Abstract
Neuroantigen-specific T cells play an important role in the disease process of multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE). These cells are encephalitogenic and in susceptible animals, these cells alone can cause EAE. However, mechanisms by which encephalitogenicity is controlled are poorly understood. This study underlines the importance of nitric oxide (NO) in the regulation of encephalitogenicity of T cells. Interestingly, reducing NO during myelin basic protein (MBP)-priming of T cells attenuated the ability of these T cells to induce EAE and EAE-associated neuroinflammation and demyelination. Consistently, increasing NO had opposite effect. Similarly scavenging NO reduced the encephalitogenicity of PLP-specific T cells isolated from female PLP-TCR transgenic mice and supplementation of NO broke the tolerance of PLP-specific T cells of male PLP-TCR mice. Reduced encephalitogenicity of neuroantigen-primed T cells isolated from iNOS (−/−) mice compared to that from wild type mice clearly defines an essential role of iNOS-derived NO in controlling the encephalitogenicity of myelin-specific T cells. This study illustrates a novel role of NO in controlling encephalitogenicity of T cells that may participate in the complex pathogenesis of MS.
Keywords: Nitric oxide, encephalitogenicity, EAE, PLP-TCR transgenic mice
Introduction
Multiple sclerosis (MS) is the most common demyelinating disease and is characterized by inflammatory demyelinating foci in the brain white matter with variable axonal damage (1–3). Although its etiology remains unknown, data from MS patients and animal models support the idea that it is an autoimmune disease mediated by myelin-specific CD4+ T cells (1, 4–6). This disease is modeled in rodents, guinea pigs or marmosets by immunization with myelin-specific proteins (myelin basic protein, MBP; proteolipid protein, PLP; myelin oligodendrocyte glycoprotein, MOG). After immunization, MBP-, PLP- or MOG-specific T cells are generated which ultimately cause an autoimmune disease characterized by initial weight loss, hind leg paralysis, perivascular infiltration, and demyelination. This condition is called experimental allergic encephalomyelitis (EAE) and myelin-specific T cells are known as encephalitogenic T cells (7–9). However, mechanisms regulating encephalitogenicity of these myelin-specific T cells are poorly understood.
Because nitric oxide (NO), a multifunctional second messenger molecule, participates in immune regulation and in the disease process of EAE and MS, we examined the role of NO in encephalitogenicity of T cells. In this study, we provide the evidence that NO plays a critical role in controlling encephalitogenicity of T cells. While excess nitric oxide increases encephalitogenicity of T cells, scavenging NO reduces encephalitogenic property of T cells.
Materials and methods
Reagents
Bovine myelin basic protein (MBP), L-glutamine and β-mercaptoethanol were obtained from Invitrogen (Carlsbad, CA). Proteolipid protein (PLP) was purchased from Tocris Bioscience (Ellisville, MO). Fetal bovine serum (FBS) and RPMI 1640 were from Mediatech (Washington, DC). Heat-killed M. tuberculosis (H37RA) was purchased from Difco Labs. Incomplete Freund’s adjuvant (IFA) was obtained from Calbiochem. Solvent Blue 38, cresyl violet acetate, and lithium carbonate were purchased from Sigma (St. Louis, MO).
Monitoring encephalitogenicity of MBP-specific T cells
MBP-specific T cells were isolated and EAE was induced in 4–6 week old naïve female SJL/J mice (Harlan Sprague-Dawley (Indianapolis, IN) by adoptive transfer as described earlier (10–13). Briefly, donor mice were immunized s.c. with 400 µg bovine MBP and 60 µg M. tuberculosis in IFA. Animals were killed 10–12 days post-immunization, and the draining lymph nodes were harvested and lymph node cells (LNC) were cultured in six-well plates in RPMI 1640 supplemented with 10% FBS, 50 µg/ml MBP, 50 µM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, 100µg/ml streptomycin. On day 4, cells were harvested and resuspended in HBSS and a total of 2 × 107 viable cells were injected into the tail vein of naive mice. Pertussis toxin (150 ng/mouse; Sigma-Aldrich) was injected once via i.p. route on 0 day post transfer (dpt) of cells. Cells isolated from donor mice immunized with CFA or IFA alone were not viable after 4 days in culture with MBP, and therefore were not transferred. Animal maintenance and experimental protocols were approved by the Rush University Medical Center. Animals were observed daily for clinical symptoms. Experimental animals were scored by a masked investigator, as follows: 0, no clinical disease; 0.5, piloerection; 1, tail weakness; 1.5, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 3.5, forelimb weakness; 4, forelimb paralysis; 5, moribund or death.
Isolation of PLP-specific T cells from PLP-TCR transgenic mice and monitoring encephalitogenicity
PLP-TCR (5B6) transgenic mice (14) were genotyped by FACS analysis of with Thy1.1, CD4, and Vbeta6. Splenocytes isolated from 4–6 week old transgenic mice were reprimed with PLP139–151 for 4 d in the presence or absence of either NO scavenger (PTIO) or NO donor (DETA-NONOate). After 4 d, CD4+ T cells were purified from PLP-primed splenocytes using MagCellect mouse CD4+ T cell isolation kit (R&D Systems) and 5 × 106 purified CD4+ T cells were injected into 4–6 week old naïve female SJL/J mice through tail-vein. Animals were observed daily for clinical symptoms as described above.
Histological analysis
On 14 dpt (peak of the acute phase), five mice from each of the following groups (HBSS-control, MBP-primed T cells, PTIO-treated MBP-primed T cells, and DETA-NONOate-treated MBP-primed T cells) were anesthetized. After perfusion with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS, cerebellum and whole spinal cord were dissected out from each mouse. The tissues were further fixed and then divided into two halves: one-half was used for histology analysis whereas the other half was used for myelin staining as described earlier (10–13). For histological analysis, routine histology was performed to obtain perivascular cuffing and morphological details of cerebellar tissues of different groups of mice. Paraformaldehyde-fixed tissues were embedded in paraffin, and serial sections (5 µm) were cut. Sections were stained with conventional H&E staining method. Digital images were collected under bright-field setting using an ×40 objective. Slides were assessed in a blinded fashion for inflammation by three examiners in different anatomical compartments (meninges and parenchyma). Inflammation was scored using the following scale as described: for meninges and parenchyma: 0, no infiltrating cells; 1, few infiltrating cells; 2, numerous infiltrating cells; and 3, widespread infiltration. For vessels: 0, no cuffed vessel; 1, one or two cuffed vessels per section; 2, three to five cuffed vessels per section and 3, more than five cuffed vessels per section. At least six serial sections of each spinal cord and cerebellar tissues from each of five mice per group were scored and statistically analyzed by ANOVA.
Staining for myelin
Serial longitudinal sections of paraformaldehyde-fixed spinal cords were stained with Luxol fast blue for myelin as described earlier (12, 13). Slides were assessed in a blinded fashion for demyelination by three examiners using the following scale: 0, normal white matter; 1, rare foci; 2, a few areas of demyelination; and 3, large areas of demyelination. At least six serial sections of each spinal cord and cerebellum from each of five mice per group were scored and statistically analyzed by ANOVA.
Immunofluoroscence analysis
Immunofluorescence analysis was performed as described earlier (15–17). Briefly, mice were perfused intracardially with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS. Dissected spleens were post-fixed in 4% formaldehyde/PBS for 2–4 days and cryoprotected in 20% sucrose/PBS overnight at 4°C. Splenic tissues were then embedded in OCT (Tissue-Tek) at −50°C and processed for conventional cryosectioning to obtain frozen longitudinal sections (8 µm) and stored at −80°C. Frozen sections were then allowed to cool to room temperature for 1.5–2 h, washed six times each for 5 min in 1x PBS, blocked in 2% BSA in 1x PBS with 0.5% Triton X-100 at room temperature and incubated with mouse anti-iNOS (1/500) along with rat anti-CD11b (1/200) for overnight at room temperature for dual immunohistochemistry. Sections were then washed six times in 1x PBS and further incubated with Cy2 and Cy3 (Jackson ImmunoResearch Laboratories) for 1.5 h at room temperature followed by overnight drying. Next, the sections were rinsed in distilled water, dehydrated successively in ethanol and xylene, and mounted and observed under an Olympus fluorescence microscope using an ×40 objective.
Semi-quantitative RT-PCR analysis
Total RNA was isolated from spinal cord and from cerebellum by using Ultraspec-II RNA reagent (Biotecx laboratories, Inc) following manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (11–13) using a RT-PCR kit from Clontech. Briefly, 1 µg of total RNA was reverse transcribed using oligo(dT)12–18 as primer and MMLV reverse transcriptase (Clontech) in a 20 µl reaction mixture. The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and following primers.
| iNOS: | Sense: 5’-CCCTTCCGAAGTTTCTGGCAGCAGC-3’ |
| Antisense: 5’-GGCTGTCAGAGCCTCGTGGCTTTGG3’ | |
| IL-1β: | Sense: 5’-CTCCATGAGCTTTGTACAAGG-3’ |
| Antisense: 5’-TGCTGATGTACCAGTTGGGG-3’ | |
| MOG: | Sense: 5’-CCTCTCCCTTCTCCTCCTTC-3’ |
| Antisense: 5’-AGAGTCAGCACACCGGGGTT-3’ | |
| PLP: | Sense: 5’-CTTTGCTTCCCTGGTGGCCA-3’ |
| Antisense: 5’-TGTTGGCCTCTGGAACCCCT-3’ | |
| CNPase: | Sense: 5’-CTACCCTCCACGAGTGCAAGA-3’ |
| Antisense: 5’-AGTCTAGTCGCCACGCTGTCT-3’ | |
| GAPDH: | Sense: 5'-GGTGAAGGTCGGTGTGAACG3' |
| Antisense: 5'-TTGGCTCCACCCTTCAAGTG-3' |
Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining. The relative expression of each gene with respect to GAPDH was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech, San Leandro, CA).
Results
Source of NO in the spleen of neuroantigen-immunized donor mice
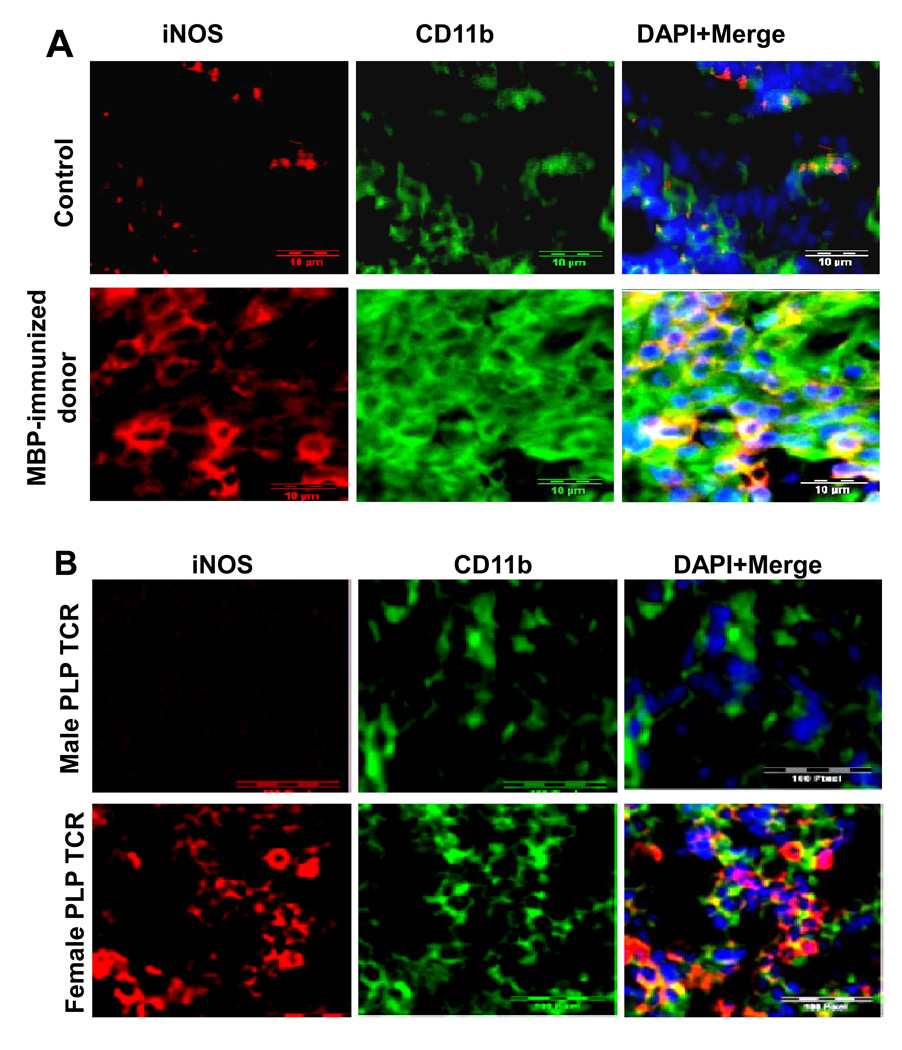
In order to understand if NO regulates encephalitogenicity of T cells, at first, we investigated the source of NO that may control encephalitogenicity in donor mice. Because spleen is an important organ in terms of initiation of immune responses, we investigated the localization of inducible nitric oxide synthase (iNOS), which synthesizes NO during immune stimulation, in splenic sections of donor mice. As evident from figure 1A, the expression of iNOS protein was much higher in spleen of MBP-immunized donor mice than that of normal mice. Double-labeling immunofluorescence analysis identified CD11b-positive macrophages as iNOS-expressing cells (Fig. 1A). Similarly we also identified marked iNOS immunoreactivity in CD11b-positive macrophages in splenic sections of female PLP-TCR transgenic mice (Fig. 1B; lower panel). Consistent to the fact that male PLP-TCR transgenic mice are much more resistant than female mice to spontaneous EAE (18), we did not notice much iNOS immunoreactivity in splenic sections of male PLP-TCR transgenic mice (Fig. 1B; upper panel). On the other hand, we could not detect iNOS in CD3-positive T cells (data not shown).
Fig. 1. Myelin protein priming induces the expression of iNOS in vivo in the spleen of donor mice.
A) Donor female SJL/J mice were immunized s.c. with 400 µg bovine MBP and 60 µg Mycobacterium tuberculosis in IFA. On 12th day of immunization, mice were perfused and splenic sections were double-immunolabeled for iNOS and CD11b. B) Splenic sections of male and female PLP-TCR transgenic mice (4 weeks old) were double-immunolabeled for iNOS and CD11b. Results represent analysis of four separate splenic sections from each of five different mice (n=5) per group.
Effect of NO scavenger and NO donor on encephalitogenicity of T cells
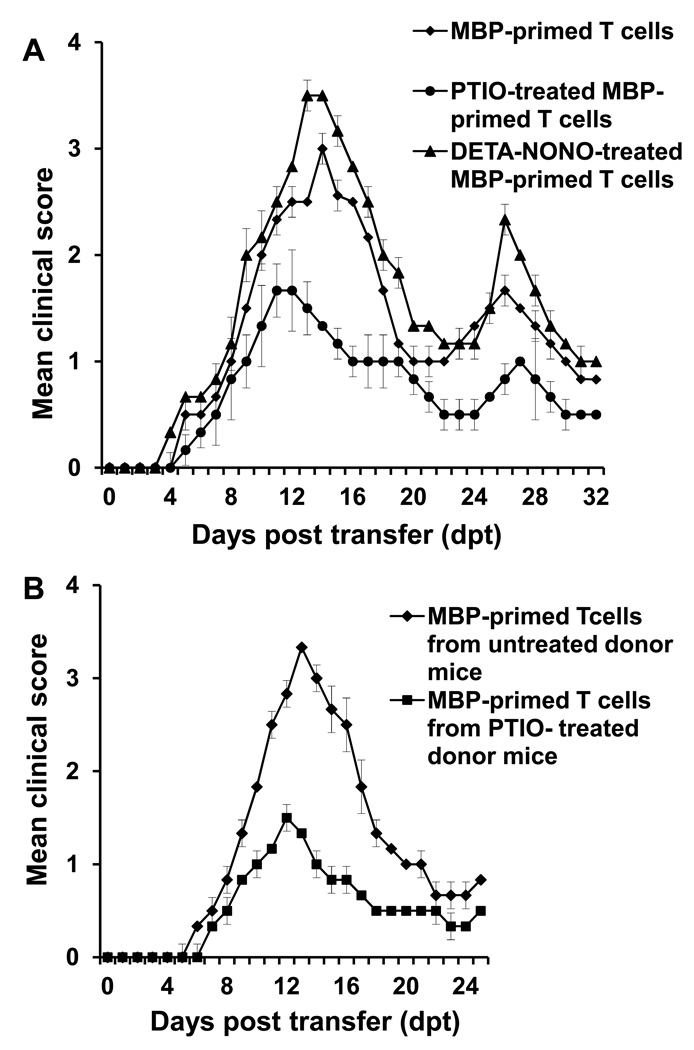
Encephalitogenicity is monitored by the EAE-causing activity of myelin protein-primed T cells in which myelin-specific T cells are transferred to naïve recipient mice followed by examining clinical symptoms of EAE. As expected, MBP-primed T cells induced clinical symptoms of EAE exhibiting the disease onset within 6–8 days post transfer (dpt) and acute phase within 14–16 dpt followed by a relapsing-remitting phase (Fig. 2), indicating encephalitogenic activity of MBP-primed T cells. However, when NO was scavenged by PTIO during MBP-priming, these PTIO-treated MBP-primed T cells became less efficient in transferring the disease to naïve recipient mice (Fig. 2A & Table-1). Clinical score and disease severity in both acute phase and relapsing phase were significantly lower in mice having PTIO-treated MBP-primed T cells than mice with MBP-primed T cells (Fig. 2A). On the other hand, when the level of NO was increased by DETA-NONOate, a NO donor, during MBP-priming, these DETA-NONOate-treated MBP-primed T cells became more efficient than MBP-primed T cells in transferring the disease in both acute and relapsing phases (Fig. 2A & Table-1). These results suggest that NO is an important regulator of encephalitogenic potential of MBP-primed T cells.
Fig. 2. Effect of NO modulators on the encephalitogenicity of MBP-specific T cells.
A) Lymph node cells (LNC) isolated from MBP-immunized donor female SJL/J mice were re-primed with MBP in the presence of either PTIO (50µM), or DETA-NONOate (DNO; 100µM). After 4 days, viable non-adherent cells (2 × 107) were adoptively transferred to naïve female SJL/J recipient mice via tail-vein injection. B) Donor mice were immunized with MBP as described under methods section, and on 2 and 8 day post immunization (dpi), mice were treated with either PTIO (100mg/kg) or saline as vehicle via i.p. injection. On 12 dpi, mice were sacrificed, and LNC were further primed with MBP (50 µg/ml) for 4 days followed by transfer of viable non-adherent cells to naïve recipient mice. Six recipient mice (n=6) were used in each group. Mice were examined for clinical symptoms daily. Data are mean ± SEM of six mice per group.
Table-1.
Effect of DETA-NONOate and PTIO on encephalitogenicity of female MBP-primed T cells
| Treatment | Incidence of EAE |
Mean peak clinical score |
Alteration of EAE | |
|---|---|---|---|---|
| Incidence | Score | |||
| MBP-primed T cells | 6/6 | 3.0 | ||
| PTIO-treated MBP-primed T cells | 5/6 | 1.66 | − 17% | − 45% a |
| DETA-NONOate-treated MBP-primed T cells | 6/6 | 3.66 | + 22% b | |
LNC isolated from MBP-immunized donor female SJL/J mice were re-primed with MBP in the presence of either PTIO (50µM), or DETA-NONOate (100µM). After 4 days, viable non-adherent cells (2 × 107) were adoptively transferred to naïve female SJL/J recipient mice via tail-vein injection. A clinical score of 1 or more was considered as the incidence of EAE in mice.
p < 0.001
p < 0.05 vs MBP-primed T cells (control).
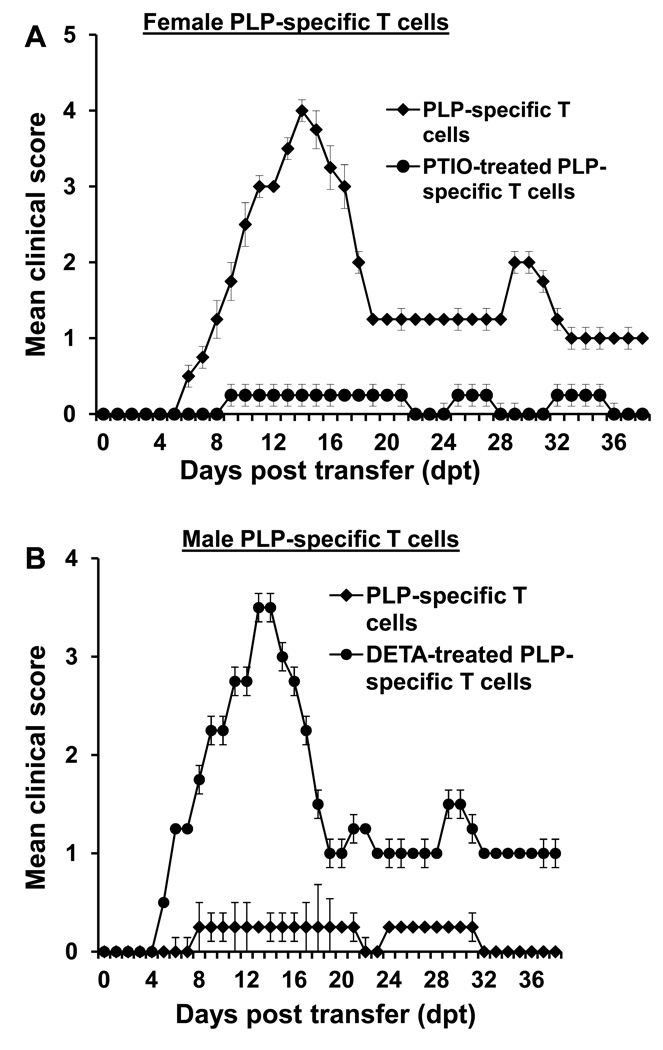
To confirm this encephalitogenic role of NO from another angle, we used PLP-TCR transgenic mice. These mice develop EAE spontaneously (14, 18). As observed with MBP-specific T cells, PLP-specific CD4+ T cells from female PLP-TCR transgenic mice also strongly induced EAE in naïve recipient mice and remained unable to do so when these cells were treated with PTIO during PLP priming (Fig. 3A & Table-2). On the other hand, male PLP-TCR transgenic mice do not develop EAE (18) and accordingly T cells from male PLP-TCR transgenic mice also did not transfer EAE to naïve recipient mice (Fig. 3B & Table-2) suggesting that male PLP-specific T cells are not encephalitogenic. Because scavenging NO reduced the encephalitogenic potential of female PLP-specific T cells, we examined if supplementation of NO could break the tolerance of male PLP-specific T cells. Therefore, splenocytes isolated from male PLP-TCR transgenic mice were primed with PLP in the presence of DETA-NONOate. After 4d, purified CD4+ T cells were transferred to naïve female SJL/J mice. As appeared from figure 3B, DETA-NONOate treatment markedly induced the encephalitogenic activity of non-encephalitogenic male PLP-specific T cells. These results clearly indicate that NO is a critical regulator of encephalitogenicity of T cells and that it is possible to break the tolerance of male T cells by NO.
Fig. 3. Effect of NO modulators on the encephalitogenicity of PLP-specific T cells.
A) Splenocytes isolated from female PLP-TCR transgenic mice were re-primed with PLP 139–151 in the presence or absence of 50µM PTIO. After 4 d, CD4+ T cells were purified and 5 × 106 PLP-specific T cells were transferred to naïve female SJL/J mice via tail vein. B) Splenocytes isolated from male PLP-TCR transgenic mice were re-primed with PLP139–151 in the presence or absence of 100µM DETA-NONOate. After 4 d, CD4+ T cells were purified and 5 × 106 PLP-specific T cells were transferred to naïve female SJL/J mice via tail vein. Six recipient mice (n=6) were used in each group. Mice were examined for clinical symptoms daily. Data are mean ± SEM of six mice per group.
Table-2.
Regulation of encephalitogenicity of male and female PLP-primed T cells by NO modulators
| Treatment | Incidence of EAE |
Mean peak clinical score |
Suppression of EAE | |
|---|---|---|---|---|
| Incidence | Score | |||
| Female PLP-primed T cells | 6/6 | 3.83 | ||
| PTIO-treated female PLP-primed T cells | 0/6 | 0.17 | 100% | 95% a |
| Male PLP-primed T cells | 0/6 | 0.25 | 100% | 93% a |
| DETA-NONOate-treated male PLP-primed T cells | 6/6 | 3.5 | ||
Splenocytes isolated from male and female PLP-TCR transgenic mice were re-primed with PLP 139–151 in the presence or absence of 50µM PTIO and 100µiM DETA-NONOate. After 4 d, CD4+ T cells were purified and 5 × 106 PLP-specific T cells were transferred to naïve female SJL/J mice via tail vein. Six recipient mice (n=6) were used in each group. While a clinical score of 1 or more was considered as the incidence of EAE in mice.
p < 0.001 vs female PLP-primed T cells.
Effect of NO scavenging on the generation of encephalitogenic T cells in vivo in donor mice
Because in vitro treatment of MBP-primed T cells with PTIO inhibited the encephalitogenicity of MBP-primed T cells, we next investigated whether PTIO treatment in donor mice was capable of inhibiting the generation of encephalitogenic T cells in vivo. Therefore, donor mice were treated with PTIO on day 2 and day 8 of immunization and T cells from these donor mice were primed with MBP for 4 days and transferred adoptively to naïve recipient mice. In the control group, EAE was induced by adoptive transfer of MBP-primed T cells from donor mice that received only PBS. Our results showed that mice that received T cells from PTIO-treated donor mice exhibited dramatically less clinical symptoms and disease severity compared with the control EAE group (Fig. 2B). These results suggest that NO plays an important role in the generation of encephalitogenic T cells in vivo in donor mice.
Effect of genetic knockdown of iNOS on the encephalitogenicity of T cells
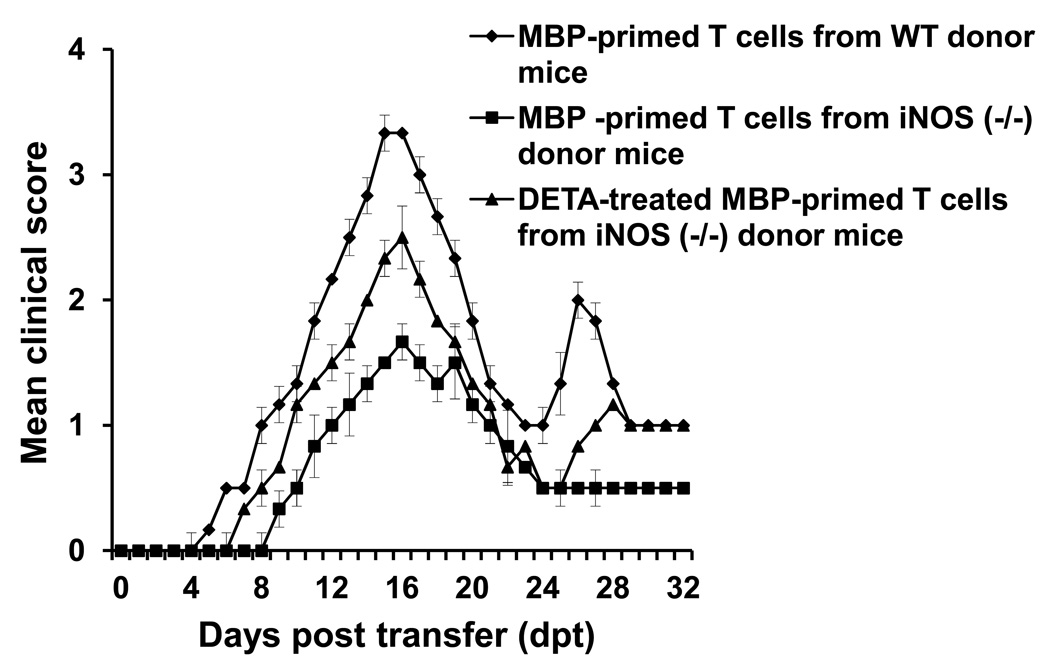
The iNOS is a major producer of NO during immune stimulation and the expression of iNOS increases in spleen of MBP-immunized donor mice as well as female PLP-TCR transgenic mice (Fig. 1). Therefore, to confirm the involvement of NO in the regulation of encephalitogenicity, we used iNOS (−/−) mice. Mice that are null for iNOS and strain-matched wild type (WT) mice were immunized with MBP followed by transferring MBP-primed T cells to naïve female SJL/J mice. Although T cells do not express iNOS, MBP-primed T cells isolated from iNOS (−/−) mice were less effective than WT MBP-primed T cells in transferring the disease to naïve female SJL/J mice (Fig. 4). Disease severity was approximately 50% lower in mice having iNOS (−/−) MBP-primed T cells than WT MBP-primed T cells (Fig. 4). To further confirm that decreased encephalitogenicity was due to the absence of NO, we supplemented NO during MBP priming of T cells isolated from iNOS (−/−) mice. In this case as well, addition of DETA-NONOate to iNOS (−/−) T cells increased their encephalitogenicity (Fig. 4), clearly establishing an important role of iNOS-derived NO in controlling the encephalitogenic potential of T cells.
Fig. 4. Knockdown of iNOS reduces the encephalitogenicity of MBP-specific T cells.
Splenocytes isolated from MBP-immunized iNOS (−/−) donor mice (B6.129) were further primed with MBP in the presence or absence of DETA-NONOate (100µM). After 4 d, CD4+ T cells were purified and 5 × 106 MBP-primed CD4+ T cells were adoptively transferred to naïve female SJL/J recipient mice. Similarly, MBP-primed T cells from WT B6.129 mice were also transferred to naïve female SJL/J mice. T cells from three different donor mice were transferred to six different recipient mice. Mice were examined for clinical symptoms daily. Data are mean ± SEM of six mice per group.
Effect of PTIO-treated and DETA-NONOate-treated MBP-primed T cells on CNS inflammation and demyelination
EAE is accompanied by the infiltration of inflammatory cells, expression of proinflammatory molecules and demyelination. Therefore, to correlate clinical score of EAE with the degree of inflammation and demyelination, we performed H&E staining, mRNA analysis of proinflammatory molecules and myelin-specific genes, and luxol fast blue staining. Recipient mice receiving MBP-primed, PTIO-treated MBP-primed and DETA-NONOate-treated MBP-primed T cells were sacrificed on 14 dpt (acute phase).
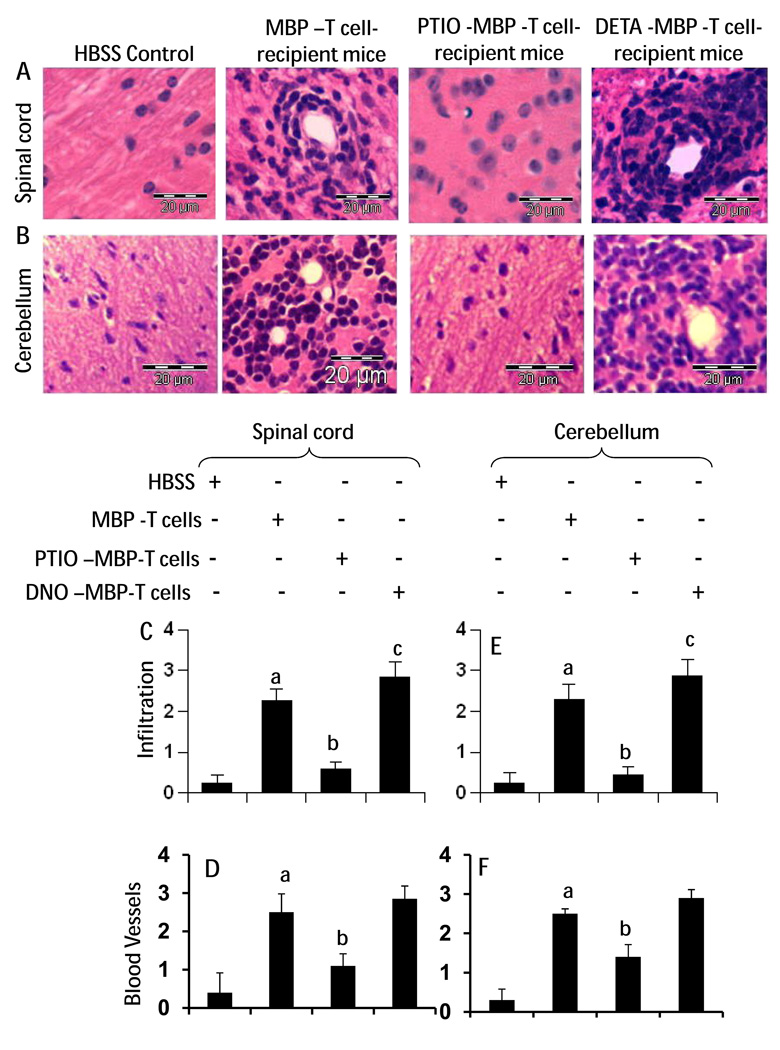
As expected, H & E staining of spinal cord (Fig. 5A) cerebellar (Fig. 5B) sections of mice receiving MBP-primed T cells showed widespread infiltration of inflammatory cells into the CNS. Adoptive transfer of DETA-NONOate-treated MBP-primed T cells resulted in increased infiltration of inflammatory cells into the spinal cord (Fig. 5A) and cerebellum (Fig. 5B) compared to the MBP group. On the other hand, mice receiving PTIO-treated MBP-primed T cells exhibited decreased infiltration of inflammatory cells into the spinal cord (Fig. 5A) and cerebellum (Fig. 5B). Quantitation of relative level of inflammatory cells (Fig. 5C–F) shows that the above group of mice exhibits dramatically reduced infiltration (Fig. 5C&E) and the appearance of cuffed vessels (Fig. 5D&F) in the spinal cord (Fig. 5C&D) and cerebellum (Fig. E&F). On the other hand, enhanced infiltration and the appearance of cuffed vessels in spinal cord (Fig. 5A, C & D) and cerebellum (Fig. 5B, E&F) were observed in mice receiving DETA-NONOate-treated MBP-primed T cells.
Fig. 5. Infiltration of mononuclear cells into spinal cord and cerebellum of mice that received MBP-specific T cells.
On 14 dpt (acute phase), spinal cord (A) and cerebellar (B) sections isolated from HBSS-treated (normal), MBP-primed T cell-treated, PTIO-incubated MBP-primed T cell-treated, and DNO-incubated MBP primed T cell-treated mice were stained with H & E. Digital images were collected under bright field setting. Infiltration (C & E) and cuffed vessels (D & F) in spinal cord (C & D) cerebellar (E & F) sections were represented quantitatively by using a scale as described under methods section. Data are expressed as the mean ± SEM of five different mice per group. ap < 0.001 vs HBSS (normal); bp < 0.001 vs MBP-primed T cells; cp < 0.05 vs MBP-primed T cells.
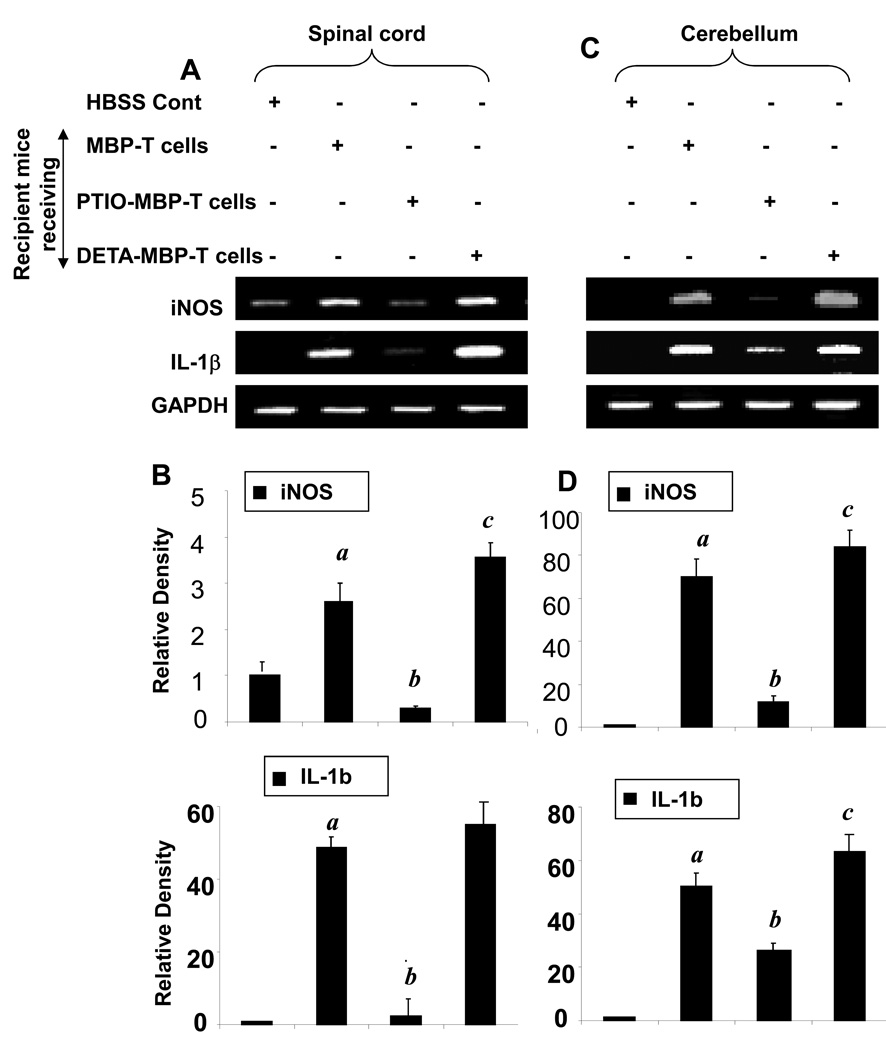
Consistently, decreased mRNA expression of iNOS and IL-1β was observed in spinal cord (Fig. 6A–B) and cerebellum (Fig. 6C–D) of mice receiving PTIO-treated MBP-primed T cells compared to mice receiving MBP-primed T cells. In contrast, the mRNA expression of iNOS and IL-1β was more in cerebellum and spinal cord of mice receiving DETA-NONOate-treated MBP-primed T cells (Fig. 6).
Fig. 6. Expression of proinflammatory molecules in spinal cord and cerebellum of mice that received MBP-specific T cells.
On 14 dpt, spinal cord (A & B), and cerebellum (C & D) of HBSS-treated (normal), MBP-primed T cell-treated, PTIO-incubated MBP-primed T cell-treated, and DNO-incubated MBP primed T cell-treated mice were analyzed for the mRNA expression of iNOS and IL-1β by semi-quantitative RT-PCR (A & C). Relative band density is presented in B & D. Data are expressed as the mean ± SEM of five different mice per group. ap < 0.001 vs HBSS (normal); bp < 0.001 vs MBP-primed T cells; cp < 0.05 vs MBP-primed T cells.
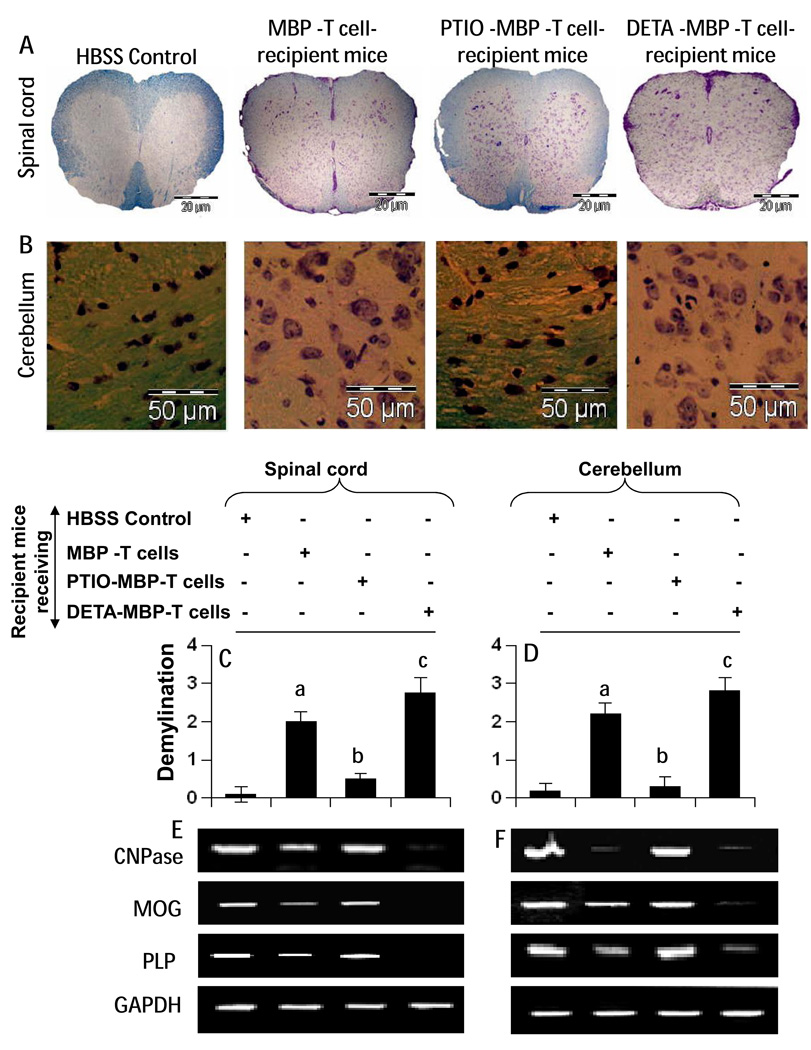
As evident from figure 7A–D, adoptive transfer of MBP-primed T cells caused widespread demyelination in spinal cord (Fig. 7&C) and cerebellum (Fig. 7B&D), which was more in case of DETA-NONOate-treated MBP-primed T cells and less in mice receiving PTIO-treated MBP-primed T cells. Earlier we have demonstrated decrease in myelin gene expression in CNS tissues of EAE mice (Brahmachari and Pahan, 2007). Accordingly, there was decreased mRNA expression of MOG, PLP and CNPase in spinal cord (Fig. 7E) and cerebellum (Fig. 7F) of mice receiving MBP-primed T cells as compared to mice receiving only vehicle. Consistent to that observed with myelin staining, this decrease in myelin gene expression was not visible in mice receiving PTIO-treated MBP-primed T cells (Fig. 7E–F) and it was higher in mice receiving DETA-NONOate-treated MBP-primed T cells (Fig. 7E–F).
Fig. 7. Demyelination and expression of myelin-specific genes in spinal cord and cerebellum of mice that received MBP-specific T cells.
On 14 dpt, spinal cord (A) and cerebellar (B) sections of HBSS-treated (normal), MBP-primed T cell-treated, PTIO-incubated MBP-primed T cell-treated, and DNO-incubated MBP primed T cell-treated mice were stained with Luxol fast blue. Digital images were collected under bright field setting. Demyelination in spinal cord (C) and cerebellum (D) was represented quantitatively by using a scale as described in materials and methods. Data are expressed as the mean ± SEM of five different mice per group. ap < 0.001 vs HBSS (normal); bp < 0.001 vs MBP-primed T cells; cp < 0.05 vs MBP-primed T cells. Spinal cord (E) and cerebellum (F) were analyzed for the mRNA expression of CNPase, MOG and PLP by semi-quantitative RT-PCR. Results represent the analysis of five different mice per group.
Discussion
Understanding mechanisms regulating the encephalitogenicity of myelin-specific T cells is an important field of research as it may lead to the development of therapeutic approaches for MS. Studies presented from various angles clearly demonstrate that NO is an important regulator of encephalitogenic potential of neuroantigen-specific T cells. Our conclusion is based on the following: First, PTIO is a scavenger of NO and PTIO-treated MBP-primed T cells were unable to induce clinical and pathological features of EAE in recipient mice. Similarly, PTIO also inhibited the generation of encephalitogenic T cells in vivo in donor mice. Second, exogenous addition of NO during MBP-priming increased the encephalitogenic property of T cells as seen by the appearance of clinical symptoms, increased expression of proinflammatory cytokines and decreased expression of myelin specific genes. Third, female 5B6 PLP-TCR transgenic mice develop spontaneous EAE and accordingly, PLP-specific T cells isolated from female PLP TCR mice also transferred EAE to female non-transgenic recipient mice. However, after PTIO treatment, PLP-specific T cells of PLP TCR mice lost their ability to induce EAE in recipient mice. Fourth, in contrast to female, male PLP TCR transgenic mice are resistant to spontaneous EAE (18). Accordingly, PLP-specific T cells from male PLP TCR mice also did not transfer EAE to non-transgenic recipient mice. However, NO could break the tolerance of these male PLP-specific T cells as after DETA-NONOate treatment, male PLP-specific T cells markedly induced EAE in non-transgenic recipient mice. These important results suggest that it is possible to break the tolerance of male T cells by NO. Finally, ablation of iNOS also reduced the encephalitogenicity of MBP-primed T cells. On the other hand, addition of NO to iNOS (−/−) T cells during MBP-priming restored the encephalitogenic activity of T cells. Taken together, these results clearly suggest that reducing the level of NO could be an effective approach for suppressing encephalitogenicity of T cells.
In fact, several studies have indicated a possible role of NO in autoimmune demyelination (20, 27–31). Apart from astroglia and microglia in the CNS, peripheral cells such as macrophages and monocytes in MS patients also express iNOS and produce NO (31, 32). Because T cells are primarily primed in periphery, NO production from peripheral cells during immune activation may play a significant role in the formation and functioning of disease-producing autoreactive T cells. Here we must remember that complete knockdown of iNOS is not beneficial for EAE and may be for MS as well. Although selective inhibition of iNOS in the CNS by intraventricular administration of antisense oligonucleotides blocks the disease process of EAE in mice (33), clinical symptoms of EAE and mortality rate increase in iNOS (−/−) mice as compared to wild-type mice (34). This is because NO produced up to the certain level from the activation of iNOS may be beneficial for EAE. It is to be noted that NO inhibits the activation of T cells and thereby acts as an immunosuppressant (31, 34). However, once iNOS is activated, it produces excessive amounts of NO for extended period of time (27, 31, 35), and NO produced in excess is cytotoxic (22, 36, 37). Therefore, partial knockdown of iNOS by pharmacological compounds to inhibit the excessive production of NO, but not the complete knockout of the iNOS gene, may be the feasible therapeutic approach for EAE and hence for MS. In this aspect, it is important to mention that NO is a key regulator of EAE-inducing activity or encephalitogenicity of T cells.
In summary, NO controls the encephalitogenicity of T cells, in which scavenging of NO reduces and supplementation of NO increases EAE-inducing activity of T cells. Furthermore, we also demonstrate that NO is a critical molecule for the development of self tolerance in male as NO alone broke the tolerance of male myelin-specific T cells. Therefore, specific targeting of NO either by iNOS inhibitors or NO scavengers may be an important step for the attenuation of MS-causing activity of pathogenic T cells.
Acknowledgement
This study was supported by grants from NIH (NS39940, NS39940-10S1 & AT6681).
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 5.Hafler DA, Weiner HL. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989;10:104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- 6.Madsen LS, Andersson EC, Jansson L, krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 7.Alvord EC, Jr, Kies MW. Clinico-pathologic correlations in experimental allergic encephalomyelitis. II. Development of an index for quantitative assay of encephalitogenic activity of antigens. J Neuropathol Exp Neurol. 1959;18:447–457. doi: 10.1097/00005072-195907000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Paterson PY. Experimental allergic encephalomyelitis and autoimmune disease. Adv Immunol. 1966;5:131–208. doi: 10.1016/s0065-2776(08)60273-4. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski HM, Keith AB. Chronic relapsing experimental allergic encephalomyelitis: an experimental model of multiple sclerosis. Ann Neurol. 1977;1:144–148. doi: 10.1002/ana.410010207. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta S, Zhou Y, Jana M, Banik NL, Pahan K. Sodium phenylacetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps. J Immunol. 2003;170:3874–3882. doi: 10.4049/jimmunol.170.7.3874. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 12.Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal S, Roy A, Pahan K. Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2009;182:5013–5023. doi: 10.4049/jimmunol.0801734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci USA. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmachari S, Pahan K. Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis. J Immunol. 184:6103–6113. doi: 10.4049/jimmunol.0804356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 19.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cross AH, Ramsbottom MJ, Lyons JA. NOS2 regulates cytokine production and VLA-4 expression in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;173:79–86. doi: 10.1016/j.jneuroim.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Pahan K. Neuroimmune pharmacological control of EAE. J Neuroimmune Pharmacol. 5:165–167. doi: 10.1007/s11481-010-9219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahan K. Immunomodulation of experimental allergic encephalomyelitis by cinnamon metabolite sodium benzoate. Immunopharmacol Immunotoxicol. doi: 10.3109/08923973.2011.561861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Paul WE. CD4+ T cell plasticity-Th2 cells join the crowd. Immunity. 32:11–13. doi: 10.1016/j.immuni.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 27.Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994;61:575–585. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 30.Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 31.Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarchielli P, Orlacchio A, Vicinanza F, Pelliccioli GP, Tognoloni M, Saccardi C, Gallai V. Cytokine secretion and nitric oxide production by mononuclear cells of patients with multiple sclerosis. J Neuroimmunol. 1997;80:76–86. doi: 10.1016/s0165-5728(97)00136-7. [DOI] [PubMed] [Google Scholar]
- 33.Ding M, Zhang M, Wong JL, Rogers NE, Ignarro LJ, Voskuhl RR. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1998;160:2560–2564. [PubMed] [Google Scholar]
- 34.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 35.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. J Immunol. 1999;163:4182–4191. [PubMed] [Google Scholar]
- 36.Parkinson JF, Mitrovic B, Merrill JE. The role of nitric oxide in multiple sclerosis. J Mol Med. 1997;75:174–186. doi: 10.1007/s001090050102. [DOI] [PubMed] [Google Scholar]
- 37.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]