Abstract
Although epidemiological data associate hypertension with a strong predisposition to develop Alzheimer’s Disease, no mechanistic explanation exists so far. We developed a model of hypertension, obtained by Transverse Aortic Constriction, leading to alterations typical of Alzheimer’s Disease, such as amyloid plaques, neuroinflammation, Blood Brain Barrier dysfunction and cognitive impairment, shown here for the first time. The aim of this work was to investigate the mechanisms involved in Alzheimer’s Disease of hypertensive mice. We focused on RAGE, that critically regulates Aβ transport at the Blood Brain Barrier and could be influenced by vascular factors.
The hypertensive challenge had an early and sustained effect on RAGE up-regulation in brain vessels of cortex and hippocampus. Interestingly, RAGE inhibition protected from hypertension-induced Alzheimer pathology, as showed by rescue from cognitive impairment and parenchymal Aβ deposition. The increased RAGE expression in TAC mice was induced by increased circulating AGEs and sustained by their later deposition in brain vessels. Interestingly, a daily treatment with an AGEs inhibitor or antioxidant prevented the development of Alzheimer’s traits.
So far, Alzheimer pathology in experimental animal models has been recognized using only transgenic mice overexpressing amyloid precursor. This is the first study demonstrating that a chronic vascular insult can activate brain vascular RAGE, favoring parenchymal Aβ deposition and the onset of cognitive deterioration.
Overall we demonstrate that RAGE activation in brain vessels is a crucial pathogenetic event in hypertension-induced Alzheimer’s Disease, suggesting that inhibiting this target can limit the onset of vascular-related Alzheimer.
Keywords: Hypertension, Alzheimer’s Disease, Receptor for Advanced Glycation Endproducts (RAGE), Cognitive Impariment, Basic science
Introduction
The central nervous system adsorbs almost 20% of the whole cardiac output and relies on an elaborate vascular network not merely to supply nutrients but also to maintain neuronal homeostasis1. Appropriate functioning of the cerebral circulation is crucial for preserving normal cognitive function. Ageing and vascular factors are the dominating causes of cerebrovascular dysfunction and, arterial hypertension, the most widespread cardiovascular risk factor accompanying mid and late life, appears to be a main challenge for the onset and progression of dementia.
In particular, hypertension is a powerful risk factor for Alzheimer’s Disease (AD), the most common cause of dementia in the elderly. Indeed, despite the old belief that AD is distinct from Vascular Dementia (VaD), having a non-vascular origin, a growing body of epidemiological studies strongly associate vascular risk factors, such as arterial hypertension, to increased probability to develop AD, reducing the boundary between AD and VaD2–5.
Many studies have focused on the possible mechanisms underlying the cognitive deterioration induced by hypertension but a pathophysiological mechanistic link is still missing.
Since brain vessels show unique structural and functional features, like the blood-brain barrier (BBB), allowing the fine crosstalk between vascular and nervous systems6, we hypothesized that hypertension should affect this complex system of brain vessels. A pathological feature of AD, underlying the cognitive impairment and dementia, is the accumulation of Amyloid-β peptide (Aβ) in the brain7, and increasing evidence points out a central role for Aβ transport across the BBB in determining CNS concentrations of Aβ, given the ability of peripheral Aβ to interact with the cerebral vasculature and influence its own deposition in brain6. The BBB maintains the right balance of the intracerebral pool of Aβ with the one of the bloodstream8. Actually, the structural composition of the BBB does not allow free exchanges of polar solutes such as Aβ between brain and blood, or the contrary. However, many mechanisms contribute to the physiologic entrance and efflux of Aβ in and out the brain. Specialized receptors at the BBB permit the shuttling of Aβ across the brain endothelium from CNS into the bloodstream or vice versa9,10.
Among these receptor systems, the Receptor for Advanced Glycation End products (RAGE) dominates the BBB transport of Aβ into the brain9. So far, RAGE activation has been associated to development of diabetes, and, only more recently, it has been demonstrated that RAGE is activated in AD murine models in which the pathology starts in the nervous system, like the transgenic models9, there is no definitive evidence of whether blood pressure challenge can activate RAGE in brain vessels, triggering and sustaining Aβ precipitation in the brain.
To elucidate this issue we have exploited a particular murine model of arterial hypertension, obtained by transverse aortic coarctation (TAC), and prone to develop AD-related brain pathology11,12. We have previously demonstrated that the hemodynamic challenge imposed by TAC on the brain led to cerebral amyloid deposition as early as 4 weeks later12, preceded by hypoperfusion and neuroinflammation11. The derived brain injury was mainly localized in selected brain areas controlling cognitive functions, such as cortex and hippocampus. We have also shown that TAC-induced hypertension increases the formation of soluble oligomers and intermediate amyloids12, the most neurotoxic forms of Aβ. Interestingly, it has been demonstrated that the severity of the cognitive defect in AD correlates with levels of oligomers in the brain, more than the total Aβ burden13,14.
Methods
For detailed description of methods please refer to http://hyper.ahajournals.org.
Animals and surgery
All experiments were conducted in conformity with European Communities Council Directive No. 86/609/EEC. 8–12 weeks old C57Bl/6J and RAGE KO (RO) male mice on a C57Bl/6J background were used for all the other experiments. Animals were kept under a constant 12-h light-dark cycle at a temperature of 22–25 °C. Standard chow and water were provided ad libitum.
Hypertension was induced by TAC, performed in anesthetized mice, between truncus anonymous and left carotid, with a 7.0 nylon suture ligature placed around the aorta. Sham mice were used as control.
Drug treatment
The AGEs inhibitor Aminoguanidine Hemisulfate (AG), 50 mg/kg/day (Sigma), and the antioxidant Tiron, 1.2 g/kg/day (Sigma) were given in drinking water starting 3 days before the induction of hypertension by TAC. The RAGE inhibitor, FPS-ZM1, was synthesized as previously described15 and given to mice via daily oral gavages at 1mg/kg b.w.
Behavioral tests
Hippocampal and cortical functions were tested by Morris Water Maze and Novel Object Recognition Task, respectively.
Immunofluorescence, histology and image analysis
All the stainings were performed on 30µm coronal brain fixed section and images were acquired with a DMI4000B Leica fluorescence/optical microscope (Leica Microsystems, Wetzlar, Germany).
Dissection of brain areas for RNA extraction
Total RNA was extracted from hippocampi and cortices using TRIzol reagent (Invitrogen, Eugene, OR) according to the manufacturer’s instructions.
Reverse transcription and quantitative PCR
Total RNA was transcribed into cDNA using the RT-PCR Superscript III kit (Invitrogen, Eugene, OR) according to the manufacturer’s instructions. Real-time PCR was performed with SYBR green PCR master mix, following the manufacturer’s instructions, using an ABI Prism 7500 Sequence Detection System (Applied Biosystems Inc, Foster City, CA).
Statistical analysis
Data are presented as mean ± SEM. Group means were evaluated by One-way ANOVA, Two-way ANOVA for factorial design, as required by study design, followed by Tukey's HSD test for the behavioral data and by Bonferroni post hoc test for all other analysis (GraphPad Prism Software 5, Inc.).
Results
Hypertension induces late brain Aβ deposition and cognitive impairment
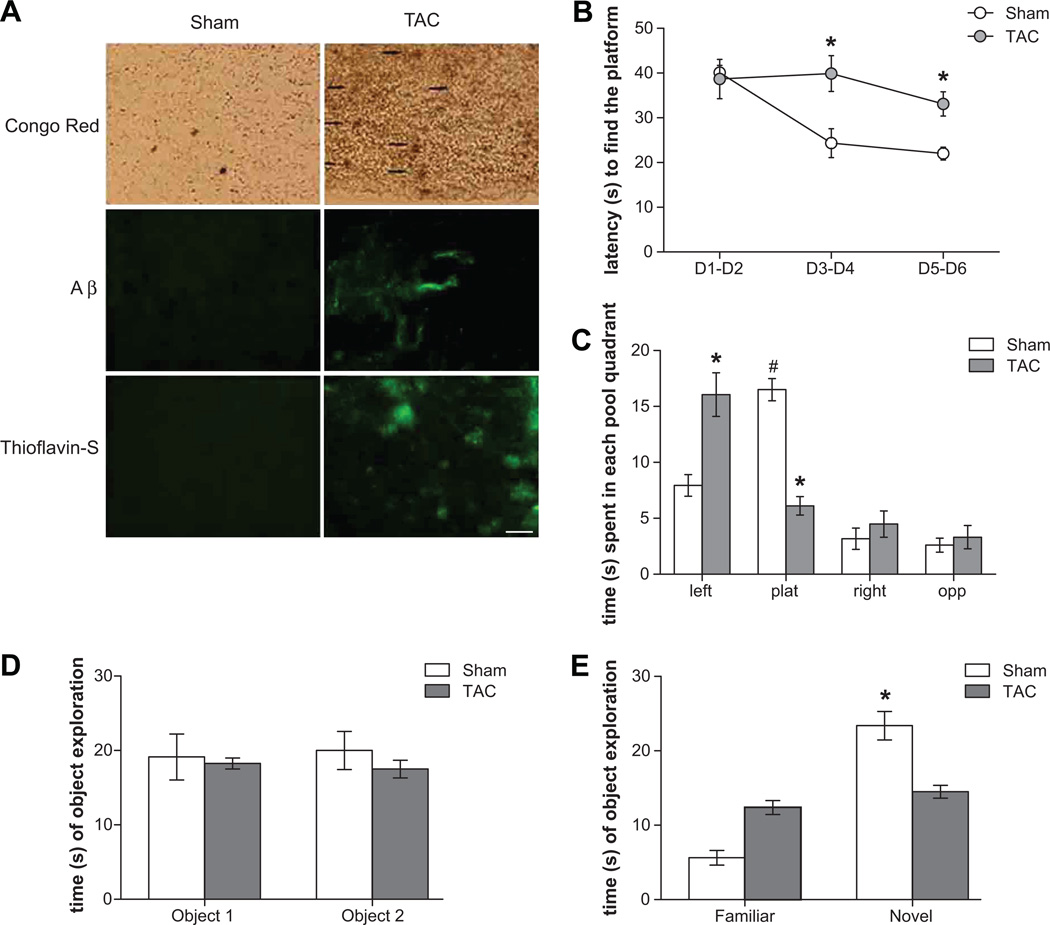
We here confirm with Congo red (Figure 1A upper panel) staining, anti-Aβ antibody (Figure 1A middle panel), and Thioflavin-S (Figure 1A lower panel) staining, that TAC-induced hypertension caused cerebral amyloid deposition in cortex (Figure 1A) and hippocampus (data not shown).
Figure 1.
TAC-induced hypertension determines (A) amyloid deposition in brain parenchyma and around blood vessels as evidenced by Congo red (upper panel, scale bar 100 µm), anti-Aβ (middle panel, scale bar 50 µm) and Thioflavin-S (lower panel, scale bar 50 µm) staining. Representative images of cortex are presented. (B–E) Impairs learning and memory abilities in MWM and NOR tests in TAC mice. (B) Learning curve in the chippocampal-dependent MWM as indicated by the latency to reach a hidden platform, during 6 days of acquisition period. The average of 3 trials per day is represented by 2-days blocks. (C) Probe phase in MWM (the platform is removed), showing the time spent in each pool quadrant (in 30s). TAC mice show higher latency to find the platform and spend less time in the target plat quadrant, i.e. the quadrant where the platform was located (* p<0.05 vs sham and # p<0.05 vs each other quadrants). (D) Basal levels of exploration and object preference in NOR test. (E) Memory retention in the cortical-dependent NOR when one object is changed and a novel one is introduced in the arena. TAC mice did not show differences in exploration between familiar and novel object (* p<0.05 vs familiar object).
More important, we here provide evidence for the first time, that chronic hypertension also affected cognitive functions, and led to behavioral alterations typical of early phases of the pathology (Figure 1B–E). In particular, since hippocampus and cortex are the brain areas mostly affected by AD, we investigated the behavioral performance of mice subjected to TAC in both hippocampus and cortex dependent tasks: the Morris Water Maze (MWM) and the Novel Object Recognition (NOR). Sham and TAC mice showed no difference in the learning performance of the visual phase of the water maze task, indicating that unexpected drawbacks due to the manipulation do not interfere with the ability to solve the maze (data not shown). By contrast, a difference in spatial learning emerged in the six days of the acquisition phase, TAC mice displaying significantly higher latencies in finding the hidden platform compared to Sham mice (Figure 1B). Such cognitive impairment shown by TAC mice was confirmed in the probe phases. In both these phases, a difference in the time spent in the four quadrants between Sham and TAC mice was found. TAC mice spent significantly less time than Sham mice in the target quadrant, i.e. the quadrant where the platform was located, indicating an impairment in the spatial memory domain (Figure 1C).
Data from NOR also confirmed the profile of cognitive deficit. Sham and TAC mice showed no difference in the objects exploration during the acquisition phase, indicating the same basal levels of exploration and object preference for all experimental groups (Figure 1D). During the retention phase, when one object was changed and a novel one was introduced in the arena, TAC mice did not show differences in exploration between familiar and novel object, compared to Sham mice, suggesting a memory impairment for TAC mice in objects recognition (Figure 1E).
So far, this animal model has the advantage to show a spontaneous evolution toward typical features of AD, starting from a hemodynamic challenge. Thus we reasoned that it could enable us to dissect the molecular mechanisms that underlies vascular related AD development.
RAGE is early activated by hypertension in brain vessels, and is crucial for Aβ deposition and cognitive impairment
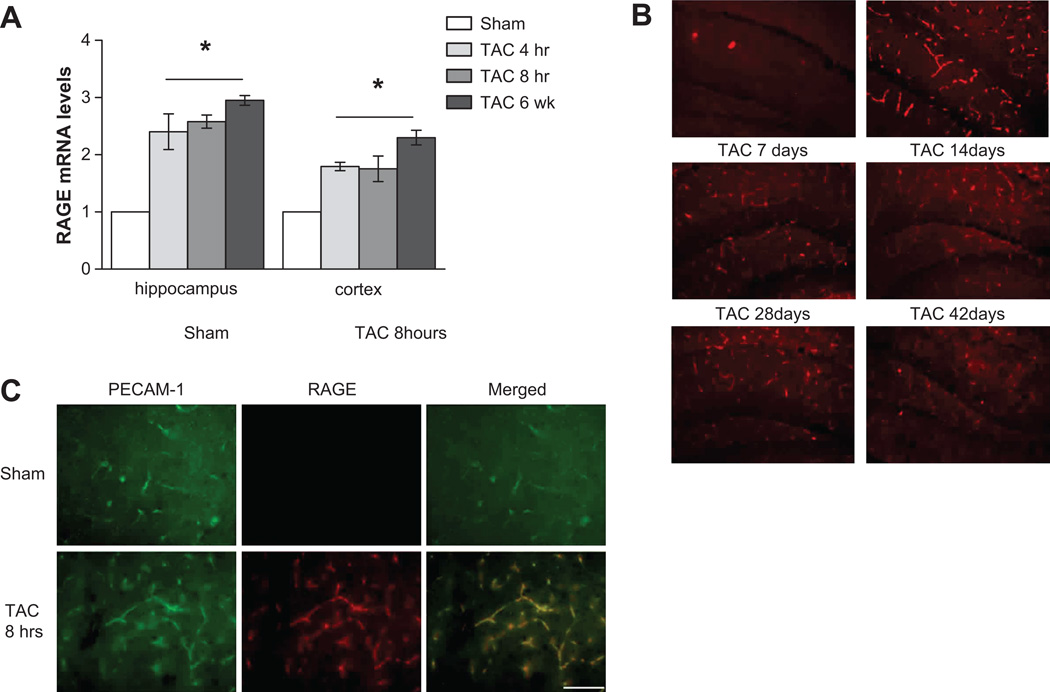
Cerebral blood vessels constitute the first line of defense for the brain from a peripheral hemodynamic challenge, like high blood pressure. Interestingly, RAGE receptor is expressed on endothelial cells and its expression can be modulated by several neurohumoral factors, as well as by the increase in the circulating levels of its ligands. On this regard, we found that the hypertensive challenge had an early and sustained effect of up-regulation of RAGE expression in cortex and hippocampus, as evidenced at mRNA (Figure 2A) and protein level (Figure 2B and S1). More important, the double staining for RAGE and PECAM-1 clearly showed that RAGE was almost exclusively localized in brain vessels (Figure 2C).
Figure 2.
TAC-induced hypertension early up-regulates mRNA (A) and protein levels (B) of RAGE in hippocampus and cortex, and sustains it over time (* p<0.05 vs Sham). Hippocampus representative images of n=4 for each group are presented, scale bar 100 µm. (C) Co-localization of RAGE and PECAM-1 double staining in cortex indicates an exclusive endothelial RAGE expression, scale 50 µm.
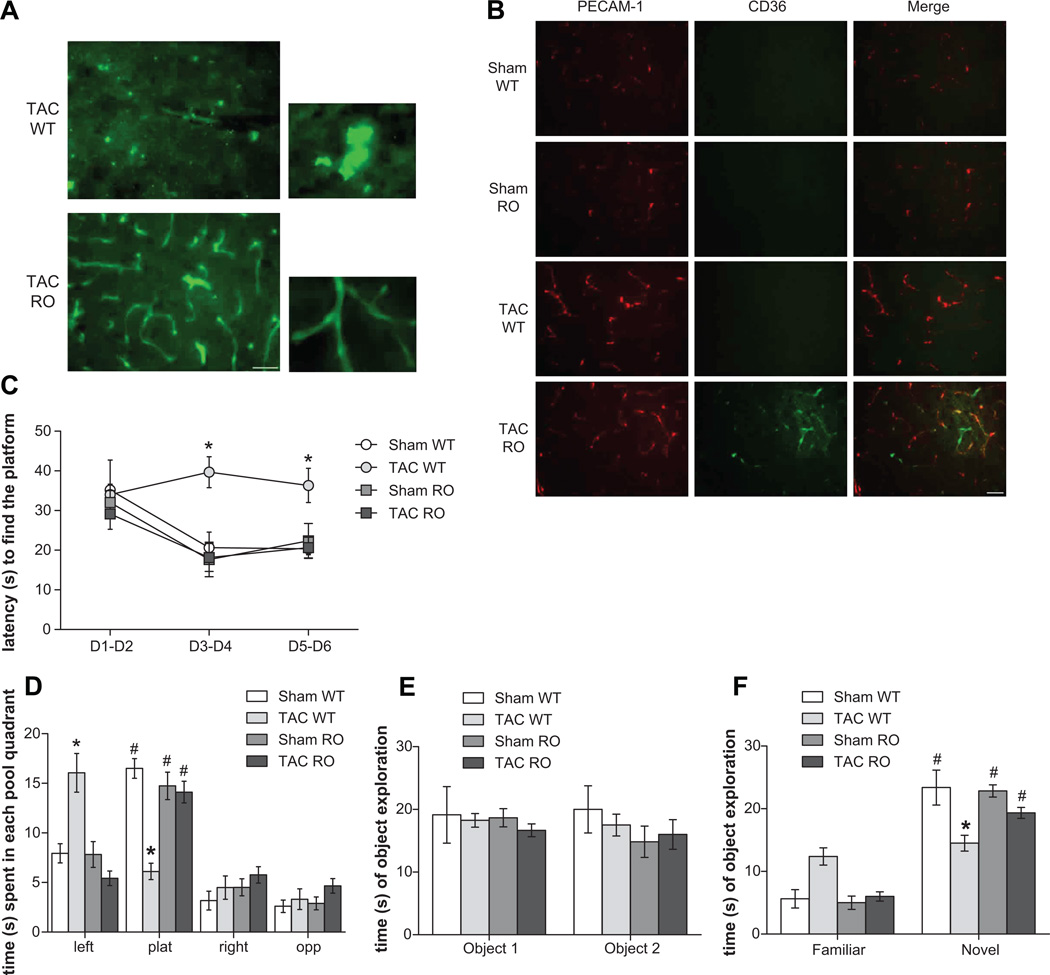
To evaluate the mechanistic role of RAGE in hypertension-induced AD pathology, we performed the hemodynamic challenge induced by TAC on mice with genetic ablation of RAGE (RO mice). When we histologically evaluated brains of TAC RO and WT mice, with Thioflavin-S, we found a clear reduction in the parenchymal Aβ deposits in TAC RO mice, as compared to WT (Figure 3A). Strikingly, RO mice displayed a strong positivity for Thioflavin-S staining confined to cerebral blood vessels. This result was confirmed by the co-localization of Thioflavin-S (Figure S2A) and Aβ (Figure S2B) with PECAM-1 staining, raising the question of whether RAGE genetic ablation, by inhibiting the overall influx of Aβ in the brain, induced a redistribution in local accumulation of the deposits (that is, from parenchyma to blood vessels).
Figure 3.
RAGE ablation (RO mice) prevents development of hypertension-induced (A) plaque formation in brain parenchyma, by shifting Aβ deposition in brain vessels (representative images of cortex are presented; scale bar 50 µm). (B) CD36 activation is marked in brain capillaries of TAC RO mice, as shown by double staining (representative images of cortex are presented; scale bar 50 µm). (C–D) RAGE ablation protects from impairment in learning and memory abilities in the hippocampus-dependent MWM in both acquisition learning (C) and in probe phases (D; * p<0.05 vs each other experimental groups; # p<0.05 vs each other quadrants) as well as prevents (E-F) memory deficits as show by retention phase of NOR (F; * p<0.05 vs familiar object).
To look whether the amyloid concentration in brain vessels found in TAC RO mice, affected brain capillaries function, we evaluated two markers of oxidative stress and inflammation. In particular, we found that the class B scavenger receptor CD36, that has been demonstrated to be involved in vascular oxidative stress and neurovascular dysfunction induced by Aβ16, is markedly increased in TAC RAGE KO mice, as compared to TAC WT mice (Figure 3B).
However, interestingly, when we analyzed learning and memory abilities in RO mice, we found that, in the MWM, TAC RO mice showed lower latency to reach the platform during acquisition phase (Figure 3C) and spent significantly more time than TAC WT in the target quadrant during the probe phase (Figure 3D). This result found support in data from NOR, in which TAC RO mice explored for a long time the novel object and their performance became similar to that observed in sham (Figure 3E and F), indicating that, besides the amyloid concentration in brain capillaries, RAGE ablation protected mice from hypertension-induced learning and memory impairment.
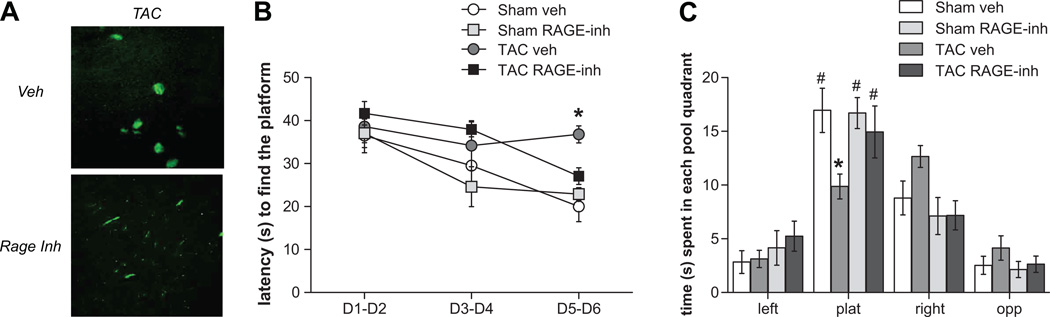
Finally, to further strengthen the data obtained in the genetic model of RAGE ablation, we chronically treated WT mice with FPS-ZM1, a recently developed high-affinity tertiary amide RAGE-specific inhibitor which was shown to block Aβ binding specifically to the V-domain of RAGE and prevented Aβ40- and Aβ42-induced cellular stress in vitro and in vivo15. In addition, FPS-ZM1 treatment has been shown to significantly reduce Aβ pathology, normalized cerebral blood flow responses and improve cognitive performance in a transgenic model of AD15. As expected, we found that FPS-ZM1 treatment elicited the same effects of rescuing amyloid deposition (Figure 4A) and cognitive impairment (Figure 4B and C) following TAC-induced hypertension as seen in the conventional RAGE knockout model.
Figure 4.
RAGE inhibition with FPS-ZM1, a high-affinity specific inhibitor, protects from (A) amyloid deposition and cognitive impairment shown by (B) acquisition learning and (C) target plat quadrant preference in probe MWM test (* p<0.05 vs Sham veh and TAC RAGE-inh groups; # p < 0.05 vs each other quadrants).
High blood pressure induces AD by activating RAGE through oxidative stress and glycation products formation
The increased expression of RAGE was associated with a peak in circulating Advanced Glycation End-products (AGEs) (Figure 5A), as measured by ELISA in serum samples from TAC mice at various time points. On the other hand, the normalization of AGEs levels observed later in TAC mice was accompanied with their accumulation in the vascular tissue, as shown by the positive staining for one of the main AGEs, Carboxymethyl-lysine (CML), that was even more marked in TAC RO mice (Figure 5B), indicating that ligand formation was independent on the presence of its receptor. To address whether the early increase in circulating AGEs, the main RAGE ligand, could effectively be the trigger of the increased RAGE expression in brain vasculature, we treated mice with Aminoguanidine, an inhibitor of AGEs formation, during the hypertensive challenge. A daily oral treatment with Aminoguanidine prevented both the early and the sustained increase in RAGE expression, as indicated by the reduced mRNA levels in cortex (Figure 5C) and hippocampus (Figure S3A), as well as the protein localized in brain vessels that was almost absent in treated mice (Figure 5E). More interestingly, the treatment rescued both the amyloid deposition (Figure 6A) and the impairment in hippocampal (Figure 6B and C) and cortical (Figure 6D and E) functions.
Figure 5.
Hypertension induces (A) an early formation of circulating AGEs and (B) sustained deposition in brain vessels. (C–D) TAC-induced RAGE in brain vessels is prevented by a treatment with Aminoguanidine (C) and Tiron (D), also shown by RAGE and PECAM-1 double-staining, scale bar 20 µm (E). * p<0.01 vs. sham and # p<0.01 vs. TAC-veh.
Figure 6.
A daily oral treatment with Aminoguanidine and Tiron rescues from hypertension-induced Aβ deposition (A) and from cognitive impairment (B–E). Mice treated with Aminoguanidine and Tiron show a behavioral performance similar to Sham veh mice in both the acquisition and probe phases of MWM (B–C; * p<0.05 vs all other groups; £ p<0.05 TAC veh vs Sham veh and TAC AG; § p<0.05 TAC Tiron vs Sham veh and TAC AG groups; # p<0.05 vs each other quadrants) as well as in memory retention of NOR test (E; * p<0.05 vs familiar object).
Finally, to address whether the AGE induced activation of RAGE was driven by an oxidant stress related mechanism, we chronically administered Tiron, an antioxidant agent, during TAC, finding not only that the increase of RAGE in brain vessels was prevented, as shown by mRNA levels in both cortex (Figure 5D) and hippocampus (Figure S3B), and double staining with PECAM-1 (Figure 5E), but also that mice were protected from amyloid deposition (Figure 6A) and cognitive impairment (Figure 6B–E).
Overall, our results obtained with AGE inhibitor and antioxidant treatment, which do not affect the degree of the hemodynamic challenge (Figure S4), indicate that hypertension triggers the activation of the AGE/RAGE pathway early in brain endothelium, leading to secondary and long-term amyloid deposition and cognitive impairment.
Discussion
In this study we show that RAGE is the molecular target activated by hypertension to induce cognitive deterioration and amyloid deposition, typical traits of AD. In particular, we have found that high blood pressure, through the induction of oxidative stress, determines the formation of circulating AGEs that, in turn, recruit RAGE activation in brain capillaries. Finally, we show that inhibition of RAGE or of the oxidant stress-related AGE/RAGE axis, is able to prevent both cognitive deterioration and amyloid deposition induced by the longstanding hemodynamic challenge to the brain, imposed by TAC.
Our results appear to have a double implication. On the one hand, we demonstrate here for the first time that chronic conditions of hypertension lead to deterioration of memory in the MWM test that assesses hippocampal functions, and in the NOR test evaluating cortical function, thus closely resembling alterations typical of AD. Thus, together with our previous observations demonstrating that TAC-induced hypertension also reproduces other typical features of AD, such as brain amyloid deposition12, hypoperfusion and neuroinflammation11, the data presented here showing memory impairment, fully characterize this experimental condition as a model of vascular-induced AD. This aspect shows an unprecedented opportunity to have a spontaneous murine model of AD that is not based on transgenic overexpression of mutant proteins related to amyloid production. Indeed, genetic models of AD greatly advanced knowledge about pathogenic processes linked to the disease, but the cause of sporadic forms of AD, affecting the vast majority of patients, still remains undiscovered, highlighting the need of novel approaches to identify the molecular targets underlying this disease.
On the other hand, we have found the molecular switch challenged by high blood pressure to induce the multifaceted aspects of AD. Starting from the consideration that RAGE: 1) has been found up-regulated in human AD brains17, 2) has a pivotal role in brain Aβ deposition in AD experimental models9,18,19, and 3) has been proposed as a fundamental mechanism signaling danger to vulnerable vasculature20 we hypothesized that high blood pressure might interfere with RAGE signaling, mediating transcytosis of plasma-derived Aβ across brain endothelium and inducing the AD-related pathology observed in TAC mice. On this regard, we have previously demonstrated that passive immunotherapy, obtained by administration of anti-Aβ IgG, is able to rescue hypertensive brains from Aβ deposition and plaques formation, supporting the fact that transport of Aβ across the BBB contributes to the overall concentrations and consequent deposition of Aβ in the CNS in our model12.
Moreover, so far RAGE has been involved in transgenic models of AD where the trigger of the disease is dependent on increased Aβ production. Moreover, recent data associate cerebral hypoperfusion to RAGE activation and cognitive deficits21. Intriguingly, brain hypoperfusion is also one of the main consequences of hypertension that continuously challenges cerebral vessels.
Here we show that mice with genetic ablation for RAGE or treated with FPS-ZM1, a recently established high-affinity RAGE-specific inhibitor15, are protected from hypertension-induced AD pathology. In particular, we show that in our model RAGE inhibition protects from transport of pathophysiologically relevant concentrations of Aβ into the CNS, hampering plaque formation, Aβ deposition around blood vessels and cognitive impairment. Despite these beneficial effects, RAGE inhibition also determines concentration of Aβ in brain capillaries, with consequent activation of oxidative stress and vascular inflammation.
As regard to how RAGE can be activated by high blood pressure, we looked at one of its main ligands, the AGEs. It has been clearly demonstrated that AGEs can be increased by hyperglycemia and, in turn, produce an activation of RAGE on vascular endothelium, generating oxidative stress22. On the other hand, recent observations put RAGE activation downstream the oxidative stress. In particular, it has been demonstrated that AngII-induced activation of RAGE is impaired by using free-radicals scavenger23 and that hyperglycemia-induced ROS production increases expression of RAGE and RAGE ligands23. Although it has been described an association among vascular stiffness and AGEs formation24,25, and recent data report that AGEs induce AD-like pathology in rats26, there are no definitive studies that correlate the hemodynamic stress to activation of the AGE/RAGE pathway. However, the development of vascular disease has its origins in an initial insult to the vessel wall by biological or mechanical factors27. In particular, the increase in pressure-induced mechanical stress represents one of the main stimuli for ROS generation in vessels28 and the oxidative stress that follows can further recruit RAGE activation, as described for hyperglycemia-induced ROS23.
In our study we identify, for the first time, that high blood pressure can activate a AGE/RAGE pathway in brain endothelium through oxidative stress. More importantly, we found that the inhibition of AGEs formation and oxidative stress prevents the deposition of amyloid plaques and the development of cognitive impairment induced by hypertension. These findings have potential clinical impact, suggesting that vascular RAGE is a target for inhibiting pathogenic consequences of hypertension-induced Aβ-vascular interactions, neuroinflammation, development of cerebral amyloidosis and cognitive impairment.
In conclusion, our data uncover for the first time that, in the current wide scenario based genetically-induced models of AD, a vascular hypertensive challenge recapitulates the main traits of AD pathology, thus fully supporting the epidemiological and molecular data obtained in humans2,5. On this regard, in the last few years, the clinical attention from the classical “amyloid cascade” has been changed to a “dynamic polygon” view where the vascular risk factors have the major impact2. In this perspective, the results of the present study demonstrate that the vascular-induced AD pathology is mediated through a high blood pressure induced RAGE mechanism, opening up a new therapeutic strategy in which switching off this molecule could allow us to cope with vascular-related AD.
Supplementary Material
Perspectives.
Despite the established clinical link between hypertension and AD, only a few basic science studies have investigated the specific molecular relationship so far. In particular, most studies use engineered animal models that mimic genetic AD, constituting only a small fraction of AD cases. In contrast, we have pursued the strategy to develop the only animal model of hypertension-related AD pathology so far. In fact, mice that have been subjected to high blood pressure show accumulation of amyloid aggregates, the main histological finding of AD and, for the first time here, impairment in learning and memory tasks. More important, we identify that a hypertensive challenge activates oxidative stress in cerebral vessels responsible for an increased expression of RAGE, leading to Aβ brain deposition and memory impairment. The involvement of the RAGE axis in AD pathology is well recognized but our observation is the first one describing that a vascular challenge can activate an oxidative molecular cascade converging on RAGE activation in brain vessels leading to the onset of the main traits of AD pathology.
Novelty and Significance.
1) What Is New?
Hypertension and AD have been strongly associated in epidemiological studies. In this paper we identify the molecular mechanism responsible for β-amyloid deposition and cognitive impairment in a hypertensive mouse model. The high translational potential of our finding is supported by the use of a novel drug inhibiting this pathway.
2) What Is Relevant?
So far most studies have used engineered animal models mimicking genetic AD, a small fraction of AD cases. In contrast, using an animal model of hypertension-related AD, we identify for the first time that hypertension activates RAGE in brain vessels, leading to Aβ brain deposition and memory impairment.
3) Summary
Although the involvement of the RAGE in AD is already recognized, our observation, describing that hypertension activates a molecular cascade converging on brain vascular RAGE, opens up a new therapeutic strategy in which targeting this molecule could allow us to cope with vascular-related AD.
Acknowledgments
Sources of fundings
The work was supported by grants from Italian Ministry of Health “Ricerca corrente 2009”, “cinque per mille”, Italian Society of Hypertension (SIIA) to GL and grant from NIH (AG17490) to SDY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- 1.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Fotuhi M, Hachinski VP, Whitehouse J. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40(3 Suppl):S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skoog D, Gustafson Update on hypertension and Alzheimer’s Disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 6.Zlokovic BV. New therapeutic targets in the neurovascular pathway in Alzheimer's disease. Neurotherapeutics. 2008;5:409–414. doi: 10.1016/j.nurt.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale D, Mascio G, Ajmone-Cat MA, D'Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, Minghetti L. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging. 2012:205.e19–229.e19. doi: 10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, Passarelli F, Landolfi A, Carullo P, Lembo G. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30:222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 14.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 15.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Meenakshisundaram T, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. Journal Clinical Investigation. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A. 2011;108:5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan SF, Ramasamy R, Schmidt AM. The RAGE Axis: A Fundamental Mechanism Signaling Danger to the Vulnerable Vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XH, Lv BL, Xie JZ, Liu J, Zhou XW, Wang JZ. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging. 2011 Mar 28; doi: 10.1016/j.neurobiolaging.2011.02.003. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi S, Matsui T, Nakamura K, Inoue H, Takeuchi M, Ueda S, Fukami K, Okuda S, Imaizumi T. Olmesartan blocks advanced glycation end products AGEs)-induced angiogenesis in vitro by suppressing receptor for AGEs (RAGE) expression. Microvasc Res. 2008;75:130–134. doi: 10.1016/j.mvr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Ihara Y, Egashira K, Nakano K, Ohtani K, Kubo M, Koga J, Iwai M, Horiuchi M, Gang Z, Yamagishi S, Sunagawa K. Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol. 2007;43:455–464. doi: 10.1016/j.yjmcc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 24.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Zieman SJ. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577–583. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Xing A, Wang X, Liu G, Li L. Regulation of β-amyloid level in the brain of rats with cerebrovascular hypoperfusion. Neurobiol Aging. 2012;826:e31–e42. doi: 10.1016/j.neurobiolaging.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17(2):48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, Antenucci G, Mascio G, Bettarini U, Landolfi A, Iorio L, Maffei A, Lembo G. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betaPIX/Rac-1 pathway. Hypertension. 2009;54:1028–1034. doi: 10.1161/HYPERTENSIONAHA.109.136572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.