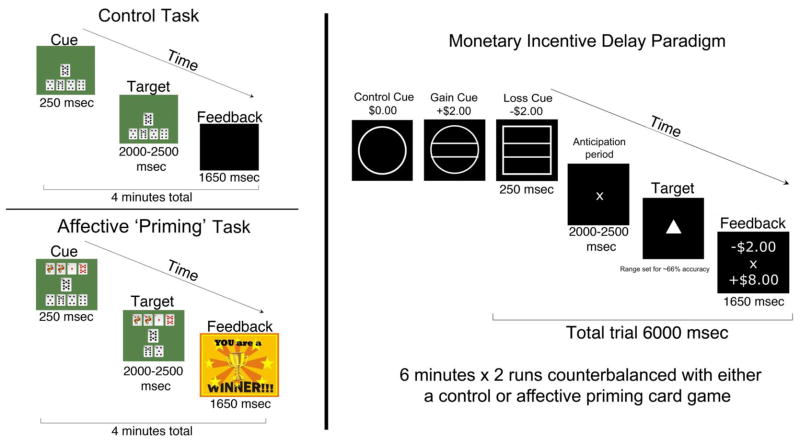
All participants practiced and were tested for their comprehension of explicit cues presented in the MID task.24 They were shown cash that they could win during the task prior to entering the scanner. The MID task was designed to probe neural responses to the anticipation and receipt of gain and loss outcomes, using a set of cues to indicate whether participants can win or avoid losing money if they respond quickly enough to a target (represented by a triangle) that follows a cue and anticipation period. On each trial participants were presented with a cue indicating that they had a chance to win or lose $0, $1, or $2. Circle cues indicated trials with the opportunity to win money, square cues indicated trials when money could be lost and lines within those shapes defined the amount of money presented for that trial. Immediately after each trial participants were shown feedback (how much money they won or lost on that trial and a running total).
Each of these six trial types appeared 9 times for 6 seconds and was pseudo-randomly distributed, for a total of 54 trials per run (approximately 6 minutes × 2 runs). Cues were displayed for 250 ms, followed by a jittered anticipatory period (2000–2500 ms). The target was displayed for a varying duration (250–350 ms), determined from reaction times collected during a practice session before scanning and set such that participants would succeed on approximately 66% of their target responses. A jittered delay period separated the offset of the target stimulus from the onset of the feedback stimulus, so that the length of the entire trial was exactly 6 seconds. After the scan, participants were tested once again on their comprehension of the cues.
Affective Priming Task
To examine the interaction between emotion and motivation, and because participants with BD were in different mood states on the day of their scan, a new fMRI task was designed to experimentally induce an elevated state of arousal in all participants. Positive mood inductions during reward processing have been previously described in adults with BD,7 and provide a relevant context for this approach; however, in this study, our mood induction could yield a positive or negative affect. Two runs of the MID task were each preceded by card games, using a traditional 52-card deck, that were designed to enhance engagement, motivation, confidence, and drive to perform in the MID task. Using a button box, participants were instructed to press a button corresponding to one of four card piles that would move a card of adjacent value into a deposit pile creating a sequence. Two versions of the card game were presented to participants in each group in counterbalanced order: one version (affective priming) in which they played against the computer and, during the last minute of the game, were given feedback that they had won the game; and another version (control task) that participants played by themselves without any feedback (Figure 1). Participants rated their levels of happiness, distraction, excitement, anxiety, and confidence about winning money in the subsequent MID task (1=Not at all, 2 = Somewhat, 3= Very, 4=Extremely) at the beginning and end of each card game.
Figure 1.
Experimental paradigm of tasks presented during functional magnetic resonance imaging. Note: The Control Task or the Affective Priming Task were counterbalanced to precede two runs of the Monetary Incentive Delay Paradigm.
MRI Data Collection and Pre-processing
After being trained on the fMRI tasks and desensitized to the scanning environment by an MRI simulator, imaging-related procedures were performed using a 3T Signa Excite scanner (GE Medical Systems, Milwaukee WI), equipped with echo-speed gradients using a standard whole-head coil (General Electric, Milwaukee) and high-resolution, T1-weighted, spoiled GRASS images. Functional images were collected with a T2*-weighted spiral pulse sequence with parameters of recovery time (TR)=2000 ms; echo time (TE)=30 ms; flip angle 803; field of view=22 cm, matrix=64×64, voxel size 3.4375 × 3.4375 mm, and slice thickness 4 mm with 1 mm spacing. An automated high-order shimming method was used before acquiring fMRI data to reduce field inhomogeneities. Structural images were collected to aid in localization of the functional data, using high-resolution, T1-weighted, spoiled gradient-recalled acquisition (SGPR) 3-dimensional MRI sequences with the following parameters: recovery time =6.4 ms, echo time=2.0 ms, inversion recovery preparation pulse=300 ms, flip angle=15°, field of view=22 cm, a 256×256 matrix, number of excitations=3, 124 slices in the coronal plane and 1.5 mm slice thickness. Seven participants (6 BD and 1 HC) originally recruited were excluded from analysis due to motion artifacts or poor behavioral performance, resulting in a sample size of 24 per group.
Functional images were processed with SPM8 (Wellcome Department of Cognitive Neurology, London, UK), including realignment, slice time correction, coregistration, normalization into MNI space with 2 mm voxel resampling, and spatial smoothing. Images were repaired by interpolation from the nearest unaffected volumes, using the ArtRepair software toolbox for SPM (http://cibsr.stanford.edu/tools/methods/artrepair-software.html) if motion exceeded a 0.5 mm/TR threshold or if global signal was greater than 3% from the mean global signal of all images. Data were coregistered and spatially normalized into standard stereotactic space using an adolescent template (https://irc.cchmc.org/software/pedbrain.php). Normalized images were then smoothed with a 7 mm FWHM Gaussian filter.
Statistical Analysis
Reaction time and accuracy was recorded on each trial of the MID task. Three-way (group [BD, HC] repeated over priming condition [prime, control] and valence [gain, loss]) analyses of variance (ANOVAs) were conducted on individual hit rates, mean reaction times, and total money gained across anticipation and feedback conditions. These were followed by two-way ANOVAs to examine the behavioral differences occurring during the gain and loss conditions.
Statistical contrasts were conducted separately from anticipation and feedback event onsets. Because reaction times did not differ as a function of incentive level, trials presenting anticipation of gain cues (i.e., $1 and $2) were combined to increase statistical power, as were trials presenting anticipation of loss cues. For the anticipation phase, trials with gain or loss cues were compared to their corresponding non-gain and non-loss trials. For the feedback phase, trials in which participants gained money were compared to non-gain feedback trials, and trials in which participants lost money were compared to non-loss trials. Statistics conducted at the individual level used a fixed effects model to define experimental (anticipation gain and loss; gain and loss outcomes) and control (non-gain and non-loss outcomes) conditions using SPM8. A high-pass filter of 120 seconds was applied and 6 motion regressor nuisance covariates were included in the model.
To investigate group differences in reward processing following an affective prime, we conducted a three way (group [BD, HC] × prime [affective priming, control] × valence [gain, loss]) ANOVA as our primary analysis. In a secondary analysis, we conducted a two-way (group [BD, HC] × prime [affective priming, control]) ANOVA for each MID condition [gain, loss]. Significant activations associated with the main effect of group, the main effect of priming, and the interaction of these terms were identified for the comparison of anticipated and received gains versus non-gains, and anticipated and received losses versus non-losses. Activation foci were superimposed on high-resolution, T1-weighted images in MNI space, and their locations were interpreted using the Talairach atlas and known neuroanatomical landmarks. The voxel level significance (p=.01) and cluster size (k=194) criteria used to hold family-wise error (FWE) at p=.05 were calculated with the AFNI program AlphaSim based upon parameters for matrix size of 91×109×91, voxel dimensions of 2 mm3, 7 mm FWHM smoothing kernal and 10,000 monte carlo simulations. This program generates null hypothesis distributions and corresponding statistical criterion values through the use of Monte Carlo simulation. Parameters known to affect the shape of null hypothesis distributions of fMRI data, such as the number of voxels compared and their effective size, the per-voxel statistical criterion, and the definition of voxel clustering used, are modeled in these Monte Carlo simulations.25
Finally, exploratory ROI analyses were conducted within the BD group to examine effects of priming on reward processing associated with the presence of high versus low manic symptoms on scan day. ROIs were defined by Automated Anatomical Labeling atlas26 and were selected based on findings from our whole-brain analysis and from the extant reward literature12,27,28,29: anterior cingulate cortex (ACC), amygdala, insula, and portions of the striatum including the caudate, putamen, NAcc, and globus pallidus. Mean Z-score values were extracted using MarsBar (http://marsbar.sourceforge.net/) and imported into SPSS18 (http://www.spss.com/) for analysis. ROI significance levels were corrected for multiple comparisons using a Bonferroni correction (0.05/#ROIs=0.007).
RESULTS
Participant Characteristics
Demographic and clinical characteristics of the two participant groups are presented in Table 1. The BD and HC adolescents did not differ significantly with respect to age, t(46)=1.51; gender, χ2(N=48)=1.34, handedness, χ2(N=48)=2.10, WASI IQ scores, t(46)=1.73; socioeconomic status, t(46)=0.32, Tanner stage, t(46)=1.08, or ethnicity, χ2(N=48)=8.74, all p>.05. Adolescents with BD reported significantly higher YMRS (t[46]=10.7, p<.0001), CDRS (t[46]=8.6, p<.0001), and trait impulsivity subscale scores in attention, t(41)=5.4, p<.0001, motor, t(41)=4.2, p<.0001), and non-planning, t(41)=3.1, p<.004) compared to the HC group. The BD group had lower scores on the CGAS (t[46]=14.8, p<.0001) compared to the HC group indicating greater functional impairment.
Table 1.
Participant Demographic and Clinical Variables
| Variable | BD (n=24) | HC (n=24) |
|---|---|---|
|
| ||
| Age, years, mean (SD) | 15.7 (1.7) | 15.0 (1.4) |
|
| ||
| Gender, Female, n (%) | 11 (46) | 15 (63) |
|
| ||
| Race, Caucasian, n (%) | 13 (54) | 14 (58) |
|
| ||
| Right Handedness, n (%) | 19 (79) | 21 (88) |
|
| ||
| Tanner Stage, mean (SD) | 3.30 (0.40) | 3.16 (0.53) |
|
| ||
| Socioeconomic Status, mean (SD) | 4.7 (0.56) | 4.61 (0.65) |
|
| ||
| Full Scale IQ, mean (SD) | 107 (10.2) | 113 (10.8) |
|
| ||
| YMRS, mean (SD)* | 17.8 (8.1) | 0.13 (0.34) |
|
| ||
| CDRS, mean (SD)* | 44.0 (14.8) | 17.9 (1.4) |
|
| ||
| CGAS, mean (SD)* | 58.3 (11.0) | 93.7 (3.2) |
|
| ||
| BIS – Attentional Impulsivity, mean (SD)* | 20.8 (4.9) | 14.0 (3.3) |
|
| ||
| BIS – Motor Impulsivity, mean (SD)* | 25.1 (3.9) | 20.4 (3.4) |
|
| ||
| BIS – Nonplanning Impulsivity, mean (SD)* | 28.9 (6.2) | 24.1 (4.2) |
|
| ||
| Mood State On Day of Scan, n (%)* | ||
| Manic | 6 (25) | 0 (0) |
| Mixed (Manic + Depressed) | 5 (21) | 0 (0) |
| Depressed | 9 (38) | 0 (0) |
| Euthymic | 4 (17) | 24 (100) |
|
| ||
| Other Lifetime Psychiatric Diagnoses, n (%)* | 9 (38) | 0 (0) |
| ADHD | 5 (21) | 0 (0) |
| Any Anxiety Disorder | 4 (17) | 0 (0) |
| Oppositional Defiant Disorder | 1 (4) | 0 (0) |
| Conduct Disorder | 9 (38) | 0 (0) |
| Marijuana Abuse | ||
|
| ||
| Lifetime Medication Exposure, n (%) | 20 (83) | 0 (0) |
|
| ||
| Weeks on meds on scan day, mean (SD) | 15.5 (25) | 0 (0) |
|
| ||
| Depressive Episode before Mania, n (%) | 14 (58) | 0 (0) |
|
| ||
| Months to Scan after Manic Episode, mean (SD) | 5.57 (3.4) | 0 (0) |
|
| ||
| Lifetime Medication Exposure,* n (mean exposure in weeks) | ||
| Lithium | 8 (1.25) | 0 (0) |
| Atypical Antipsychotics | 17 (4.6) | 0 (0) |
| Antidepressants | 12 (4.2) | 0 (0) |
| Psychostimulants | 7 (5) | 0 (0) |
| Any medication | 20 (16) | 0 (0) |
Note: ADHD = attention-deficit/hyperactivity disorder; BD = Adolescents with Bipolar I disorder; BIS = Barratt Impulsiveness Scale; CGAS = Clinical Global Assessment Scale; CDRS = Childhood Depression Rating Scale; HC = Healthy Control Adolescents; YMRS = Young Mania Rating Scale.
p<0.05
Behavioral Results
A three-way (group by prime by valence) ANOVA conducted on reaction times yielded a significant interaction of group and valence, F(1,48)=3.99, p=0.05. Subsequent two-way (group by prime) ANOVAs indicated that adolescents with BD had significantly faster reaction times than did HC adolescents for anticipation of gain and gain control trials during the MID task following the affective prime, F(1,48)=5.734, p=.021; analyses of hit rates and reaction times for anticipation of loss trials yielded no significant main effects or interactions, indicating comparable performance of the two groups on the task. Three-way (group by prime by order [i.e., pre/post-task rating]) ANOVAs conducted on self-report measures yielded significant interactions of prime and order for positive affect F=34.71, p<0.001, excitability, F=5.35, p=0.025, and confidence, F=6.686, p=0.013. Subsequent paired t-tests showed significant increases across the priming task from before to after the priming task for positive affect (t=5.38, p<0.001), excitability (t=2.12, p=0.04), and confidence (t=4.23, p<0.001); there were no significant pre-post task differences across the control task for these measures (all ps>0.05) (Table 2).
Table 2.
Behavioral Results and Mean Affective Ratings before and after Prime and Control Tasks
| Variable | 3rd Factor | Bipolar Group | Control Group | ANOVA significant interactions | ||
|---|---|---|---|---|---|---|
| Prime | Control | Prime | Control | |||
| MID RT | Gain | 193.11 (54.60) | 205.60 (38.56) | 224.65 (36.22) | 222.53 (31.11) | Interaction group × valence F=3.99, p=0.05 |
| Loss | 203.84 (39.77) | 207.36 (46.09) | 223.90 (30.19) | 225.96 (26.71) | ||
| MID accuracy | Gain | 0.71 (0.16) | 0.72 (0.16) | 0.69 (0.16) | 0.69 (0.11) | No significant interactions |
| Loss | 0.66 (0.14) | 0.69 (0.14) | 0.68 (0.15) | 0.66 (0.11) | ||
| Happiness | Before | 2.18 (0.50) | 2.21 (0.58) | 1.96 (0.69) | 2.13 (0.74) | Interaction prime × order F=34.71, p<0.001 |
| After | 2.79 (0.50) | 2.33 (0.56) | 2.38 (0.82) | 2.04 (0.55) | ||
| Excitement | Before | 2.04 (0.35) | 2.13 (0.45) | 1.87 (0.95) | 1.92 (0.77) | Interaction prime × order F=5.35, p=0.025 |
| After | 2.46 (0.65) | 2.08 (0.50) | 1.92 (0.78) | 1.79 (0.83) | ||
| Anxiety | Before | 1.62 (0.64) | 1.58 (0.58) | 2.08 (1.06) | 2.00 (0.97) | Interaction group × order F=4.51, p=0.039 |
| After | 2.46 (0.65) | 2.08 (0.50) | 1.92 (0.78) | 1.79 (0.83) | ||
| Confidence | Before | 2.42 (0.65) | 2.50 (0.72) | 2.21 (0.83) | 2.42 (0.92) | Interaction prime × order F=6.69, p=0.013 |
| After | 2.71 (0.62) | 2.46 (0.72) | 2.54 (0.83) | 2.21 (0.83) | ||
| Distraction | Before | 1.74 (0.61) | 1.78 (0.73) | 2.09 (0.95) | 1.96 (0.80) | No significant interactions |
| After | 1.62 (0.71) | 1.71 (0.69) | 1.96 (0.86) | 2.09 (0.95) | ||
Note: 1st factor of analysis of variance (ANOVA) = group, 2nd factor of ANOVA = Prime task, 3rd factor of ANOVA = Valence of Monetary Incentive Delay Task Behavioral Performance (MID) condition or Order of self-report scores. BD = Adolescents with Bipolar I disorder; HC = Healthy Comparison Adolescents; RT = reaction time.
Neuroimaging Results
Primary Three-way ANOVA Results with Post-hoc Analyses
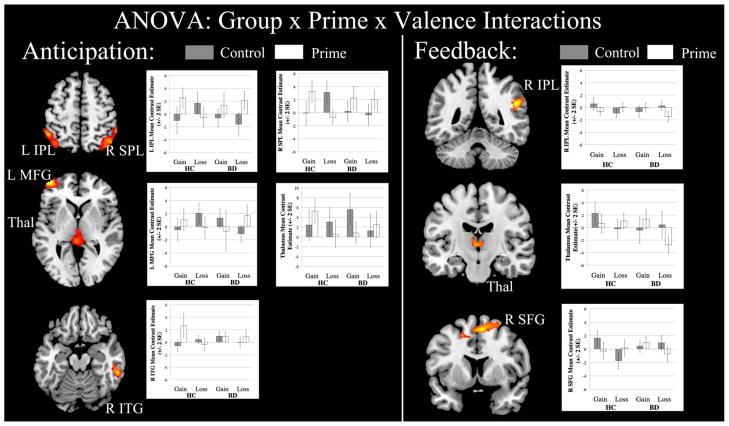
A three-way (group by prime by valence) ANOVA during the anticipation condition yielded a significant three-way interaction; activations in this interaction included the left middle frontal gyrus (BA 10), left and right inferior parietal lobule (BA 40), right inferior temporal gyrus (BA 20), and left thalamus (Table 3, Figure 2). Post-hoc tests in these regions indicated that, compared to the HC group, the BD group showed decreased activation in the thalamus and inferior temporal gyrus while anticipating gains after priming but increased activations in the middle frontal gyrus and parietal cortices while anticipating losses after priming.
Table 3.
Significant Clusters of Activation using a Three-way analysis of variance (ANOVA)
| ANOVA | Cluster Location | BA | extent | F | Primary Peak (x,y,z) | Direction of significant post hoc analyses |
|---|---|---|---|---|---|---|
| Anticipation: Group × Prime × Valence Three-way ANOVA | ||||||
| Interaction group × prime × valence | Right superior parietal lobule | 7 | 632 | 11.38 | 56, −42, 50 | Interaction group × prime; F(46)=4.17, p=0.047 Interaction prime × valence; F(46)=9.49, p=0.003 Group Differences: Loss after prime; BD>HC, p=0.006 Loss after control; HC>BD, p=0.006 Prime Differences: HC; Gain; prime>control, p=0.021 HC; Loss; control>prime, p=0.001 BD; Loss; prime>control, p=0.013 Valence Differences: HC; Prime; gain>loss, p=0.001 HC; Control; loss>gain, p=0.015 |
| Left inferior parietal lobule | 40 | 445 | 9.28 | −50, −48, 56 | Main effect of prime; prime>control, F(46)=8.76, p=0.005 Group Differences: Loss after prime; BD>HC, p=0.016 Loss after control; HC>BD, p=0.014 Prime Differences: HC; Gain; prime>control, p=0.005 BD; Loss; prime>control, p=0.006 Valence Differences: HC; Prime; gain>loss, p=0.009 HC; Control; loss>gain, p=0.022 |
|
| Left middle frontal gyrus | 10 | 617 | 12.01 | −43, 56, −3 | Group Differences: Loss after control; HC>BD, p=0.003 Prime Differences: HC; Loss; control>prime, p=0.039 BD; Loss; prime>control, p=0.015 Valence Differences: HC; Control; loss>gain, p=0.002 BD; Control; gain>loss, p=0.013 |
|
| Thalamus | 226 | 11.46 | −3, 31, 2 | Main effect of valence; prime>control, F(46)=5.04, p=0.030 Group Differences: Gain after prime; HC>BD, p=0.009 Prime Differences: BD; Gain; control>prime, p=0.012 Valence Differences: HC; Control; gain>loss, p=0.004 BD; Control; gain>loss, p=0.010 |
||
| Right inferior temporal gyrus | 20 | 197 | 10.26 | 58, −36, −15 | Main effect of prime; prime>control, F(46)=4.17, p=0.048 Main effect of valence; gain>loss, F(46)=4.43, p=0.041 Interaction of prime × valence; F(46)=3.87, p=0.055 Group Differences: Gain after control; BD>HC, p=0.016 Prime Differences: HC; Gain; prime>control, p=0.013 Valence Differences: HC; Prime; gain>loss, p=0.019 BD; Control; gain>loss, p=0.050 |
|
| Feedback: Group × Prime × Valence Three-way ANOVA | ||||||
| Interaction group × prime × valence | Right inferior parietal lobule | 40 | 409 | 17.52 | 49, •43, 28 | Group Differences: Loss after prime; HC>BD, p=0.006 Loss after control; HC>BD, p=0.035 Prime Differences: HC; Gain; prime>control, p=0.035 BD; Loss; control>prime, p=0.008 Valence Differences: HC; Control; gain>loss, p=0.012 BD; Prime; gain>loss, p=0.008 |
| Right superior frontal gyrus | 8 | 1101 | 13.79 | 7, 24, 49 | Main effect of valence; gain>loss, F(46)=8.75, p=0.005 Group Differences: Loss after control; BD>HC, p=0.003 Prime Differences; HC; Gain; control>prime, p=0.024 HC; Loss; prime>control, p=0.035 Valence Differences: HC; Control; gain>loss, p<0.001 BD; Prime; gain>loss, p=0.028 |
|
| Thalamus | 377 | 11.75 | •3, •13, 4 | Main effect of group; HC>BD, F(46)=4.10, p=0.049 Main effect of valence; gain>loss, F(46)=4.48, p=0.04 Group Differences: Loss after prime; HC>BD, p>0.001 Prime Differences: BD; Loss; control>prime, p=0.019 Valence Differences: BD; Prime; gain>loss, p=0.001 |
||
Note: BA = Brodmann Area; BD = Adolescents with Bipolar I Disorder; HC = Healthy controls.
Figure 2.
Significant neural activations from whole brain 3-way analysis of variance (ANOVA) (threshold of p=0.05, k=194 or > voxels). Note: IPL = Inferior parietal lobule; ITG = Inferior temporal gyrus; L = Left; MFG = Middle frontal gyrus; R = Right; SFG = Superior frontal gyrus; SPL = Superior parietal lobule; Thal = Thalamus.
During the feedback phase of the task, the three-way ANOVA also yielded a significant three-way interaction, with activations in the right inferior parietal lobule (BA 40), thalamus, and right superior frontal gyrus (BA 8) (Table 3). Post-hoc t-tests revealed that, when compared to the HC group, the BD group showed less activation in all three of these regions while receiving losses after priming.
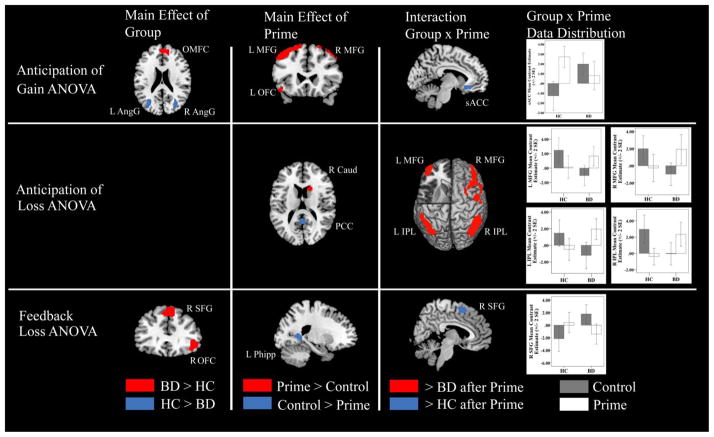
Secondary Whole-Brain Two-way ANOVA Results
A significant main effect of valence from the three-way ANOVA showed that the ventral striatum was significantly more activated during anticipation of gain than during anticipation of loss. To pursue our hypotheses about differential prefrontal and subcortical dysfunction during gain and loss conditions as shown in previous studies using the MID task24–25,27, we conducted exploratory two-way whole brain ANOVAs comparing BD and HC participants in gain and loss conditions. These secondary analyses were not derived from the three-way ANOVA, so should be viewed with caution as exploratory and yielded the following results (p=0.05, FWE-corrected; Table 4, Figure 3).
Table 4.
Significant Clusters of Activation using a Two-way analysis of variance (ANOVA)
| ANOVA | Cluster Location | BA | extent | Direction of posthoc t-test | Posthoc t-test p-value | F | Primar y Peak (x,y,z) |
|---|---|---|---|---|---|---|---|
| Anticipation Gain ANOVA | |||||||
| Main effect of Group | Right orbitomedial frontal cortex | 10 | 403 | BD>HC | p=0.003 | 9.94 | 11, 55, 14 |
| Right angular gyrus | 39 | 202 | HC>BD Prime>control |
p=0.001 p=0.02 |
11.88 5.53 |
31, −65, 27 | |
| Left angular gyrus | 39 | 233 | HC>BD Prime>control |
p=0.005 p=0.013 |
8.92 6.73 |
−31, −63, 18 | |
| Main effect of prime | Left middle frontal gyrus | 6 | 1363 | Prime>control | p<0.001 | 20.45 | −37, 11, 57 |
| Left inferior parietal lobule | 40 | 1271 | Prime>control | p<0.001 | 14.67 | −52, −44, 43 | |
| Right superior parietal lobule | 7 | 808 | Prime>control | p=0.002 | 10.44 | 29, −69, 55 | |
| Right superior frontal gyrus | 8 | 454 | Prime>control | p=0.001 | 12.93 | 41, 24, 49 | |
| Right middle temporal gyrus | 21 | 371 | Prime>control | p=0.001 | 12.02 | 66, −28, −14 | |
| Left inferior occipital gyrus | 18 | 223 | Prime>control | p=0.001 | 12.39 | −43, −84, −3 | |
| Right parahippocampal gyrus | 30 | 207 | Prime>control | p=0.002 | 10.45 | 29, −54, 58 | |
| Right precentral gyrus | 6 | 205 | Prime>control | p=0.001 | 11.98 | 60, 5, 31 | |
| Left orbitofrontal gyrus | 11 | 203 | Prime>control | p=0.001 | 12.53 | −29, 34, −17 | |
| Interaction of group × prime | Subgenual cingulate | 25 | 213 | HC>BD | p<0.001 | 14.97 | −13, 22, −14 |
| Anticipation Loss ANOVA | |||||||
| Main effect of group | No significant clusters identified | p>0.05 | |||||
| Main effect of prime | Right posterior cingulate | 31 | 408 | Control>Prime | p=0.002 | 10.34 | 1, −39, 31 |
| Right inferior parietal lobule | 40 | 491 | Prime>control | p<0.001 | 14.38 | 62, −29, 46 | |
| Right caudate | 249 | Prime>control | p=0.002 | 11.19 | 15, 16, 16 | ||
| Interaction of group × prime | Right inferior parietal lobule | 40 | 1083 | BD>HC | p<0.001 | 17.30 | 54, −42, 52 |
| Right middle frontal gyrus | 10 | 894 | BD>HC | p<0.001 | 15.54 | 41, 44, 16 | |
| Left inferior parietal lobule | 40 | 823 | BD>HC | p=0.001 | 11.98 | −49, −50, 56 | |
| Left parahippocampal gyrus | 19 | 381 | HC>BD | p<0.001 | 18.47 | −35, −43, −3 | |
| Left middle frontal gyrus | 46 | 372 | BD>HC | p=0.001 | 13.71 | −43, 42, 15 | |
| Right middle frontal gyrus | 6 | 223 | BD>HC | p=0.002 | 10.40 | 50, 1, 52 | |
| Left middle temporal gyrus | 20 | 195 | BD>HC | p=0.002 | 11.38 | −60, −45, −11 | |
| Feedback Gain ANOVA | |||||||
| Main effects and Interactions | No significant clusters identified | p>0.05 | |||||
| Feedback Loss ANOVA | |||||||
| Main effect of group | Right superior frontal gyrus | 6 | 446 | BD>HC Prime>control |
p<0.001 p=0.042 |
15.08 4.39 |
7, 30, 55 |
| Right orbitofrontal gyrus | 47 | 355 | BD>HC | p<0.001 | 17.81 | 43, 28, −7 | |
| Main effect of prime | Left parahippocampal gyrus | 30 | 211 | Control>Prime | p<0.001 | 27.11 | −21, −48, 6 |
| Interaction of group × prime | Right superior frontal gyrus | 6 | 214 | HC>BD | p=0.001 | 11.95 | 5, 12, 53 |
Note: BA = Brodmann Area; BD = Adolescents with Bipolar I Disorder; HC = Healthy controls.
Figure 3.
Significant neural activations from secondary 2-way whole brain analysis of variance (ANOVA) (threshold of p=.05, 194 or > voxels) depicted in red and blue. Note: No significant activations during the Feedback Gain ANOVA were identified. AngG = Angular gyrus; BD = bipolar disorder; Caud = Caudate; HC = healthy controls; L = left; MFG = Middle frontal gyrus; OFC = Orbitofrontal cortex; OMFC = Orbitomedial frontal cortex; PCC = posterior cingulate cortex; Phipp = Parahippocampal gyrus; R = right; sACC = subgenual anteriors cingulate cortex; SFG = Superior frontal gyrus.
Anticipation of Gain minus Non-gain
The main effect of group showed the BD group to have increased orbitomedial frontal cortex (OMFC) activation relative to controls whereas HC youth had increased bilateral angular gyrus activation relative to the BD group during this contrast. The main effect of priming while anticipating gains showed significant activations throughout the brain relative to the control condition (Table 3). In the two-way interaction of group and prime, the HC group had significantly greater activation in the subgenual anterior cingulate cortex (sgACC) than did the BD group following affective priming.
Anticipation of Loss minus Non-loss
The main effect of group yielded no significant clusters of activation during this contrast. The main effect of priming during anticipation of loss showed significant activations in the right posterior cingulate, inferior parietal lobe, and caudate relative to the control condition. In the interaction of group and prime, adolescents with BD showed greater activation than did the HC group in the bilateral inferior parietal lobules, bilateral middle frontal gyrus, and left middle temporal gyrus, whereas HC adolescents showed greater activation in the left parahippocampal gyrus relative to BD following affective priming.
Gain minus Non-gain Outcomes
This contrast yielded no significant main effects or interactions.
Loss minus Non-loss Outcomes
The main effect of group showed adolescents with BD to have greater activation than the HC group in the right superior and orbitofrontal gyri. The main effect of priming showed significant activations in the left parahippocampal gyrus relative to the control condition. In the interaction of group and prime, HC adolescents had greater activation in the right superior frontal gyrus than did the BD group following affective priming.
Exploratory ROI analysis from the 2-way ANOVAs showed that during the anticipation of gain following affective priming, youth with BD with high levels of mania at the scan (YMRS>20, n=11) exhibited less activation in the NAcc bilaterally than did youth with BD with low levels of mania (YMRS≤20, n=13) (p=0.003, uncorrected). Levels of depression were not associated with a priming effect.
DISCUSSION
The present study is the first to compare neural activation during reward processing after affective priming in adolescents with BD and healthy adolescents. Youth with BD were found to show aberrant neurobehavioral responses during anticipation and receipt of reward and loss with affective priming. Our results point to anomalous mechanisms of reward processing in youth with BD that may be related to aberrant goal pursuit and motivation. Specifically, compared with their HC peers, BD youth showed decreased activation in the thalamus and inferior temporal gyrus while anticipating gains after priming, but increased activations in the middle frontal gyrus and parietal cortices while anticipating losses after priming. Youth with BD also showed less activation in the inferior parietal lobule, thalamus, and superior frontal gyrus while receiving losses after priming. Given the significant effect of valence, we conducted an exploratory examination of gain and loss conditions separately in a secondary analysis. This analysis yielded increased activation in prefrontal and subcortical regions that have been documented to subserve functions related to reward processing, such as motivation, goal-pursuit (caudate, OMFC) and emotion regulation (OMFC, ACC). Increased orbitomedial frontal activation (BA 10) in BD relative to HC youth during reward anticipation was consistent with our prediction. It is noteworthy, however, that the BD group also showed decreases in sgACC activation following affective priming, suggesting interference in cognitive control over reward processing during primed reward anticipation. In addition, compared with HC adolescents, youth with BD showed speeded reaction times and decreases in activation in the parahippocampal gyrus during loss anticipation after affective priming. In summary, the profile of aberrant activation observed in our adolescent BD sample provides an initial picture of developmental brain dysfunction that may underlie disordered goal pursuit and motivation in individuals at the earliest stages of this serious neuropsychiatric disorder.
Our study showed that in BD youth, processing of reward after affective priming results in aberrant activations in regions important in information relay to the prefrontal cortex (thalamus), visuospatial processing (parietal cortex, inferior temporal gyrus), uncertainty and self-awareness (superior frontal and middle frontal gyrus). These regions have been previously implicated in individuals with BD while they are performing tasks assessing visuospatial emotion processing and reactivity,30 working memory,31 sustained attention with emotional and neutral distracters,32,33,34 response inhibition,35 and reversal learning.36 Most relevant to bipolar symptomatology, however, particularly in the context of hedonic drive, grandiosity, and poor insight were the findings of prefrontal activation in the middle and superior frontal gyri. Contrary to our prediction, we found increased activation in the middle frontal gyrus in BD youth during the primed loss compared with the primed gain condition, suggesting that priming has differential effects on gain and loss conditions in pediatric BD. It is noteworthy, however, that decreased activation in the superior frontal gyrus after primed loss receipt is consistent with prior studies that have associated this region with aberrant attention to negative emotional information37 and decreased top down control of emotional reactivity30 in youth with bipolar symptoms. Our results point to potential neural mechanisms that may underlie disordered self-awareness or insight during goal pursuit and motivation in BD.38 That similar activation patterns have been observed in youth with BD across a variety of cognitive tasks39 suggests that these candidate neural regions that are likely involved in the pathophysiology of BD.
Although exploratory and not derived from the primary three-way analysis, the findings from the secondary two-way whole brain ANOVAs examining activations within each condition, combined with literature on reward processing, provide a relevant context within which to understand the role of the medial prefrontal cortex in the processing of rewarding stimuli in youth with BD.13 Prior research suggests that mesocorticolimbic brain regions subserve a hierarchical model of reward processing that may be relevant to several possible pathways leading to dysregulated goal pursuit and motivation in BD. Whereas striatal regions represent an affective component expressed as arousal and action, cortical regions represent a probabilistic component that may be related to confidence in goal attainment and may manifest in BD as grandiosity and dysregulated goal pursuit.27 In the secondary two-way ANOVA, the main effect of group suggests that typical mechanisms of reward processing are altered in BD. Whereas youth with BD showed increased OMFC activation, HC youth activated the angular gyrus bilaterally during reward anticipation. OMFC activation may be related to the perceived likelihood of attaining a reward25 whereas the angular gyrus has been found to be involved in calculations concerning arithmetic fact retrieval.40 Although the probability of reward in the MID task is titrated individually to be held constant across all participants,27 the subjective perception of higher probability of reward outcomes may underlie illness-associated characteristics such as unrealistic outcome expectations3,41 and impaired decision making42 that may make individuals with BD particularly vulnerable to increased reward reactivity during reward anticipation. For example, when youth with BD received feedback about losing money after priming, both primary and secondary analyses yielded deficits in the recruitment of superior frontal regions relative to HC adolescents, putatively indicating a lack of self-awareness that would otherwise strengthen executive control to improve performance. Importantly, BD and HC groups did not differ in ventrostriatal activation during reward anticipation in either the primary or secondary analyses, suggesting a lack of differential regard for reward magnitude during this condition.10,11,27 Together, these findings suggest that reward magnitude is less salient for youth with BD than is OMFC-mediated reward probability, and that BD youth have aberrant prefrontal executive control when primed to process rewards.
This study was also a proof-of-concept investigation to examine whether an affective prime has neurobehavioral effects. Behaviorally, we found that affective priming resulted in increased reaction times in BD relative to HC youth, particularly during reward anticipation followed by affective priming. Self-report ratings of increased happiness, excitability, and confidence were observed after priming across all participants, permitting neural comparisons without the confound of heterogeneous mood states within the BD group. Regarding neural activation effects associated with priming, which have not previously been studied, we obtained main effects of priming that included increased frontostriatal activation during anticipation of reward and loss. Reductions in activation after priming were found in the thalamus and inferior temporal gyrus during gain anticipation and in the thalamus, inferior parietal lobule, and superior frontal gyrus during loss feedback, suggesting task condition-specific effects that might aid in understanding how different mood states (versus traits) influence reward processing. In a previous study using positive mood inductions to examine mood state and trait factors that mediate cognitive changes associated with emotional processing in BD, investigators reported that positive mood inductions were more effective in individuals with BD than in controls.43 In that study, individuals with BD showed a positive emotional bias on an affective Go/No-Go task and performed more slowly than did controls on a Cambridge Gambling Task (CGT), especially while making more difficult decisions. That study was limited, however, by lack of a neutral control condition, so latency differences on the CGT could not be explained as being due to state or trait effects in BD. Together, these data underscore the strengths of our experimental design.
In the present study, speeded reaction times and neural interactions of group and priming support the notion that an affective prime interferes with typical processing of reward stimuli. The fact that the interaction of group and prime in self-report ratings was not significant suggests that neural differences between BD and HC groups are unlikely to be explained by any state-related positive affect that was induced with priming. Significant reductions in thalamic and inferior temporal activations following affective priming in BD relative to HC during reward anticipation, and greater NAcc deactivation during reward anticipation following affective priming in youth with BD with higher levels of manic symptoms, provide supporting evidence that errors in reward prediction signaling may be due to desensitization or downregulation of NAcc during reward activation.10 Thus, deficits in thalamic, temporal and striatal activation may be due to interference of typical reward processing by trait affective symptoms, or may reflect abnormal top-down modulation of these regions by higher cortical regions, a phenomenon that has been documented with other emotion processing30,31,44,45,46 and reversal learning47 tasks in youth with BD.
We should note a number of limitations of this study. First, to minimize motion artifact and limit the strain of scanning teenagers with significant mood symptoms, the MID task we administered only permitted nine replications of each trial type, which may have reduced power to obtain significant effects. Second, our cross-sectional design does not permit a separation of mania- versus depression-dependent contributions to our findings that may center on state-dependent enhanced arousal to reward during mania and on avoidance of punishment or loss during depressed and euthymic states. Indeed, although the affective priming task influenced self-reported affect and neural activation, it did not intensify activation in reward- or emotion-related regions across conditions. Importantly, however, an advantage of adding an affective prime is that it may have neutralized any confound of heterogeneity of opposing mood states on the day of scan by inducing a comparable affective state in all participants; moreover, a control condition that counterbalanced the affective prime permitted us to make inferences about trait versus state features of BD. Third, although we attempted to minimize heterogeneity in our sample, there was still variation in levels of medication and substance exposure, and in time elapsed since the first episode of mania. Although it is possible that group differences in neural responses are due to differential exposure to medications, this is unlikely in the present sample in which participants had an average of only 16 weeks of lifetime medication exposure, and in which length of lifetime exposure to each of atypical antipsychotics, lithium, antidepressants, or psychostimulants did not correlate with any significant activations within the BD group. Finally, although not within the scope or goals of the current study, further investigation is needed to relate these findings to true trait features of BD that could be evaluated in adolescents at risk for BD preceding the onset of illness, in order to examine the specificity of these findings to adolescent-onset BD as opposed to other psychiatric disorders in this age group, and to determine whether these characteristics predict long-term clinical outcome. Nevertheless, we found group differences in key brain regions involved in cognition, self-awareness, motivation, and affect in adolescents with and without mania providing an initial step in understanding the role of reward processing in the neurophysiology of pediatric BD.
In summary, mania affects neural mechanisms underlying the anticipation and receipt of reward. In this study we present evidence that early after the onset of mania, adolescents with BD exhibit anomalies in prefrontal and subcortical regions during reward processing. These regions may be promising candidates for biological markers for the development and progression of BD into adulthood. Future research is needed to examine the longitudinal trajectories of these characteristics and their ability to predict the clinical progression of this disorder over time.
Clinical Guidance.
Youth with bipolar disorder (BD) show aberrant neurobehavioral responses during anticipation and receipt of reward and loss with affective priming.
Compared with typically developing adolescents, youth with BD had speeded reaction times while anticipating primed rewards, and showed aberrant patterns of activation in regions important for information relay to the prefrontal cortex (thalamus), visuospatial processing (parietal cortex, inferior temporal gyrus), and self-awareness (superior frontal and middle frontal gyrus).
Youth with BD who had higher levels of manic symptoms showed decreases in nucleus accumbens activation compared to those with lower levels of manic symptoms.
Together, these patterns of activation may clinically manifest in BD as grandiosity and dysregulated goal pursuit, and distinguish psychopathology from processes associated with typical adolescence.
Acknowledgments
The authors thank Melissa Pease, Erica Weitz, Elizabeth Adams, Tenah Acquaye, and Layla Bararpour of Stanford University for their assistance with recruitment and data collection, and Allison Libby of Palo Alto University for help with data entry. This research was supported by the Klingenstein Third Generation Foundation Depression Fellowship and the National Institute of Mental Health grant MH085919 to Manpreet K. Singh. This paper was presented as a poster at the annual meeting for the American College of Neuropsychopharmacology in Hollywood, Florida on December 9, 2009.
Footnotes
This paper was presented as a poster at the annual meeting for the American College of Neuropsychopharmacology in Hollywood, Florida on December 9, 2009.
AUTHOR DISCLOSURES
Dr. Singh. Drs Singh, Cui, and Gotlib, Mr. Kelley, Ms. Sherdell, and Ms. Howe report no biomedical financial interests or potential conflicts of interest. Dr. Chang is a consultant for GlaxoSmithKline, Eli Lilly and Company, Merck, and Bristol-Myers Squibb; receives research support from GlaxoSmithKline, Merck, National Institute of Mental Health, and National Alliance for Research in Schizophrenia and Depression. Dr. Reiss is a consultant for Novartis for development of neuroimaging biomarkers in Fragile X Syndrome.
FINANCIAL DISCLOSURES
Dr. Chang is a consultant for GlaxoSmithKline, Eli Lilly and Company, Merck, and Bristol-Myers Squibb; receives research support from GlaxoSmithKline, Merck, National Institute of Mental Health, and National Alliance for Research in Schizophrenia and Depression. Dr. Reiss is a consultant for Novartis for development of neuroimaging biomarkers in Fragile X Syndrome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perlis RH, Dennehy EB, Miklowitz DJ, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SL. Mania and dysregulation in goal pursuit: a review. Clin Psychol Rev. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic Patients with Bipolar Disorder Show Decreased Reward Learning in a Probabilistic Reward Task. Biological Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller SC, Ng P, Temple V, et al. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. J Psychopharmacol. 2010;24:1779–1784. doi: 10.1177/0269881109353462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorrindo T, Blair RJ, Budhani S. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 7.Farmer A, Lam D, Sahakian B, et al. A pilot study of positive mood induction in euthymic bipolar subjects compared with healthy controls. Psychol Med. 2006;36:1213–1218. doi: 10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- 8.Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ernst M, Dickstein DP, Munson S, et al. Reward-related processes in pediatric bipolar disorder: a pilot study. J Affect Disord. 2004;82(Suppl 1):S89–S101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal Reward System Activation in Mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermpohl F, Kahnt T, Dalanay U, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nusslock R, Almeida JR, Forbes EE, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar disorders. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linke J, King AV, Rietschel M, et al. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169:316–325. doi: 10.1176/appi.ajp.2011.11050711. [DOI] [PubMed] [Google Scholar]
- 14.Geller B, Zimerman B, Williams M, Frazier J. Washington University at St Louis Kiddie and Young Adult Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St. Louis, MO: Washington University School of Medicine; 1996. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL). Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 17.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 18.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 19.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Hollingshead AB. Four Factor Index of Social Status. New Haven: Yale Press; 1975. [Google Scholar]
- 21.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace Inc; 1999. [Google Scholar]
- 23.Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 24.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 25.Gotlib IH, Hamilton JP, Cooney RE, et al. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 29.Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 32.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleck DE, Eliassen JC, Durling M, et al. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17:325–336. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullin BC, Perlman SB, Versace A, et al. An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Res. 2012;201:196–205. doi: 10.1016/j.pscychresns.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010;71:1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 201(50):1173–1185. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich BA, Carver FW, Holroyd T, Rosen HR, Mendoza JK, Cornwell BR, Fox NA, Pine DS, Coppola R, Leibenluft E. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011;45:1283–94. doi: 10.1016/j.jpsychires.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin JM, Jeanningros R, Goodwin GM. Lack of insight may predict impaired decision making in manic patients. Bipolar Disord. 2008;10:829–837. doi: 10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 39.Fusar-Poli P, Howes O, Bechdolf A, Borgwardt S. Mapping vulnerability to bipolar disorder: a systematic review and meta-analysis of neuroimaging studies. J Psychiatry Neurosci. 2012;37:170–184. doi: 10.1503/jpn.110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 41.Meyer B, Beevers CG, Johnson SL. Goal appraisals and vulnerability to bipolar disorder: A personal projects analysis. Cognitive Therapy and Research. 2004;28:173–182. [Google Scholar]
- 42.Murphy FC, Rubinsztein JS, Michael A, et al. Decision-making cognition in mania and depression. Psychol Med. 2001;31:679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- 43.Roiser J, Farmer A, Lam D, et al. The effect of positive mood induction on emotional processing in euthymic individuals with bipolar disorder and controls. Psychol Med. 2009;39:785–791. doi: 10.1017/S0033291708004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 45.Dickstein DP, Rich BA, Roberson-Nay R, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12:707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]