Abstract
For many years, clinicians have commented on the development of signs of parkinsonism among their essential tremor (ET) patients but the links between ET and parkinsonism are not well understood. We report 11 of 89 ET patients (12.4%) who were prospectively collected at the Essential Tremor Centralized Brain Repository over the course of its first 9 years. All patients had longstanding ET (median duration = 38 years); there was a 5- to 49-year latency from the onset of ET to the development of either parkinsonism or dementia. Despite the presence of parkinsonism or dementia during life, none had been diagnosed clinically with progressive supranuclear palsy (PSP). All 11 received the postmortem diagnosis of PSP. The prevalence of PSP in this ET sample (12.4%) is clearly larger than the population prevalence of PSP (0.001% to 0.0065%). It is also 2 to 5 times the proportion of normal cases with incidental PSP in 2 prior autopsy series. This case series raises the questions of an association between ET and PSP, whether ET patients are at increased risk of developing PSP, and what the proportion of ET patients who develop presumed PD or AD in life actually have PSP (i.e. ET+PSP).
Keywords: Essential tremor, Glial cytoplasmic inclusion, Movement disorder, Neurodegeneration, Parkinsonism, Progressive supranuclear palsy, Tau
INTRODUCTION
Essential tremor (ET) is a disease or group of diseases the central defining clinical feature of which is action tremor (1, 2). Rest tremor may also develop in a small proportion of ET patients with longstanding disease or severe action tremor (3). Progressive supranuclear palsy (PSP) is a syndrome variably characterized by supranuclear palsy, postural instability and, in many cases, some degree of parkinsonism. It may also be characterized by dementia (4, 5). It is defined neuropathologically by the accumulation of tufted astrocytes and tau-positive neurofibrillary tangles in regions of relative vulnerability in the striatum, brainstem, cerebellum, and cerebral cortex (4). Action tremor is not a prominent or well-described clinical feature of PSP.
For many years, clinicians have commented on the development of signs of parkinsonism and presumed Parkinson disease (PD) among their ET patients (ET+PD) (6, 7), and both cross-sectional and longitudinal studies have reported increased odds of PD and increased risk of PD among prevalent ET patients (8, 9). In some postmortem series, brainstem Lewy bodies are more prevalent in ET patients than in controls (10, 11). This furthers interest in the pathological and mechanistic basis of a possible connection between the 2 disorders. However, the links between ET and parkinsonism are not well understood.
Here, we report the clinicopathological findings of 11 of 89 ET patients (12.4%) who were prospectively collected at the Essential Tremor Centralized Brain Repository (ETCBR) over the course of its first 9 years (late 2003 to early 2012). All 11 patients received a postmortem diagnosis of PSP.
MATERIALS AND METHODS
Clinical Evaluation
The ETCBR of the New York Brain Bank, Columbia University Medical Center, is a centralized repository for the prospective collection of brains from ET patients living in the United States. ET patients may learn about the repository through several sources including advertisements on ET organizational websites (International Essential Tremor Foundation, Tremor Action Network), and through an ETCBR study website. Potential participants signed an informed consent form approved by the Columbia University Medical Center Internal Review Board.
ET diagnoses were carefully assigned using each of the following 3 sequential methods. First, nearly all (88/89 = 98.9%) patients were diagnosed clinically with ET by their treating physician (83% of these physicians were neurologists); 1/89 (1.1%) was diagnosed by his spouse who was a registered nurse. Second, patients were asked to complete a series of semi-structured clinical questionnaires (i.e. demographic data, general medical data, tremor-specific data), which included data on age of onset (i.e. age at first symptoms and signs of ET) and family history information (i.e. reportedly affected relatives). Each patient then submitted 4 standardized hand-drawn Archimedes spirals (2 right and 2 left, each on a 8.5 × 11 inch sheet of paper). This was supplemented with additional clinical information (from clinical records, treating physicians, family members) in each patient. ET diagnoses were then confirmed by a senior neurologist specializing in movement disorders (E.D.L.) who used the following criteria: 1) moderate or greater amplitude arm tremor (rating of 2 or higher on the Washington Heights Inwood Genetic Study of Essential Tremor rating scale [12]) in at least 1 of the submitted Archimedes spirals; 2) no history of PD or dystonia; 3) no other etiology for tremor (e.g. medications). ET patients then underwent a standardized, videotaped neurological examination, including a detailed assessment of tremor (13). The videotape protocol included assessment of postural, kinetic, and rest tremor of the limbs as well as neck, voice and jaw tremors. In total, the videotaped examination included 1 test to elicit postural tremor (sustained arm extension) and 5 tests to elicit kinetic tremor (e.g. writing, pouring). The videotaped examination also included the motor portion of the Unified Parkinson's Disease Rating Scale, including assessments of speech, reading out loud, facial expression, rest tremor (with arms in 3 positions: resting in the lap, relaxed at sides while standing, and while walking), bradykinesia, posture, arising from a chair, and gait while walking and turning (14). Horizontal and lateral smooth pursuit eye movements were assessed, as were vertical saccades. Each videotape was reviewed (E.D.L.), and based on the questionnaire and videotape data, the diagnosis of ET was re-examined in each patient using published diagnostic criteria (moderate or greater amplitude kinetic tremor [tremor rating ≥2] during 3 or more activities or a head tremor in the absence of PD) (12).
Patients completed a follow-up telephone questionnaire, which included a series of screening questions for PD and dystonia every 6 months; they also submitted 4 new standardized Archimedes spirals (2 right and 2 left) on 8.5 × 11 inch sheets of paper. A brief cognitive screen was administered (Telephone Interview for Cognitive Status) (15). A follow-up videotaped neurological examination was performed if any screening question was positive for PD or dystonia, or if the spiral showed signs of micrographia. Development of ET+PD or ET and dementia was not an exclusionary criterion for further follow-up or brain donation. Patients also continued with follow-up by their treating physicians. Between late 2003 and early 2012, 89 ET patients died and their brains were prospectively collected.
Neuropathology
Brains were characterized at the New York Brain Bank, which operates under the approval of the Columbia University Medical Center Internal Review Board. Each brain underwent a comprehensive neuropathological assessment and determination of detectable pathological findings (website: nybb.hs.columbia.edu). Standardized measurement of brain weight (grams) and postmortem interval (i.e. hours between death and placement of brain in a cold room or upon ice) was performed. All brains underwent Braak PD staging of Lewy bodies (i.e. neuropathologic stage of PD related changes [NSPD]) (16), and NIA-Reagan Criteria for AD (17).
Blocks were taken from standardized brain regions and embedded in paraffin; 7-μm-thick sections were stained with Luxol fast blue counterstained with hematoxylin and eosin (LH&E) (10, 18). Additional sections from selected blocks were stained with modified Bielschowsky silver technique and others were immunostained with antibodies directed against α-synuclein (1:40, Leica, Buffalo Grove, IL) (including cerebral cortex, hippocampal formation, globus pallidus, putamen, amygdala, midbrain with substantia nigra, pons with the locus ceruleus, medulla with the dorsal vagal nucleus, and olfactory bulbs); β-amyloid (1:400, Biocare Medical, Concord, CA) (including cerebral cortex, hippocampal formation, caudate nucleus, putamen, and thalamus); hyperphosphorylated tau (AT8) (1:200, Thermo Scientific, Rockford IL) (including hippocampus, globus pallidus, putamen, caudate nucleus, amygdala, thalamus, subthalamic nucleus, mesencephalon with red nucleus, pons, medulla oblongata, cerebellum with dentate nucleus, and cerebral cortex); Tar-DNA binding protein-43 (TDP-43) (1:2000, Protein Tech Group, Chicago, IL); and glial fibrillary acidic protein (Ventana, Tucson, AZ) proteins. The following selected blocks were stained with antibodies directed against ubiquitinated proteins (1:300, Dako, Carpinteria, CA): superior frontal cortex; posterior frontal cortex; parietal cortex; calcarine cortex; hippocampal formation with lateral geniculate body and tail of caudate nucleus; caudate, putamen, and nucleus accumbens; globus pallidus and putamen with claustrum; cerebellum; subthalamic nucleus with anterior thalamus; anterior hippocampal formation; and pituitary gland.
A standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was also harvested from the neocerebellum (10); the block included the cerebellar cortex, white matter and dentate nucleus. A senior neuropathologist who was blinded to all clinical information counted torpedoes throughout 1 LH&E-stained, 7-um-thick section and, when available, 1 entire Bielschowsky 7-um-thick section, and counted and averaged Purkinje cells in 5 100x fields (LH&E) (10). A semiquantitative (0–3) rating of the appearance of the basket cell plexus surrounding Purkinje cell bodies throughout Bielschowsky preparations was carried out by the same neuropathologist, as previously described (19).
In all patients, the neuropathological diagnosis of PSP included 1) the presence of tau-positive tufted astrocytes in the cerebral cortex, neostriatum, and amygdaloid nucleus; 2) globose neuronal tangles in at least 7 of the 9 following sites: cerebral cortex, neostriatum, globus pallidus, subthalamic nucleus, red nucleus, pars compacta of the substantia nigra, pontine nuclei, inferior olivary nucleus, and in the dentate nucleus of the cerebellum; and 3) scattered tau-positive glial cytoplasmic inclusions (20-22). Neuronal loss was rated as 0 (none), 1 (mild), 2 (moderate), or 3 (severe) in the dentate nucleus, globus pallidus, substantia nigra pars compacta and subthalamic nucleus. We compared the tau pathology, torpedo counts, Purkinje cell counts, and basket process ratings in these 11 ET+PSP patients to 10 pure PSP patients ascertained from the New York Brain Bank during the same time period.
RESULTS
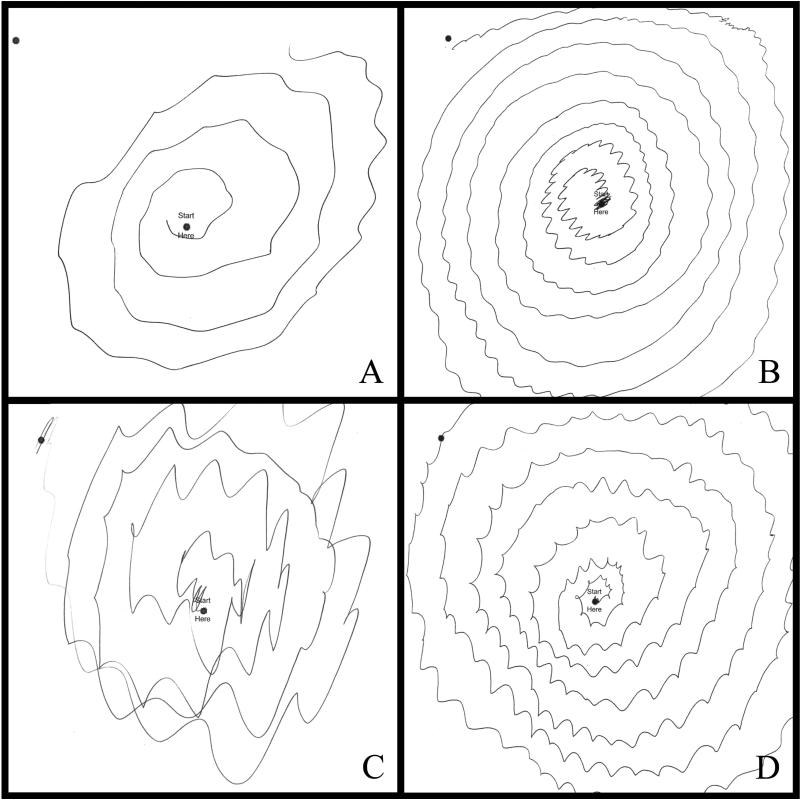
Between late 2003 and early 2012, 89 ET patients died and their brains were prospectively collected. At postmortem, 1/89 had PD and 11 others had PSP, including 1 with PSP+PD. None of the remaining 77 ET brains had PD or PSP. There were 11 patients with ET+PSP (Tables 1–3), including 9 (81.8%) women. All had had an ET diagnosis during life assigned by their treating neurologist; all had had moderate or greater amplitude arm tremor (rating of 2 or higher) in at least 1 of the submitted Archimedes spirals (Fig. 1A-D); 8 had 1 or more videotaped neurological examinations reviewed by a senior neurologist specializing in movement disorders (E.D.L.) and their ET diagnoses were confirmed. Age of onset of ET (action tremor) ranged from 8 to 88 years (median = 41 years), and in 8 of the 11 (72.7%) this occurred in mid-life (between 32 and 56 years). Duration of ET at death ranged from 10 to 75 years (median = 38 years), and in 9 (81.8%) the duration was 30 or more years. All patients had clear bilateral action tremor in the arms that was moderate or severe, and 8 (72.7%) had tremor involving cranial structures (neck, jaw or voice). Ten patients (90.9%) had taken 1 or more medication for ET; 2 (18.2%) had had surgery (deep brain stimulation [DBS] or gamma knife) for ET. Eight (72.7%) had 1 or more reportedly affected first-degree relative. Aside from arm tremor, 3 patients had other features that had been noted in the chart as worthy of comment but not diagnostically inconsistent with ET: head tremor that was more marked than arm tremor (case 3); head tremor that was more marked than arm tremor, and forehead tremor (case 5); and forehead tremor and lip tremor (case 9).
Table 1.
Demographic Features and Clinical Features of Essential Tremor Patients
| Case Number | Gender | Age at Death (y) | Age at ET Onset (y) | Duration of ET at Death (y) | Spiral Ratings1 | HT, JT, VT | Additional Comments on Clinical Features of ET | ET Medications | Family History of ET |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 89 | 56 | 33 | R = 2 L = 2 |
HT, JT | None | Prim, Dz | None |
| 2 | M | 98 | 88 | 10 | R = 3 L = 3 |
None | None | Prim, Top | Sister |
| 3 | F | 92 | 41 | 51 | R = 2 L = 2 |
HT | HT > arm tremor. “Yes- yes” HT. | Gab | Sister |
| 4 | F | 99 | 53 | 46 | R = 3 L = 3 |
HT | None | None | Son, Granddaughter |
| 5 | F | 85 | 55 | 30 | R = 1.5 L = 2 |
JT, VT | Head > arm tremor. Forehead tremor | Prop, Prim Cz | None |
| 6 | M | 77 | 35 | 42 | R = 2 L = 1 |
None | None | Prop, Gab2 | Mother |
| 7 | F | 74 | 36 | 38 | R = 3 L = 3 |
HT, JT, VT | None | Prop, Prim, Top, Dz, Cz, Mir | Father, Brother, Sister |
| 8 | F | 88 | 32 | 56 | R = 2.5 L = 3 |
HT, JT, VT | None | Prop, Prim, Gab, Dz, botulinum toxin injections3 | Mother, Sister, Niece |
| 9 | F | 83 | 8 | 75 | R = 2 L = 2 |
JT | Forehead tremor. | Yes, but details not known | Father, Son, Sister, |
| 10 | F | 87 | 74 | 13 | R = 2 L = 1.5 |
None | Lip tremor None | Prim | Grandfather Mother |
| 11 | F | 91 | 55 | 36 | R = 1 L = 2 |
HT | None | Prop, Top, Gab | None |
Cz = clonazepam, Dz = diazepam, ET = essential tremor, F = female, gab = gabapentin, HT = head (i.e., neck) tremor, JT = jaw tremor, L = left arm, M = male, Mir = mirtazapine, Prim = primidone, Prop = propranolol, R = right arm, Top = topiramate, VT = voice tremor, y = years
R = mean of two 0 – 3 ratings from right hand; L = mean of two 0- 3 ratings from left hand. Washington Heights Inwood Genetic Study of Essential Tremor rating scale (0 = none, 1 = mild, 2 = moderate, 3 = severe).
Case 6 also had left thalamic deep brain stimulation surgery for tremor control (at age 74).
Case 8 also had left gamma knife radiosurgical thalamotomy for ET (age 83) and right Vim brain stimulation surgery for tremor (age 84).
Table 3.
Postmortem Fiindings
| Case # | Tauopathic changes / PSP distribution1 | Tufted Astrocytes2 | Neuronal loss3 | LBs NSPD | Postmortem PD Diagnosis | NIA-Reagan Criteria for AD | Torpedo Count | PC Count | Basket Process Rating | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNc | STN | GP | |||||||||
| D | |||||||||||
| 1 | 1+ | 1+ | 2 | 0 | 0 | 0 | No | Low | 14 (LH&E) | 7.4 | 3 |
| 1 | 30 (Biel) | ||||||||||
| 2 | 1+ | 1+ | 2 | 0 | 0 | 0 | No | 0 | 6 (LH&E) | 6.6 | NA |
| 1 | NA (Biel) | ||||||||||
| 3 | 2+ | 2+ | 2 | 0 | 0 | 0 | No | 0 | 6 (LH&E) | 6.9 | 2.5 |
| 1 | 24 (Biel) | ||||||||||
| 4 | 1+ | 1+ | 1 | 0 | 0 | 0 | No | Low | 23 (LH&E) | 4.6 | NA |
| 1 | NA (Biel) | ||||||||||
| 5 | 1+ | 1+ | 1 | 0 | 0 | 0 | No | Low | 4 (LH&E) | 12.6 | 3 |
| 1 | 15 (Biel) | ||||||||||
| 6 | 2+ | 2+ | 2 | 1 | 1 | 4 | Yes4 | 0 | 20 (LH&E) | 5.5 | 1 |
| 1 | 37 (Biel) | ||||||||||
| 7 | 1+ | 1+ | 1 | 0 | 1 | 0 | No | Low | 7 (LH&E) | 9.2 | NA |
| 0 | NA(Biel) | ||||||||||
| 8 | 2+ | 2+ | 2 | 0 | 1 | 0 | No | Low | 13 (LH&E) | 9.0 | 3 |
| 0 | 27 (Biel) | ||||||||||
| 9 | 1+ | 2+ | 2 | 0 | 0 | Yes5 | No | 0 | 5 (LH&E) | 9.7 | 2 |
| 0 | 15 (Biel) | ||||||||||
| 10 | 3+ | 3+ | 3 | 3 | 3 | 0 | No | Intermediat | 13 (LH&E) | 7.5 | NA |
| 2 | e | NA (Biel) | |||||||||
| 11 | 2+ | 2+ | 2 | 0 | 0 | 0 | No | 0 | 10 (LH&E) | 6.8 | 2.5 |
| 1 | 21 (Biel) | ||||||||||
AD = Alzheimer disease, Biel = Bielschowsky impregnation, D= dentate nucleus (cerebellum), GP = globus pallidus, LBs = Lewy bodies, LH&E = Luxol fast blue counterstained with hematoxylin and eosin, NA = not available, NSPD = neuropathologic stage of Parkinson disease related changes, PC = Purkinje cell count, PD = Parkinson disease, PSP = progressive supranuclear palsy, SNc = substantia nigra pars compacta, STN = subthalamic nucleus
refers to globose neuronal tangles found in at least 7 of the following 9 sites: cerebral cortex, neostriatum, globus pallidus, subthalamic nucleus, red nucleus, pars compacta of the substantia nigra, pontine nuclei, inferior olivary nucleus, and dentate nucleus of the cerebellum. 1+ (1 per 100X microscopic field), 2+ (up to 3 per 100X microscopic field) 3+ (> 3 per 100X microscopic field).
Relative densities of tufted astrocytes within the cerebral cortex, neostriatum, globus pallidus, amygdala, and subthalamic nucleus. 1+ (1 per 100X microscopic field), 2+ (up to 3 per 100X microscopic field) 3+ (> 3 per 100X microscopic field).
Neuronal loss was rated as 0 (none), 1 (mild), 2 (moderate), 3 (severe).
Case 6 had a postmortem diagnosis of both PSP and PD. The neuropathologic stage of PD related changes (NSPD), as proposed by Braak and Braak, was 4 out of 6.
Lewy body-containing neurons confined to the substantia innominata and pars compacta of the substantia nigra, not found within the dorsal nucleus of vagus or within the nucleus coeruleus, thus the distribution of Lewy body-containing neurons in this brain does not recapitulate the NSPD scheme as proposed by Braak and Braak.
Figure 1.
Archimedes spirals drawn with the right hand in cases 2 (A), 6 (B), 7 (C), and 8 (D). There is moderate or greater tremor (rating ≥2) in each case.
Three patients (27.2%) were diagnosed during life with PD (Table 2); the remaining 8 were not diagnosed during life with PD or atypical parkinsonism (e.g. PSP). The latency from onset of ET to PD was 20 years (case 1), 35 years (case 11), and 41 years (case 6). Seven of the remaining 8 patients had rest tremor; 6 of these had some other subtle feature of mild parkinsonism (e.g. mild bradykinesia, mild hypophonia, positive pull test), which was evident on examination later in life, 11, 28, 35, 45, 49 and 68 years after the onset of ET. One of these 6 cases (case 3) had had minor early indicators suggestive of atypical parkinsonism (i.e. early postural instability, deep nasolabial folds, rest tremor that seemed more severe than kinetic tremor) that were noted 45 years after the onset of ET; this patient also had a clinical diagnosis of dementia, diagnosed 47 years after the onset of ET.
Table 2.
Clinical Features of Parkinsonism and Dementia in Cases with Essential Tremor
| Case # | Parkinsonism on Neurological Examination | Latency from ET to Parkinsonism on Neurological Examination (y) | PD or Atypical Parkinsonism Diagnosed During Life | Treated with carbidopalevodopa | Cognitive Complaints | Normal Cognition on Examination | Clinical Diagnosis of Dementia | Latency from ET to Dementia (y) | Latency from last neurological examination to death (m or y) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | RT, B, ePI | 20 | Yes (PD, age 76) | Yes | No | Yes | No | NA | 1m |
| 2 | None | NA | No | No | Yes | No | Yes (AD, age 93) | 5 | 5.5 y |
| 3 | RT, B, ePI, deep NLFs | 45 | No | No | Yes | No | Yes, (Dementia, age 88) | 47 | 3.5 y |
| 4 | RT only | NA1 | No | No | Yes | No | Yes (AD, age 96) | 44 | 1 y |
| 5 | RT, + pull test | 28 | No | No | No | Yes | No | NA | 1 y |
| 6 | RT, B, R, PI | 41 | Yes (PD, age 76) | Yes | Yes | No | Yes (Dementia, 75) | 40 | 9 m |
| 7 | RT, +pull test | 35 | No | No | No | Yes | No | NA | 8 m |
| 8 | RT, hypophonia | 49 | No | No | Yes | Yes | No | NA | 3 m |
| 9 | RT, mild B, + pull test | 68 | No | No | No | Yes | No | NA | 1.5 y |
| 10 | RT, mild B, mild hypomimia | 11 | No | No | No | Yes | No | NA | 1 y |
| 11 | RT, R | 35 | Yes | Yes | No | Yes | No | NA | 4 m |
AD = Alzheimer disease, B = bradykinesia, ePI = early postural instability, ET = essential tremor, F = female, L = left, NA = not applicable, m = months, NLFs = nasolabial folds, PD = Parkinson disease, PI = postural instability, R = right, RT = rest tremor, y = years, + = positive
Case 4 only had mild rest tremor in the setting of severe, longstanding ET. There was no bradykinesia or other features of parkinsonism.
Five patients had cognitive complaints later in life and 4 of these patients were diagnosed by their treating physician as having had dementia. Cognitive screen scores were low in these. The latency from onset of ET to dementia was 5 years (case 2), 40 years (case 6), 44 years (case 4), and 47 years (case 3). In total, all patients had some parkinsonism or dementia later in life; 7 (63.6%) were diagnosed with PD or dementia during life. None of the patients reported double vision or difficulties with reading (e.g. reduced speed). None of the patients were reported by treating physicians to have eye movement abnormalities and eye movement abnormalities were not noted in the videotaped examinations. The latency from last neurological examination to death was 1 year or less in 8 patients, but ranged from 1 month to 5.5 years (median = 1 years).
All patients had the neuropathological features of PSP (Table 3, Figs. 2-5). The neuropathological diagnosis of PSP was assigned based on the presence of tufted astrocytes, AT8-labeled glial cytoplasmic inclusions, and the presence of globose neuronal tangles. Furthermore, globose neuronal tangles had to be documented within at least 7 of the 9 following sites: cerebral cortex, neostriatum (caudate nucleus and putamen), globus pallidus, subthalamic nucleus, red nucleus, pars compacta of the substantia nigra, pontine nuclei, inferior olivary nucleus, and dentate nucleus of the cerebellum. A semiquantitative assessment of the density of tufted astrocytes and neuronal tangles was performed (Table 3). All cases had neuronal loss in the substantia nigra pars compacta, which was moderate or severe in 8 cases (72.7%), moderate in 7 (63.4%), and severe in 1 (9.1%). There was neuronal loss in the dentate nucleus in 8 (72.7%) cases.
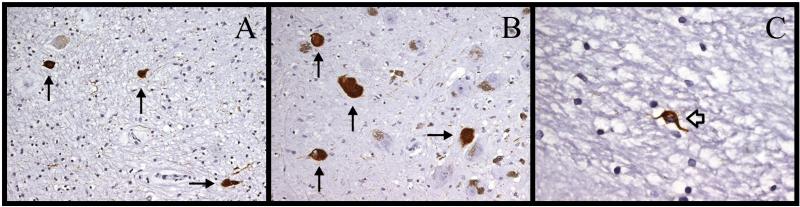
Figure 2.
Immunostaining for hyperphosphorylated tau (AT8) in case 1. (A) There are 3 neurofibrillary tangles ([NFTs], arrows) in the ventral arm of the dentate nucleus. (B) There are 4 NFTs (arrows) and normal pigmented neurons in the caudal substantia nigra pars compacta. (C) This is a glial cytoplasmic inclusion (open arrow) in the anterior limb of internal capsule (630x). Magnifications: A, B, 200x; C, 630x.
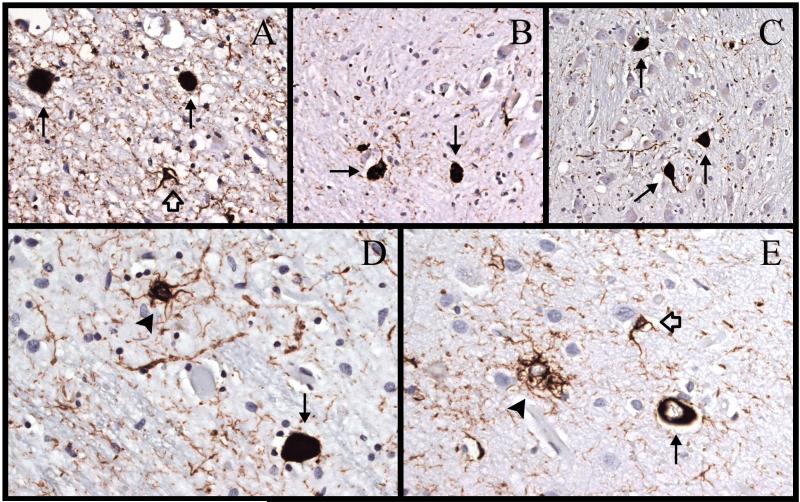
Figure 3.
Immunostaining for hyperphosphorylated tau (AT8) in case 3. (A) There are 2 neurofibrillary tangles ([NFTs], arrows) and a glial cytoplasmic inclusion (GCI) (open arrow) in the subthalamic nucleus. (B) There are 2 NFTs (arrows) and scattered neuropil threads in the dorsal arm of the inferior olivary nucleus. (C) There are 3 NFTs (arrows) in the pontine nuclei. (D) There is an NFT (arrow) and a tufted astrocyte (arrowhead) in the red nucleus. (E) There is an NFT (arrows), a tufted astrocyte (arrowhead), and a GCI (open arrow) in the superior parietal lobe. Magnifications: A, D, E, 400x; B, C, 200x.
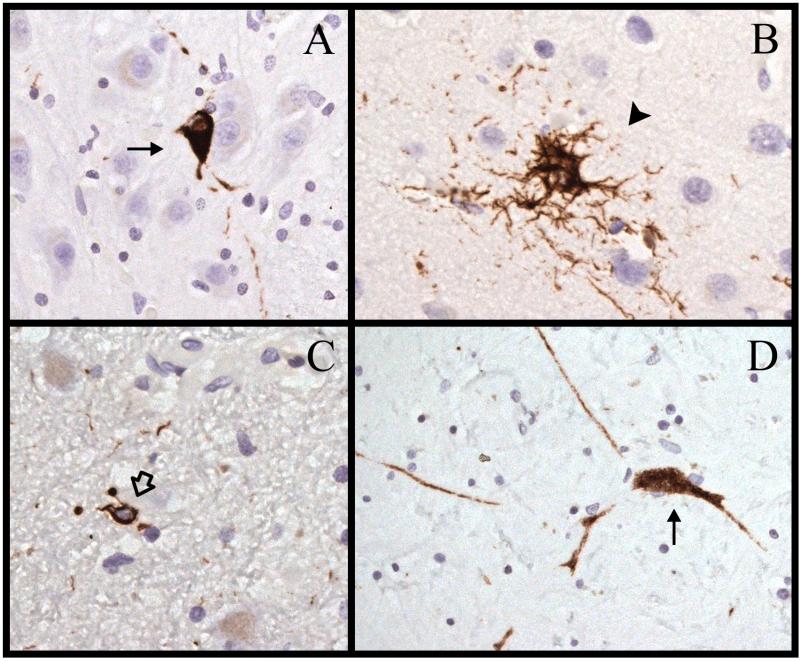
Figure 4.
Immunostaining for hyperphosphorylated tau (AT8) in case 5. (A) There is a neurofibrillary tangle ([NFT], arrow) in the pontine nuclei. (B) There is a tufted astrocyte (arrowhead) in the motor cortex. (C) There is a glial cytoplasmic inclusion (open arrow) in the subthalamic nucleus. (D) There is an NFT with labeled processes (arrow) in the internal segment of the globus pallidus. Magnifications: A, C, D, 400x; B, 630x.
Figure 5.
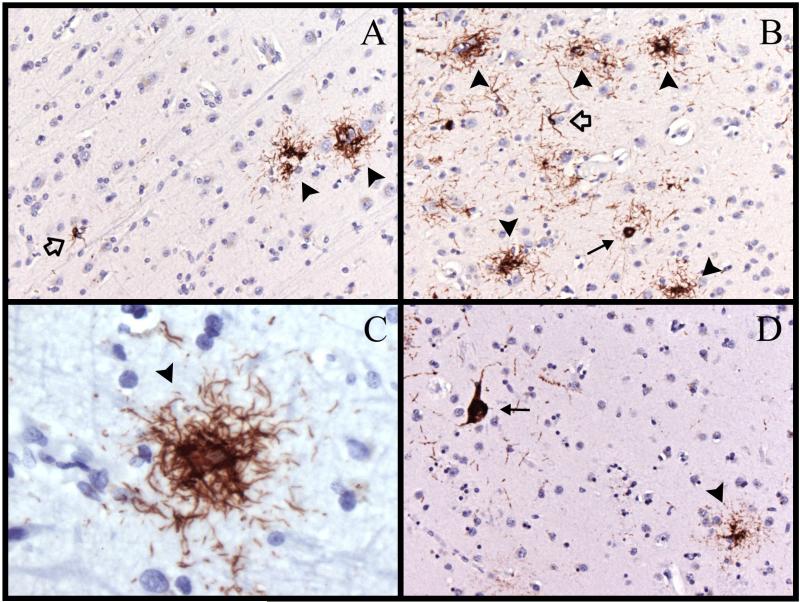
Immunostaining for hyperphosphorylated tau (AT8) in case 8. (A) There are 2 tufted astrocytes (arrowheads) and a glial cytoplasmic inclusion (GCI) (open arrow) in the prefrontal cortex. (B) There are 5 tufted astrocytes (arrowheads), a neurofibrillary tangle ([NFT], arrow) and a GCI (open arrow) in the precuneus. (C) A tufted astrocyte (arrowhead) in the motor cortex. (D) A tufted astrocyte (arrowhead) and an NFT (arrow) in the head of the caudate nucleus. Magnifications: A, B, D, 200x; C, 630x.
We compared the tau pathology in the 11 ET+PSP patients to 10 pure PSP patients ascertained from our brain bank during the same time period; all patient tissue samples were processed using an identical protocol by the same laboratory. The mean neuronal tangle rating was similar (1.5 ± 0.7 in current series vs. 1.7 ± 0.7 in 10 pure PSP patients, t = 0.65, p = 0.53), as was the mean tufted astrocyte rating (1.6 ± 0.7 in current series vs. 1.3 ± 0.5 in 10 pure PSP patients, t = 1.12, p = 0.28). By contrast, the basket process rating of the 11 ET+PSP cases (mean = 2.4 ± 0.7, median = 2.5) was higher than that of the 10 pure PSP cases (mean = 1.1 ± 0.7, median = 1.0) (Mann-Whitney z = 2.87, p = 0.004). The mean/median torpedo counts were also compared and were 11.1/10 (LH&E) and 24.1/24 (Bielschowsky) in ET+PSP vs. 12.4/8 (LH&E) and 13.9/7.5 (Bielschowsky) in pure PSP (for Bielschowsky, Mann Whitney z = 2.06, p = 0.04). The mean/median Purkinje cell counts were similar: 7.8/7.4 in ET+PSP vs. 7.5/9.2 in pure PSP.
We also assessed patients for the possibility of argyrophilic grain disease; only 2 of the 11 patients (cases 4 and 7) had argyrophilic grains in the parahippocampal gyrus; the changes were mild in both patients. Both of these patients also had tau pathology in 7 or more of 9 extra-temporal regions: cerebral cortex, neostriatum, globus pallidus, subthalamic nucleus, red nucleus, pars compacta of the substantia nigra, pontine nuclei, inferior olivary nucleus, and dentate.
Two patients (cases 6 and 9) also had Lewy body-containing neurons. One of these (case 6) had been diagnosed with PD during life and had the postmortem diagnosis of PSP and PD. The NSPD assigned was 4/6 although no Lewy body-containing neurons were detected within 3 levels of the nucleus coeruleus; however, Lewy neurites were present. Furthermore, Lewy body-containing neurons and neurites were found within the dorsal nucleus of vagus. The changes characteristically encountered in PSP were clearly the predominant ones. Lewy body-containing neurons in case 9 were confined to the substantia innominata and to the pars compacta of the substantia nigra; thus, the regional pattern of Lewy body-containing neurons of case 9 did not match the one proposed by Braak et al (16).
DISCUSSION
Epidemiological studies suggest that the crude prevalence of PSP is on the order of 0.001% to 0.0065% (23), indicating that it is a rare disorder. The prevalence of PSP in our ET sample (11 of 89 or 12.4%) is clearly larger than the population prevalence of PSP. It is also 2 to 5 times larger than the proportion of normal cases with “incidental” PSP in the 2 prior autopsy series. The Harvard Brain Tissue Center reported incidental PSP in 1 of 39 (2.6%) normal controls (24). In another series, 5 of 76 (6.6%) autopsies of “clinically normal subjects” had mild pathological changes of PSP (21). Two of those patients (cases 2 and 4) had postural changes and/or mild postural instability on examination and a third had a diagnosis of amnestic mild cognitive impairment (case 3). While not severe enough to qualify clinically for a diagnosis of PSP, as discussed by the authors, these signs may have been early markers of an emerging neurodegenerative process. The proportion we report (12.4%) is 2 to 5 times larger than these previous proportions (2.6%–6.6%), and many times larger than the population prevalence of PSP.
In an earlier series, we reported the neuropathological findings of 33 ET cases (10). The present series of 89 ET cases includes 18 ET cases from the prior series; thus, 71 ET cases were not reported in that earlier series. Of the 11 ET+PSP cases we now report, only 1 was present in the earlier series (10). It was not reported in that earlier series because it was considered to be an example of an early comorbid diagnosis on top of longstanding ET.
In the Canadian ET postmortem series, 2 of 20 (10%) ET patients were also reported to have had PSP (25), which is similar to the 11 of 89 or 12.4% in this report; however, given the small numbers (n = 2 with ET+PSP), the authors did not draw attention to this possible connection. In the Arizona postmortem series, 1 of 24 (4.2%) had PSP (26).
Our patients had an onset of parkinsonism on neurological examination when they were in their 70s and 80s. While the typical age of onset of PSP is in the 60s and 70s (5), considerable clinical heterogeneity has been described, with ages of onset in the 70s and well into the 80s in some series (27).
The present study raises a number of interesting questions. First, is there an association between ET and PSP, and are ET patients at increased risk of developing PSP? Cross-sectional and prospective epidemiological studies are needed to address these questions of association and risk. Through such epidemiological studies, ET has already been linked with increased risk of several neurodegenerative disorders including PD (9) and AD (28, 29); such a study would add to the current discussion over the possible neurodegenerative nature of ET (2, 30). Second, what proportion of ET patients who develop what is presumed during life to be PD or AD (i.e. ET+PD or ET+AD) actually have PSP (i.e. ET+PSP)? This is currently unknown and it expands the debate as to whether ET and “PD” are linked. Third, what proportion of “ET+PD” is actually ET+PSP? This too is unknown but a closer link between ET and PSP is worthy of additional scrutiny in longitudinal, prospective, epidemiological studies and clinical-pathological studies. Neuroimaging would also help to address this issue.
The mean/median torpedo counts (Table 3) are similar to the mean counts reported previously in ET patients (10.5 and 16.5, respectively) and higher than those seen in controls (1.7 and 3.3, respectively) (10). The mean/median Purkinje cell count was 7.8/7.4 (Table 3), which is similar to the count noted in ET previously (7.2) and lower than that observed in controls (9.6) (10).
An issue with all brain bank series is whether they are representative of the typical disease patient. In general, patients may have more severe disease or atypical features. We have shown, for example, that a large proportion of ET patients self-referred to ETCBR have severe tremor and a high proportion have a family history of ET (31). On the other hand, the ETCBR patients do not have atypical ET; indeed, their 3-tiered diagnostic process ensured that their ET diagnosis was confirmed. Three of our 11 patients were diagnosed with parkinsonism, but the latency from onset of ET to PD was 20 to 41 years. We did not select ET patients based on having ET+PD, nor did we exclude them during follow-up if this developed.
Most of our patients had a family history of ET. A recent study showed that a microtubule-associated protein tau gene (MAPT) H1 haplotype, which is a risk factor for PSP, is also a risk factor for ET (32). Whether there are susceptibility genes for both ET+PSP is not known, although these recent findings seem to be a promising avenue for future exploration.
This study had limitations. Although 4 of our 11 patients were clinically demented, none of their brains showed changes that met neuropathological criteria for AD. The assessment of cognitive features was not a prominent part of our study protocol, and future studies are needed to characterize the clinical features of the cognitive deficit patterns in these patients. Although horizontal and lateral smooth pursuit eye movements were assessed clinically, as were vertical saccades, eye movement recordings were not performed. The presence of vertical supranuclear gaze palsy, fixation instability, lid retraction, blepharospasm and apraxia of eyelid opening and closing may occur in patients with PSP (33). None of our patients reported double vision, difficulties with reading (e.g. reduced speed) or problems with eye opening or closure, however, and none were reported by treating physicians to have eye movement abnormalities; eye movement abnormalities were also not noted in the videotaped examinations. The latency from last neurological examination to death was 1 year or less in 8 patients, but was more than 1 year in 3 patients; it is possible that 1 of these patients may have developed eye movement abnormalities in the terminal years of life. It is also possible that square wave jerks may have been present as this was not routinely assessed.
In summary, we report a series of 11 of 89 ET patients with longstanding ET who after many years also developed either parkinsonism or dementia and were found to have the neuropathological features of PSP. This case series raises several interesting questions about the possible links between ET and PSP and, more broadly, between ET and parkinsonism.
Acknowledgments
Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R21 NS077094 (co-investigator), NINDS #R01 NS36630 (co-investigator), NIEHS P30 ES09089 (co-investigator, and CTSA grant number UL1 RR024156. He has also received support from Parkinson's Disease Foundation, the Arlene Bronstein Essential Tremor Research Fund (Columbia University), and the Claire O'Neil Essential Tremor Research Fund (Columbia University).
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369:1152–4. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–8. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–10. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- 4.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–9. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri KR, Buxton-Thomas M, Dhawan V, et al. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76:115–7. doi: 10.1136/jnnp.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankovic J. Essential tremor and Parkinson's disease. Ann Neurol. 1989;25:211–20. doi: 10.1002/ana.410250226. [DOI] [PubMed] [Google Scholar]
- 8.Tan EK, Lee SS, Fook-Chong S, et al. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord. 2008;23:993–7. doi: 10.1002/mds.22005. [DOI] [PubMed] [Google Scholar]
- 9.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80:423–5. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 11.Ross GW, Dickson D, Cerosimo M, et al. Pathological investigation of essential tremor. Neurology. 2004;62:A537–8. [Google Scholar]
- 12.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–33. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Zheng W, Applegate L, et al. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–6. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED, Asabere N, Agnew A, et al. Rest tremor in advanced essential tremor: a post-mortem study of nine cases. J Neurol Neurosurg Psychiatry. 2011;82:261–5. doi: 10.1136/jnnp.2010.215681. [DOI] [PubMed] [Google Scholar]
- 15.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychi Neuropsychol Beh Neurol. 1988;1:111–7. [Google Scholar]
- 16.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 17.The National Institute on Aging a Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(S4):S1–2. [PubMed] [Google Scholar]
- 18.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115:509–32. doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson-Davis CR, Faust PL, Vonsattel JP, et al. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 21.Evidente VG, Adler CH, Sabbagh MN, et al. Neuropathological findings of PSP in the elderly without clinical PSP: possible incidental PSP? Parkinsonism Relat Disord. 2011;17:365–71. doi: 10.1016/j.parkreldis.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 23.Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain. 2001;124:1438–49. doi: 10.1093/brain/124.7.1438. [DOI] [PubMed] [Google Scholar]
- 24.Cantuti-Castelvetri I, Keller-McGandy CE, Albers DS, et al. Expression and activity of antioxidants in the brain in progressive supranuclear palsy. Brain Res. 2002;930:170–81. doi: 10.1016/s0006-8993(02)02244-8. [DOI] [PubMed] [Google Scholar]
- 25.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: A clinicopathologic study of 20 cases. Neurology. 2004;62:932–6. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- 26.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–5. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto R, Tsuchiya K, Mimura M. Clinical heterogeneity in progressive supranuclear palsy: problems of clinical diagnostic criteria of NINDS-SPSP in a retrospective study of seven Japanese autopsy cases. Neuropathology. 2010;30:24–35. doi: 10.1111/j.1440-1789.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 28.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: A population-based study. Mov Disord. 2007;22:1573–80. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 29.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73:621–5. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bermejo-Pareja F. Essential tremor - a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. 2011;7:273–82. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 31.Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361–5. doi: 10.1002/mds.20583. [DOI] [PubMed] [Google Scholar]
- 32.Vilarino-Guell C, Soto-Ortolaza AI, Rajput A, et al. MAPT H1 haplotype is a risk factor for essential tremor and multiple system atrophy. Neurology. 2011;76:670–2. doi: 10.1212/WNL.0b013e31820c30c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong RA. Visual signs and symptoms of progressive supranuclear palsy. Clin Exp Optom. 2011;94:150–60. doi: 10.1111/j.1444-0938.2010.00504.x. [DOI] [PubMed] [Google Scholar]