Abstract
The biological processes underlying stroke are complex, and patients have a narrow repertoire of therapeutic opportunities. After the NIH convened the Stroke Progress Review Group in 2001, a paradigm shift took place that shifts the emphasis of stroke research from a purely neurocentric focus to a more integrated view wherein dynamic interactions between all cell types contribute to function and dysfunction in the brain. This so-called “neurovascular unit” provides a conceptual framework that emphasizes cell-cell interactions between neuronal, glial and vascular elements. Under normal conditions, signaling within the neurovascular unit helps manintain homeostasis. After stroke, cell-cell signaling is disturbed leading to pathophysiology. More recently, emerging data now suggest that these cell-cell signaling mechanisms may also mediate parallel processes of neurovascular remodeling during stroke recovery. Since plasticity is a signature feature of the young and developing brain, these concepts may have special relevance to how the pediatric brain responds after stroke.
Keywords: stroke, neurovascular unit, brain remodeling, neurovascular signaling, biphasic response
Introduction
Stroke is now the second leading cause of death and is one of the worst devastating diseases worldwide. While the disease burden is enormous, there are still few therapeutic opportunities for stroke patients. For ischemic stroke, acute treatments are limited to reperfusion with tPA or mechanical catheter-devices. For acute intracerebral hemorrhage, current surgical and medical therapies are extremely limited in efficacy.
For drug discovery in stroke field, early investigations were mostly focused on the prevention of neuronal death. However, the complex pathophysiology of stroke ultimately involves interactions between multiple cell types, so a focus on saving neurons alone might not be sufficient. In 2001, the National Institutes of Neurological Disorders and Stroke convened the Stroke Progress Review Group to explore new directions for stroke research, and at this workshop, the concept of “neurovascular unit” was introduced (http://www.ninds.nih.gov/find_people/groups/stroke_prg/04_2002_stroke_prg_report.htm). Fundamentally, this concept emphasized that brain function and dysfunction arises from integrated interactions between a network of neurons, glia, and cerebral endothelium. Coordinated responses at the neurovascular interface will mediate not only acute but also chronic events in ischemic and hemorrhagic brain tissue 1, 2. During the early acute phase after stroke onset, loss of cell-cell signaling in the neurovascular unit would lead to pathophysiology. During the delayed phase, cell-cell signaling between neurons, glia and vessels may provide the critical neurovascular substrates for functional plasticity and remodeling.
In this mini-review, we will briefly survey this basic idea, i.e. cell-cell crosstalk in the neurovascular unit mediates both acute injury as well as delayed recovery. Although data in this field are still dominated by findings in adult brains, these concepts will surely be even more important in the pediatric brain because plasticity is a signature feature of the young and developing CNS system.
Neurovascular damage in the acute phase
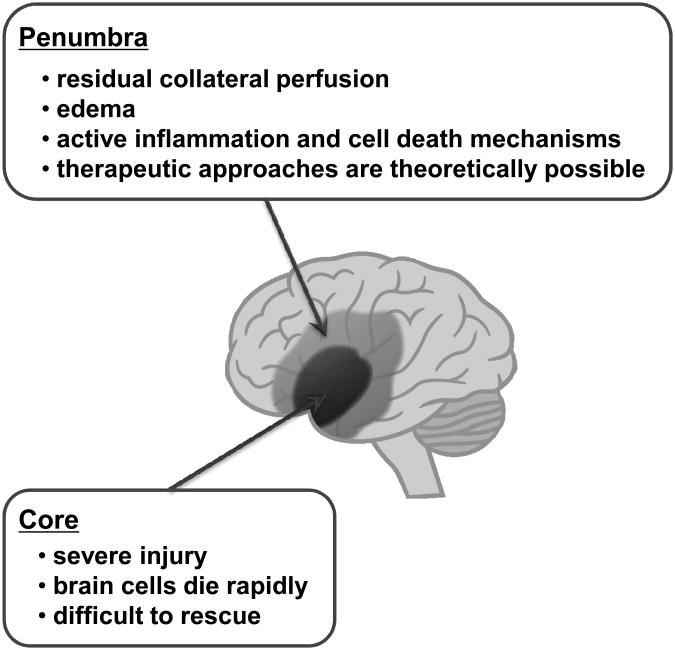
Stroke is a heterogeneous spectrum of conditions caused by the interruption of blood vessels supplying the brain 3, 4. In ischemic strokes, severe blood flow deficits in the core rapidly decrease ATP levels and energy stores. Because cell death processes in this area will occur in minutes, it may be difficult to protect the cells by drug treatments. In contrast, cells in the surrounding region (so-called penumbra) suffer milder insults due to residual perfusion from collateral blood vessels. In the penumbra, cells die more slowly by active cell death mechanisms, and therefore therapeutic approaches are theoretically possible (Figure 1). In hemorrhagic strokes, areas surrounding the central hematoma may also suffer from expanding edema and progressive inflammation and cell death, although the molecular mechanisms are less well defined compared to ischemic strokes.
Figure 1.
In central core areas of stroke, blood flow deficits and/or hemorrhagic lesions are severe and brain cells die rapidly. In peripheral areas (the penumbra), it has been proposed that cell death, inflammation and neurovascular perturbations proceed at a slower pace. Hence, therapeutic salvage is theoretically possible.
The fundamental mechanisms of brain cell death in the acute stroke phase are complex. But accumulated data over the past two decades have implicated excitotoxicity, oxidative stress and in some circumstances, apoptotic-like pathways 3, 5. When brain fails to generate sufficient ATP by reduction of blood flow supply, energy failure occurs and ionic gradients are lost. Glutamate reuptake processes are impaired, and accumulated promotes excessive calcium entry and release. Calcium-dependent synthases and proteases contribute to neuronal death by degrading key cytoskeltal and enzymatic proteins. Abnormality of calcium homeostasis also generates nitric oxide and peroxynitrite, which directly strike neighboring cells. Moreover, mitochondrial functions such as oxidative phosphorylation fail and reactive oxygen radicals are released that further compromise cells by attacking proteins, lipids, and nucleic acids. In parallel with these ionic and free radical pathways, deleterious molecules such caspases may also promote cell death by suicidal endogenous mechanisms. However, most of the cell death pathways outlined here are well documented for neurons. Whether similar mechanisms should be targeted for glial and vascular compartments remains to be carefully assessed.
Beside basic cell death mechanisms, one of the most important facets of early neurovascular damage is manifested as perturbations in blood-brain barrier (BBB) function. The BBB homeostasis is remarkably dependent on endothelial-astrocyte-matrix interactions 6, 7. Perturbation of the neurovascular matrix (type IV collagen, heparan sulphate proteoglycan, laminin, fibronectin, etc.) disrupts the cell-matrix and cell-cell signaling that maintain neurovascular functions. Many proteinases might contribute to extracellular matrix proteolysis, and the extracellular protease systems become dysregulated under diseased conditions. In particular, roles of the matrix metalloproteinase (MMP) family have been focused in the neurovascular damage after stroke 8. MMP levels are increased in both experimental models of stroke 9-11 and stroke patients 12, 13. Those excessive MMP activities might be deleterious. MMPs can degrade the extracellular matrix that comprises the basal lamina, thus damaging the BBB directly. In experimental stroke models, MMP inhibition reduces infraction and edema 14, 15. In addition to BBB disruption, MMP-induced proteolysis of the neurovascular matrix might also promote programmed cell death by detachment of cells from the extracellular matrix (so-called “anoikis”) 16, 17. These findings suggest that MMPs (and other extracelluar proteases) mediate neurovascular damage during the acute stages of stroke.
Ultimately, these neurovascular perturbations can also be interpreted as dysfunctional crosstalk between components of the neurovascular unit. Lack of proper signaling between endothelium and astrocytes may underlie blood-brain barrier leakage. Lack of proper signaling between neurons and endothelium may interfere with hemodynamic coupling required for functional brain activation. Lack of proper signaling between neurons and glia may impede normal neurotransmitter release-reuptake kinetics as well as communications along axons. Hence, preventing neuronal death along will surely not suffice. Saving all these functional interactions across multiple cell types will be required for any truly effective therapy.
Despite convincing experimental evidence, none of above cell death pathways or neurovascular mechanisms have been successfully exploited for treating acute stroke patients. Although many translational barriers are involved 18, 19, the heterogeneity of patients and tight timelines during acute pathology makes it difficult to block these early targets efficiently. Therefore, a recent emphasis in the field is beginning to assess opportunities for promoting neurovascular recovery after stroke.
Neurovascular repair in the chronic phase
Most stroke patients show some degree of recovery over time. For example, functional MRI studies demonstrate that peri-infarct areas are highly plastic 20, 21. Representational areas shift as latent networks are unmasked and parallel circuits are recruited adjacent to damaged regions 22. However, the underlying cellular mechanisms for these recovery processes remain to be fully understood.
The primary neurovascular responses during stroke recovery phase are thought to involve angiogenesis and neurogenesis. Molecular mechanisms of angiogenesis and neurogenesis have been evolutionarily conserved so that similar mediators and pathways are involved in both phenomena 23. It is now accepted that cell-cell signaling between cerebral endothelium and neuronal precursor cells help mediate and sustain pockets of ongoing angiogenesis and neurogenesis in adult brain 7, 21, 24-26. These endothelial-brain interactions comprise the neurovascular niche. Crosstalk between the vascular and neuronal compartments in the neurovascular niche is mediated by an exchange of soluble signals. This phenomenon is partly mediated by the ability of cerebral endothelium to secrete a rich repertoire of trophic factors 27-29. In the normal brain, the neurovascular nich defines these complex mechanisms of cell-cell signaling between cerebral endothelium and neural precursors in the subventricular and subgranular zones of ongoing neurogenesis. In the context of post stroke recovery, these close relationships between neurogenesis and angiogenesis are maintained. Neuroblasts migrate along perivascular routes 30. Promotion of neurogenesis enhances vascular regrwoth and, conversely, angiogenic stimulation enhances neurogenesis 31, 32. Angiogenesis in peri-infarct regions have been detected in rodent models of cerebral ischemia 33 as well as in human stroke 34. Hence, brain recovery after stroke comprises interdependent neurovascular plasticity and remodeling processes that recruit multiple common mediators and signals 23.
Therapies that can boost these endogenous signals and substrates of neurovascular remodeling might be a new direction for stroke treatments 35. However, it remains to be fully elucidated how these approaches can be utilized in clinic. It is worth noting that most molecular targets for stroke therapy have biphasic roles in stroke pathophysiology 1, 36. As mentioned in the previous section, during the acute phase, MMPs mediate neurovascular damage through interruption of cell-cell trophic coupling. During the chronic phase, in the other hand, the same mediators may contribute to neurovascular recovery. In a mouse stroke model, endothelial and glial cells in peri-infarct areas showed a secondary elevation in MMP-9, and pharmacological inhibition of MMPs during the delayed phase made outcomes worse 37. Furthermore, secondary MMP-9 signals co-localized with streams of migrating neuroblasts from the subventricular zone, and MMPs inhibition also blocked the movement of these neuroblasts, which headed towards damaged region 38. In addition, VEGF and HMGB1 are other examples for biphasic mediators in neurovascular responses after stroke. VEGF increases BBB permeability in the acute phase, and accelerates angiogenesis/neurogenesis in the delayed stroke phase 39, 40. HMGB1 is normally present in the nucleus, and is released extracellulary in response to ischemic stress. During the acute stroke phase, HMGB1 promotes necrosis and influx of damaging inflammatory cells. In contrast, during the delayed phase, HMGB1 mediates beneficial plasticity and recovery in the neurovascular unit 41. Likewise, many mediators in the neurovascular unit have biphasic roles, i.e. harmful effects in the acute phase and beneficial in the chronic phase. Hence, neurovascular protection may not be achieved without considering the timing when the initial deleterious responses transits into recovering mechanisms.
Cell-cell trophic coupling in white matter
For the most part, the concept of the neurovascular unit is used to guide investigation in gray matter. However, cell-cell trophic interactions are likely to be important in white matter as well. White matter is vulnerable to ischemic stress and white matter damage is a clinically important part of stroke 3, 42. Therefore, without considering oligo-protection, we may not be able to protect the brain against stroke insult. Compared to the cellular mechanisms of neurovascular damage/repair in gray matter, white matter pathophysiology remains relatively understudied and poorly understood. However, the idea of the neurovascular unit are now applied to the white matter stroke research.
The main components of white matter are the neuronal axon, oligodendrocyte (myelin), astrocyte, and endothelium. As in the neurovascular unit in gray matter, astrocytes and cerebral endothelial cells work together to maintain blood-brain barrier in white matter 42. In addition, astrocytes are in close apposition to OLGs within white matter 43, and couple with OLGs through gap junctions to maintain their functions 44. Furthermore, astrocyte-derived soluble factors were also reported to nourish oligodendrocyte lineage cells 45, 46. And, of course, myelin-axon interactions are essential for white matter homeostasis. OLGs not only myelinate axons but also maintain their functional integrity and survival through OLG-specific proteins and/or trhophic factor release 47, 48. Similar to gray matter, during the acute phase of stroke, several deleterious factors/cascades are activated. For example, MMPs are upregulated, and direct attack of MMPs on myelin components affect OLG survival and function 49. Even if outright cell death does not occur, metabolic dysfunctions in OLGs might still affect the normal replenishment of myelin and synthesis of myelin-associated proteins, which eventually impair myelin-axon interactions.
In the chronic phase, some endogenous responses might work for repairing white matter damage. White matter in the adult brain primarily consists of axonal bundles ensheathed with myelin by mature OLGs, and plays an important role in passing signals between different areas of gray matter. During development, oligodendrocyte precursor cells (OPCs) migrate from the ventricular zone to their final destination, and then differentiate to from myelin sheaths 50, 51. OPCs are now well-known to widely distributed even in adult brain 52. And importantly, after brain injury, they are guided to the site for contributing to myelin repair 52. It remains to be fully elucidated how oligodendrogenesis occurs during the chronic phase, however, cell-cell trophic interactions might be involved in this phenomena. As in the so-called neurovasucular niche, an oligovascular niche might play an important role in supporting trophic interactions between brain endothelium and OPCs 53. Hence, cell-cell trophic coupling in white matter may provide an essential mechanism for repairing damaged white matter in the chronic stroke phase.
Conclusions and potential implications for the developing brain
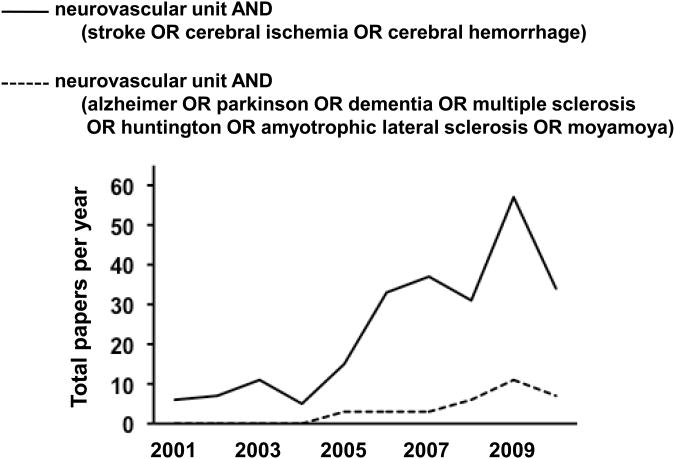
It is now a decade since the concept of neurovascular unit was first introduced. This concept has provided a novel framework for both basic and clinical stroke research. A purely neurocentric focus has been shifted into a more integrated view wherein dynamic interactions between all cell types contribute to function and dysfunction in the brain. As we have seen in this mini-review, multiple mediators induce neurovascular dysfunction in the acute stroke phase. In contrast, the same mediators in turn may underlie neurovascular repair processes in the chronic stroke phase. Understanding cellular mechanisms of the transition zones between injury and repair will give us new directions for stroke treatments. More recently, this concept of the neurovascular unit has grown far beyond its original roots in stroke. The idea that cell-cell signaling in all brain cells comprises the basis for both function and dysfunction is now well accepted in many other CNS disorders (Figure 2).
Figure 2.
Trends in neurovascular unit research compiled from publications between 2001 and 2010. An online PubMed search based on the terms “neurovascular unit AND (stroke OR cerebral ischemia OR cerebral hemorrhage)” demonstrated a rapid growth of stroke-related neurovascular unit articles over the past 5 years. The PubMed search based on the terms “neurovascular unit AND (alzheimer OR parkinson OR dementia OR multiple sclerosis OR huntington OR amyotrophic lateral sclerosis OR moyamoya)” suggested that this concept is gradually applying to other CNS disorders.
Although most patients suffering from stroke and neurodegenerative diseases typically comprise an elderly population, lessons from ongoing studies suggest that these concepts should also be relevant for the developing CNS. Crosstalk and signaling between multiple cell types is a central event for CNS development and maturation. Hence, the notion that damaged brain is surprisingly plastic will surely present many parallels with the young and developing brain. Simultaneous neural and vascular remodeling has been observed in neonatal brain injury 54, 55. And in contrast to the older brain, post-stroke profiles in the developing brain will encompass long periods of recovery, so proper remodeling of the neurovascular unit will be extremely important. However, it is also useful to remember that there will be key differences in how the young and older brain respond to stroke and injury, especially in the context of inflammation 56. This will be an area that warrants further investigations.
Acknowledgments
Supported in part by the Deane Foundation, American Heart Association and NIH. Ideas and concepts discussed here are drawn from previous papers from the authors, including Arai et al, FEBS J 2009, Lok et al, Neurochem Res 2007, Moskowitz et al, Neuron 2010, Lo, Nat Med 2010.
References
- 1.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009 Sep;276(17):4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lok J, Gupta P, Guo S, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007 Dec;32(12):2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 3.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003 May;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010 Jul 29;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005 Feb;36(2):189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007 Nov;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008 Jan 24;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007 Feb;38(2 Suppl):748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 9.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000 Dec;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Gasche Y, Fujimura M, Morita-Fujimura Y, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999 Sep;19(9):1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999 Jun;19(6):624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Montaner J, Alvarez-Sabin J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001 Aug;32(8):1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 13.Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008 Jan;39(1):100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- 14.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001 Oct 1;21(19):7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998 Oct;29(10):2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 16.Tagaya M, Haring HP, Stuiver I, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001 Jul;21(7):835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997 Dec 26;91(7):917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 18.Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008 Mar;153(1):S396–405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher M. New approaches to neuroprotective drug development. Stroke. Jan;42(1 Suppl):S24–27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 20.Dijkhuizen RM, Singhal AB, Mandeville JB, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003 Jan 15;23(2):510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007 Feb;38(2 Suppl):827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009 Dec;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 23.Snapyan M, Lemasson M, Brill MS, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009 Apr 1;29(13):4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008 Mar;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 25.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004 May;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005 Dec 15;438(7070):954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 27.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999 Jun;13(6):450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 28.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004 May 28;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 29.Guo S, Kim WJ, Lok J, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007 Nov;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 31.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006 Dec 13;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004 Aug;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding G, Jiang Q, Li L, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008 May;39(5):1563–1568. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996 May;27(5):852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009 May;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008 May;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 37.Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006 Apr;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 38.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006 Mar 29;26(13):3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004 Sep;35(9):2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 40.Hansen TM, Moss AJ, Brindle NP. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res. 2008 Nov;5(4):236–245. doi: 10.2174/156720208786413433. [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010 Oct;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009 Oct;32(10):1639–1644. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butt AM, Ibrahim M, Ruge FM, Berry M. Biochemical subtypes of oligodendrocyte in the anterior medullary velum of the rat as revealed by the monoclonal antibody Rip. Glia. 1995 Jul;14(3):185–197. doi: 10.1002/glia.440140304. [DOI] [PubMed] [Google Scholar]
- 44.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci. 2008 May;35(1):101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corley SM, Ladiwala U, Besson A, Yong VW. Astrocytes attenuate oligodendrocyte death in vitro through an alpha(6) integrin-laminin-dependent mechanism. Glia. 2001 Dec;36(3):281–294. doi: 10.1002/glia.1116. [DOI] [PubMed] [Google Scholar]
- 46.Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J Neurosci Res. 2010 Mar;88(4):758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg SS, Ng BK, Chan JR. The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol. 2006 Oct;16(4):288–294. doi: 10.1111/j.1750-3639.2006.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995 Dec 15;201(3):223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 50.Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000 Apr;30(2):105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Bhat MA. Molecular organization of axo-glial junctions. Curr Opin Neurobiol. 2003 Oct;13(5):552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009 Jan;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 53.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009 Apr 8;29(14):4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J, Sha B, Zhou W, Yang Y. VEGF-mediated angiogenesis stimulates neural stem cell proliferation and differentiation in the premature brain. Biochem Biophys Res Commun. 2010 Mar 26;394(1):146–152. doi: 10.1016/j.bbrc.2010.02.132. [DOI] [PubMed] [Google Scholar]
- 55.Iwai M, Ikeda T, Hayashi T, et al. Temporal profile of neural stem cell proliferation in the subventricular zone after ischemia/hypoxia in the neonatal rat brain. Neurol Res. 2006 Jun;28(4):461–468. doi: 10.1179/016164105X49283. [DOI] [PubMed] [Google Scholar]
- 56.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31(5):378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]