Abstract
Rationale: Unprecedented pollution control actions during the Beijing Olympics provided a quasi-experimental opportunity to examine biologic responses to drastic changes in air pollution levels.
Objectives: To determine whether changes in levels of biomarkers reflecting pulmonary inflammation and pulmonary and systemic oxidative stress were associated with changes in air pollution levels in healthy young adults.
Methods: We measured fractional exhaled nitric oxide, a number of exhaled breath condensate markers (H+, nitrite, nitrate, and 8-isoprostane), and urinary 8-hydroxy-2-deoxyguanosine in 125 participants twice in each of the pre- (high pollution), during- (low pollution), and post-Olympic (high pollution) periods. We measured concentrations of air pollutants near where the participants lived and worked. We used mixed-effects models to estimate changes in biomarker levels across the three periods and to examine whether changes in biomarker levels were associated with changes in pollutant concentrations, adjusting for meteorologic parameters.
Measurements and Main Results: From the pre- to the during-Olympic period, we observed significant and often large decreases (ranging from −4.5% to −72.5%) in levels of all the biomarkers. From the during-Olympic to the post-Olympic period, we observed significant and larger increases (48–360%) in levels of these same biomarkers. Moreover, increased pollutant concentrations were consistently associated with statistically significant increases in biomarker levels.
Conclusions: These findings support the important role of oxidative stress and that of pulmonary inflammation in mediating air pollution health effects. The findings demonstrate the utility of novel and noninvasive biomarkers in the general population consisting largely of healthy individuals.
Keywords: air pollution, inflammation, oxidative stress, respiratory health, the Beijing Olympics
At a Glance Commentary
Scientific Knowledge on the Subject
Increased air pollution concentrations have been associated with increased mortality and morbidity. The overlapping mechanisms underpinning pulmonary inflammation and respiratory and systemic oxidative stress in relation to air pollution exposure remain unclear.
What This Study Adds to the Field
We observed significant improvements in biomarkers of pulmonary inflammation and biomarkers of respiratory and systemic oxidative stress during the Beijing Olympic air pollution control period in young healthy subjects. The findings from this real-world study provide quasi-experimental, mechanistic data to support that air pollution adversely affects young and healthy individuals through acute changes in pulmonary inflammation, airway, and systemic oxidative damage. This study also demonstrates the utility of novel and noninvasive biomarkers of pathophysiologic pathways using human breath specimen.
Increased air pollution concentrations have previously been associated with increased cardiorespiratory mortality and morbidity (1–5). However, observational and experimental studies in humans or animals have generated limited and somewhat inconsistent data supporting several postulated pathophysiologic pathways (6–10). One of these is the hypothesis that inhaled pollutants can react rapidly with extracelluar macromolecules or cell constituents in the airway epithelium to generate reactive oxygen or nitrogen species (e.g., free radicals and peroxides), inducing local and systemic oxidative or nitrosative stress and subsequent inflammation (11).
Pulmonary inflammation and oxidative stress responses to air pollution have been examined in human studies using several noninvasive biomarkers in exhaled breath and exhaled breath condensate (EBC) (7, 12–18). Increased air pollution levels have been associated with increased levels of fractional exhaled nitric oxide (FeNO), reflecting pulmonary inflammation, in children and the elderly (12–15, 19–22). Traffic pollution exposure has been associated with increased airway acidity (lowered EBC pH) in persons with asthma (16), reflecting inhibition of local epithelial proton pumps during airway inflammation (23). Furthermore, several novel EBC biomarkers reflecting oxidative and nitrosative stress in the respiratory tract (e.g., EBC nitrite, nitrate and 8-isoprostane) have been associated with air pollution exposure (17, 24–26). Recent studies also suggest that an increased whole-body burden of oxidative stress may induce or exacerbate pulmonary or systemic inflammation. These studies often use urinary 8-hydroxy-2-deoxyguanosine (8-OHdG) as a biomarker of systemic oxidative stress (27, 28). However, the overlapping mechanisms underpinning pulmonary inflammation, local (respiratory tract) and systemic (whole-body) oxidative stress, in relation to air pollution exposure, remain unclear. Major obstacles include the lack of “controlled” contrasting exposure conditions and the lack of noninvasive pathway-specific biomarkers that are sensitive to day-to-day changes in air pollution levels, as done in typical panel studies (9, 14, 16).
Unprecedented pollution control actions during the 2008 Beijing Olympics resulted in substantial temporary air quality improvements, providing a quasi-experimental opportunity to assess whether the drastic change in air quality would lead to changes in pathway-specific biomarkers, thereby elucidating mechanisms underlying air pollution health effects. Taking advantage of this opportunity, we have designed a study to examine several prominently hypothesized mechanisms of cardiorespiratory health effects from air pollution, including pulmonary inflammation, systemic inflammation, thrombosis, oxidative stress, and antonomic dysfunction (29). In the present paper, we focus on three interrelated pathways including pulmonary inflammation, pulmonary oxidative and nitrosative stress, and systemic oxidative stress. We measured a suite of noninvasive biomarkers, some of which are novel (e.g., EBC markers), reflecting these pathways in a panel of healthy adults. We hypothesized that each of these biomarkers would improve (reduced level of inflammation and oxidative stress) from the pre-Olympic period (without pollution controls) to the during-Olympic period (with pollution controls), followed by worsening from the during-Olympic period to the post-Olympic period (without pollution controls). We further hypothesized that changes in biomarker levels would be associated with changes in concentrations of pollutants. The results on air pollution associated with systemic inflammation and thrombosis, along with heart rate and blood pressure, have been reported elsewhere (29).
Methods
Study Design and Participants
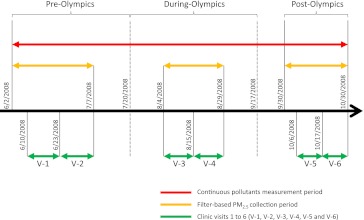
This panel study, nested within a quasi-experimental design with “high-low-high” pollution levels, was designed according to the timelines of the Olympic pollution control measures (30). The whole study period included three subperiods: (1) the pre-Olympic period (June 2–July 20); (2) the during-Olympic period (July 21–September 17) when industrial and commercial combustion facility operation and vehicle use were strictly controlled; and (3) the post-Olympic period (September 30–October 30) when the pollution control measures were relaxed (Figure 1). Participants were repeatedly measured for biomarkers twice, at least 1 week apart, within each subperiod.
Figure 1.
Operational definition of the pre-, during-, and post-Olympic periods in relation to the start and end dates for air pollutant measurements and clinical visits within each period. 0 = 0–23 hours before clinic visit; 1 = 24–47 hours before clinic visit; 2 = 48–71 hours before clinic visit; 3 = 72–95 hours before clinic visit; 4 = 96–119 hours before clinic visit; 5 = 120–143 hours before clinic visit; 6 = 144–167 hours before clinic visit.
Participants were a homogenous group of nonsmoking medical residents at Peking University First Hospital located in central Beijing, with regimented work and living lifestyles (29). Enrollment required each participant to complete a medical history, physical examination, routine blood chemistries, spirometry, and electrocardiogram to rule out any medical conditions that would preclude participation. Through on-site advertisements and with informed consent, we screened 137 persons, among which 128 met the inclusion criteria (never smoked and had no chronic diseases) and agreed to participate. Three participants withdrew from the study after taking part in the first one or two clinical visits, and thus were excluded from data analyses. Among the remaining 125 participants, 119 completed all six visits and six participants only missed one visit. These participants (62 female and 63 male, from 19–33 yr old) and their 744 person-visits were used in statistical analyses.
The study was approved by the joint Ethics Committee of Peking University Health Sciences Center and Peking University First Hospital, and the University of Medicine and Dentistry of New Jersey Institutional Review Board.
Clinical Visits and Biomarker Measurements
Participants could not have an active upper respiratory illness (either infection or allergy) and could not use antiinflammatory medication for allergies or other respiratory conditions for at least a week before a clinic visit. Each experimental session was about 60 minutes for each participant, starting with electrocardiogram monitoring in the supine position to measure heart rate and heart rate variability. Then we measured blood pressure and the following protocols without a particular order. Fasting blood samples were drawn to measure biomarkers of systemic inflammation and thrombosis, as described previously (29). FeNO and EBC samples were collected to measure for markers of pulmonary inflammation and oxidative stress. A spot urine sample was collected during each visit at a convenient time.
We measured FeNO using an offline sampling method, following the recommendations of the American Thoracic Society/European Respiratory Society (31), which has been described previously (21). All measurements were made with participants being seated, after 3–5 minutes of rest. Participants inhaled from functional residual capacity through a mouthpiece with a NO-scrubber attached, thereby inhaling NO-free air, followed by a controlled expiration through the mouthpiece. A resistive pressure of 13 cm H2O was applied to the exhaled air flow to ensure the closure of the nasopharyngeal vellum, thus preventing contamination by NO from the nose and sinuses. Hence, we collected the exhaled air containing only NO released from bronchial epithelial cells into a NO-impermeable aluminum foil bag. The exhaled breath samples were analyzed for FeNO using a NO/NO2/NOx chemiluminescence analyzer (Model 42i; ThermoScientific, Rockford, IL) within 3 hours of collection. The analyzer had a detection limit of 0.40 ppb and an accuracy of ± 0.40 ppb. The analyzer was calibrated daily with five concentrations of NO (ranging from 0–80 ppb).
We collected EBC samples using a Jaeger EcoScreen collector (Erich Jaeger, Hoechberg, Germany). The machine was switched on at least 30 minutes before collection to allow the cooling cuff to stabilize at the operating temperature of −20°C. The sealing cap was applied to the cuff to insulate the internal cooling area and to avoid condensation of ambient moisture. The EBC collection was made following the recommendations of the American Thoracic Society/European Respiratory Society Task Force on EBC (32). After collection, EBC samples were deaerated with inert argon gas (350 ml/min for 10 min), and pH was measured using a pH meter with a resolution of 0.01 and a working range from −2.00 to 16.00. The pH meter was calibrated using pH buffer solutions daily. Samples were then aliquoted in labeled crytotubes and an antioxidant mixture (butylated hydroxytoluene, 2 mM in 99% ethanol, 10 μl per ml sample) was added. Samples were immediately stored at −80°C for later analyses of the following biomarkers. The samples were analyzed for nitrite and nitrate using an HPLC-UV system at 214 nm (Waters, Milford, MA). The detection limits were 7.22 ng/ml for nitrite and 4.43 ng/ml for nitrate. Finally, the samples were analyzed for 8-isoprostane using an ELISA assay with a method detection limit of 1.56 pg/ml (Rapidbio, West Hills, CA).
We measured urinary 8-OHdG using an HPLC equipped with an electrochemical detector (Waters, Milford, MA) (33). The method detection limit was 0.5 ng/ml. Concentrations of urinary 8-OHdG were normalized by urinary creatinine concentrations and reported in mg/mol.
Air Pollution and Weather Measurements
We measured 24-hour mean concentrations of fine particles (particulate matter [PM]2.5) and several of its constituents: elemental carbon (EC), organic carbon (OC), and sulfate (SO42−) (30). We also measured 24-hour mean concentrations of sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), carbon monoxide (CO), and ambient temperature and relative humidity (RH). All these measurements were made on the roof top of a seven-story building in the center of the hospital campus. The methods have been described in more detail previously (29).
Statistical Analyses
We calculated period-specific descriptive statistics for each air pollutant, meteorologic variable, and biomarker by adding indicator variables for period (i.e., pre-, during-, and post-Olympics) into statistical models. Specifically, we used time-series regression models to estimate the period-specific means and between-period difference in air pollutant concentrations and meteorologic variables. We log-transformed FeNO, nitrite, nitrate, nitrite plus nitrate, and 8-OHdG because these biomarkers had right-skewed distributions. We estimated changes in biomarker levels across the periods (pre-, during-, and post-Olympic period) using linear mixed models for all the biomarkers except 8-isoprostane. Because there were a substantial fraction of data below the detection limit for 8-isoprostane, we did not perform this analysis. In this set of models, we used each biomarker as the dependent variable and period as the independent variable.
We then used the next set of linear mixed models to estimate changes in biomarker concentration associated with each interquartile range (IQR) increase in pollutant concentration in the 24 hours before the clinic visit (lag day 0), and the six previous 24-hour periods (lags 1–6, respectively). This was done for all the biomarkers, except 8-isoprostane, each of which was assessed as a continuous variable. We dichotomized 8-isoprostane data into above (and equal to) and below 75th percentile (6.21 pg/ml) and used random-effects logistic regression models to assess odds ratios for getting a “higher” (≥75th percentile) value associated with one IQR increase in pollutant concentration.
In all the models, we used the Akaike Information Criterion (AIC) to assess correlations between repeatedly measured data using the equicorrelated structure because this was the best structure consistently chosen across biomarkers among alternative correlation structures. Temperature, RH, sex, and day of week of clinic visits were controlled as covariates. Temperature and RH measured in the 24 hours before a visit were included in the models using natural splines with degrees of freedom chosen to minimize the AIC. We also accounted for additional seasonal differences by including cumulative averages of temperature and RH of up to 7 days using natural splines if these terms resulted in lower AICs. Because partial regression plots suggested that allowing more than three degrees of freedom resulted in over-fitting, we only allowed up to three degrees of freedom for all splines.
We examined adjusted associations between biomarker levels and pollutant concentrations averaged over the last 24 hours (lag 0), 24–48 hours (lag 1), 48–72 hours (lag 2) and so on, up to a 7-day lag. For all pollutant-biomarker combinations, “lag plots” were created to show the changes in biomarker level associated with one IQR increase in pollutant for lag 0 through lag 6. These were a series of single-lag models. Because some pollutants were correlated, we conducted two-pollutant models to examine whether the biomarker–pollutant associations from the single pollutant analyses were consistent with the effect of that pollutant while controlling for another pollutant. In these two-pollutant models, we only included one lag for each pollutant that showed the strongest statistical significance.
We conducted three sets of sensitivity analyses to evaluate the robustness of our models with respect to the adjustment of temperature and RH: (1) without any adjustments; (2) with full adjustments as described previously (our main analysis); and (3) with adjustments only when temperature or RH was significantly associated with biomarker in the main model. Furthermore, we examined potential confounding effects of seasonality by excluding post-Olympic period observations, and other time varying factors across periods by including period indicators in the single pollutant analyses.
We used R Version 2.12.1 (available on CRAN at http://cran.r-project.org) to perform all the analyses.
Results
Descriptive statistics of air pollutant concentrations and meteorologic variables are summarized in Table 1. Across the entire study period, we observed large day-to-day variations in pollutant concentrations, as reflected in large IQR for each pollutant. From the pre-Olympic to the during-Olympic period, we observed significant decreases in mean concentrations of SO2 (−60%), CO (−48%), NO2 (−43%), and EC (−36%) and nonsignificant reductions in mean concentrations of PM2.5 (−27%), SO42 (−13%), and OC (−22%). The mean O3 concentration and mean temperature increased from the pre-Olympic to the during-Olympic period. In contrast, from the during-Olympic to the post-Olympic period, we observed significant increases in pollutant concentrations for all the pollutants except O3 and SO42. Throughout the study period, several pairs of pollutants had correlation coefficients greater than 0.7: PM2.5 and SO42; EC and OC; EC and NO2; OC and NO2; OC and SO2; and PM2.5 and SO2. O3 was negatively correlated with NO2, EC, OC, and CO, but was weakly correlated with SO2, PM2.5, and SO42 (r < 0.35) (29).
TABLE 1.
PERIOD-SPECIFIC MEANS AND SE FOR AIR POLLUTANTS, AMBIENT TEMPERATURE, AND RH AND PERIOD COMPARISON RESULTS
| Period Comparison |
|||||||||||
| Pre-Olympics (June 2 to July 7) |
During-Olympics (July 28 to August 29) |
Post-Olympics (September 29 to October 31) |
Difference (during–pre) |
Difference (post–during) |
|||||||
| Pollutant/Meteorologic Parameter | IQR | Mean | SE | Mean | SE | Mean | SE | Mean | 95% CI | Mean | 95% CI |
| SO2, ppb | 5.4 | 7.45 | 1.17 | 2.97 | 1.33 | 6.81 | 1.22 | −4.48* | (−7.94 to −1.02) | −0.64 | (−3.95 to 2.67) |
| NO2, ppb | 18.7 | 25.60 | 3.66 | 14.61 | 3.76 | 41.39 | 3.81 | −10.99* | (−21.26 to −0.71) | 15.79* | (5.44 to 26.14) |
| O3, ppb | 25.4 | 31.84 | 3.75 | 39.60 | 3.85 | 15.12 | 3.91 | 7.75 | (−2.78 to 18.29) | −16.73 | (−27.34 to −6.11) |
| CO, ppm | 0.65 | 1.23 | 0.13 | 0.64 | 0.14 | 0.81 | 0.14 | −0.59* | (−0.97 to −0.22) | −0.42* | (−0.80 to −0.04) |
| PM2.5, μg/m3 | 76.8 | 98.9 | 14.7 | 71.9 | 15.1 | 85.3 | 15.3 | −27 | (−68.3 to 14.3) | −13.7 | (−55.3 to 27.9) |
| EC, μg/m3 | 1.4 | 2.2 | 0.3 | 1.4 | 0.3 | 3.4 | 0.3 | −0.80 | (−1.7 to 0.1) | 1.1* | (0.3 to 2) |
| OC, μg/m3 | 5.1 | 8.8 | 1.6 | 6.8 | 1.7 | 15 | 1.7 | −1.97 | (−6.6 to 2.6) | 6.2* | (1.7 to 10.7) |
| Sulfate, μg/m3 | 28 | 26.5 | 5.8 | 23 | 6.4 | 13.7 | 6.2 | −3.5 | (−20.4 to 13.5) | −12.8 | (−29.5 to 3.9) |
| Temp, °C | 25.2 | 0.9 | 27.8 | 0.9 | 16.7 | 0.9 | 2.52 | (0.02 to 5.02) | −8.56† | (−11.08 to −6.04) | |
| RH, % | 65.6 | 4 | 64.9 | 4.1 | 49.5 | 4.2 | −0.69 | (−11.93 to 10.54) | −16.10* | (−27.42 to −4.78) | |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; RH = relative humidity; T = temperature.
The results were derived from time-series regression models.
P < 0.05.
P < 0.01.
Changes in Biomarker Levels by Period
Period-specific means and SE of the biomarkers, accounting for the repeated measures, are shown in Table 2. From the pre-Olympic to the during-Olympic period, we observed decreases in mean concentrations of FeNO, nitrate, nitrite, nitrate plus nitrite, and 8-OHdG, respectively. We also observed an increase in EBC pH and a decrease in the fraction of above-detection-limit for EBC 8-isoprostane. These changes were reversed (increase vs. decrease) for all the biomarkers except nitrite from the during-Olympic to the post-Olympic period.
TABLE 2.
PERIOD-SPECIFIC MEANS AND SE FOR PULMONARY INFLAMMATION AND OXIDATIVE STRESS BIOMARKERS
| Pre-Olympics |
During-Olympics |
Post-Olympics |
||||
| Biomarkers | Mean | SE | Mean | SE | Mean | SE |
| FeNO, ppb* | 11.76 | 1.04 | 5.80 | 1.04 | 12.51 | 1.04 |
| EBC nitrite, μM* | 7.33 | 1.03 | 4.71 | 1.03 | 4.69 | 1.04 |
| EBC nitrate, μM* | 2.79 | 1.04 | 2.61 | 1.04 | 4.23 | 1.05 |
| EBC nitrite + nitrate, μM* | 10.48 | 1.03 | 7.68 | 1.03 | 10.37 | 1.03 |
| EBC pH | 7.43 | 0.03 | 7.46 | 0.03 | 7.61 | 0.03 |
| EBC 8-isoprostane, % ≥DL 1.56 pg/ml | 68 | N/A | 44 | N/A | 74 | N/A |
| Urinary 8-OHdG, mg/mol* | 3.70 | 1.12 | 2.22 | 1.12 | 3.34 | 1.12 |
Definition of abbreviations: DL= detection limit; EBC = exhaled breath condensate; FeNO = fractional exhaled nitric oxide; 8-OHdG = 8-hydroxy-2-deoxyguanosine.
The results are based on period estimates from mixed-effects models, accounting for the repeated measures but not adjusting for covariates.
Biomarkers had skewed data distributions, for which geometric means were reported.
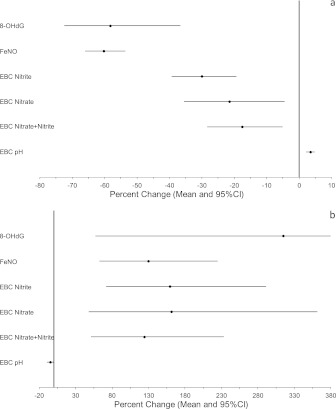
After adjusting for ambient temperature, RH, sex, and day of week for measurement, pre-Olympic to during-Olympic changes in biomarker levels were statistically significant for all the biomarkers in the hypothesized direction. As shown in Figure 2a, we observed large and significant decreases, ranging from −72.5 to −4.5%, in FeNO, nitrite, nitrate, nitrite plus nitrate, and 8-OHdG, respectively. We also observed a significant increase in EBC pH (3.5% with 95% confidence interval, 2.2–4.9%), which corresponds to a large decrease (−46%) in EBC hydrogen ion. Likewise, during-Olympic to post-Olympic changes after adjusting for the covariates were similar to the results from unadjusted analysis shown in Table 2 (EBC nitrite change became statistically significant in the adjusted analysis). As shown in Figure 2b, we observed large increases, ranging from 48–362%, in FeNO, nitrite, nitrate, nitrite plus nitrate, and 8-OHdG, respectively. We also observed a decrease in EBC pH (−4.8% with 95% confidence interval, −9.4 to −0.2%) corresponding to a large increase (146%) in EBC hydrogen ion concentration.
Figure 2.
Point estimates and 95% confidence interval (CI) of percent changes in biomarker levels (a) from pre-Olympic to during-Olympic period, and (b) from during-Olympic to post-Olympic period. Estimates were derived from linear mixed-model analysis controlling for ambient temperature, relative humidity, sex, and day of week of measurement. EBC = exhaled breath condensate; FeNO = fractional exhaled nitric oxide; 8-OHdG = 8-hydroxy-2-deoxyguanosine.
Biomarker–Pollutant Associations
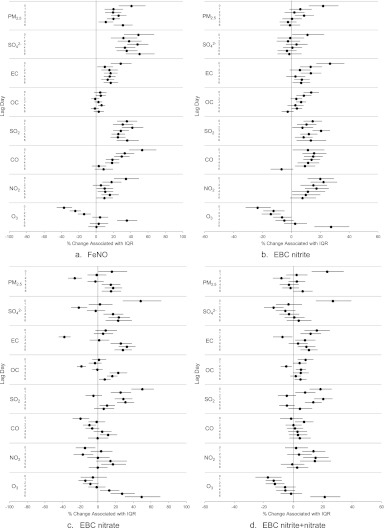
As hypothesized, we observed significant increases in FeNO (ranging from 10–75%) associated with IQR increases in PM2.5, SO42−, EC, SO2, CO, and NO2 at most lags (Figure 3a). The FeNO changes per IQR increases (effect estimates) in most pollutants were largest at lag 0 (0–24 h before biomarker measurement), except SO2, which had the largest effect estimate at lag 3. The effect estimates generally decreased with each increasing lag. However, for O3, the direction of the association was negative at lags 0–2.
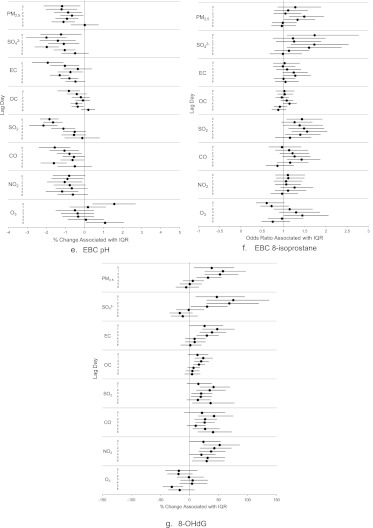
Figure 3.
Percent changes and 95% confidence intervals in biomarker levels (odds ratios for having a >75th percentile value of exhaled breath condensate [EBC] 8-isoprostane) associated with one interquartile range (IQR) increase in pollutant concentration, by 24-hour lag period (lag Day 0–6), for the whole study period. (a) Fractional exhaled nitric oxide (FeNO). (b) EBC nitrite. (c) EBC nitrate. (d) EBC nitrite and nitrate. (e) EBC pH. (f) EBC 8-isoprostane. (g) 8-hydroxy-2-deoxyguanosine (8-OHdG).
Consistent with the hypothesis, EBC nitrite was positively associated with all pollutants except O3 at one or more lags (Figure 3b). Across the lags, the largest effect estimates per IQR increase in pollutant concentration were 21.9% for PM2.5, 11.1% for SO42−, 26.7% for EC, 13.7% for OC, 20.5% for SO2, 15.7% for CO, and 22.2% for NO2. Pollutant associations of EBC nitrate varied more substantially across lags than those of EBC nitrite, in terms of variation directions and effect size (Figure 3c). As expected (Figure 3d), the pattern for nitrite plus nitrate was between that for nitrite and that for nitrate. The effect estimates were positive, significant, and largest for PM2.5, SO42−, EC, OC, and SO2 at lag 0, and CO and NO2 at lag 1. However, the association with O3 was significant and negative at lags 0–2.
Consistent with the hypothesized mechanism that air pollution exposure leads to airway acidification, we observed significant negative associations between EBC pH and all the pollutants at most lags except O3 at lags 0 and 6 (Figure 3e). The largest effect estimates were observed at lags 0 and 1 for PM2.5; lags 1 and 4 for SO42−; lag 0 for EC and OC; lag 2 for SO2; lags 0 and 5 for CO; lag 5 for NO2; and lag 0 for O3 (at the opposite direction).
Consistent with the hypothesis that increased exposure to air pollution leads to increased lipid peroxidation in the lungs, we observed an increased odds ratio of a high EBC 8-isoprostane level (≥75th percentile relative to <75th percentile values) associated with each IQR increase in all the pollutant except O3 at multiple lags (Figure 3f). The increased odds ratios were statistically significant for PM2.5 at lags 3 and 4; SO42− at lag days 4 and 5; EC at lag day 4; OC at lag 4; SO2 at lag days 0, 3, and 4; CO at lag 4; and O3 at lag 4 (but in the opposite direction).
As shown in Figure 3g for urinary 8-OHdG, we observed mostly positive and significant associations for all pollutants (except O3) across multiple lags, which is consistent with the hypothesis that increased air pollution exposure leads to increased whole-body burden of oxidative stress. The largest changes in 8-OHdG concentration per IQR increase in pollutant concentration were 57.6% for PM2.5, 75.4% for sulfate, 47.7% for EC, 23.6% for OC, 41.4% for SO2, 42.2% for CO, and 51.9% for NO2 (all at lag 1). The largest change in 8-OHdG level associated with an IQR increase in O3 concentration was −30.5% (opposite direction) at lag 5.
In two-pollutant model analyses, when adjusting for a second pollutant in the same model, we observed small changes in the individual pollutant-biomarker effect estimates for most of the biomarkers, compared with those in the single-pollutant model analysis. However, no substantial changes were observed in the overall pattern of the associations (see Figure E1 in the online supplement).
Sensitivity Analyses
Table E1 provides degrees of freedom selected for each meteorologic parameter in the statistical analysis. When nonsignificant temperature and RH were deleted from the models or without controlling for temperature and RH, the changes in the between-period and the overall pattern in the pollutant-biomarker associations remained similar to those observed in the main model (primary) analyses (see Figure E2).
Results from single-pollutant models excluding the post-Olympic observations for selected biomarkers (FeNO, EBC nitrite, and urinary 8-OHdG) are summarized in Figure E3. As expected, the reduced sample size in the two-period-only analysis resulted in wider confidence intervals of the effect estimates but did not change the main findings. To assess within-period effects of the single pollutants, we added the “period” indicator as a covariate to the single-pollutant models. Results from this set of analysis are shown in Figure E4 for selected biomarkers. We observed, in general, more conservative (reduced) effect estimates of the pollutant-biomarker associations compared with the effect estimates when “period” was not adjusted, except for EBC nitrite–nitrate, for which this difference was less notable.
Findings from these sensitivity analyses further support our main findings, indicating that changes in biomarker levels between periods and in association with pollutant concentrations were unlikely driven by other time-varying factors than changes in pollution levels.
Discussion
Taking advantage of a unique experimental opportunity where the Chinese government mandated temporary closures or relocations of industry and reductions in motor vehicle use during the Beijing Olympics, we observed significant changes in multiple biomarkers in healthy and young adults in response to drastic changes in air pollution levels. We also observed significant associations of each of these biomarkers with multiple pollutants after adjusting for meteorologic parameters. The biomarkers we used in the study, reflecting pulmonary inflammation, respiratory, and whole-body oxidative stress, were sensitive to changes in air pollution levels across the three periods of the quasi-experimental design and in the day-to-day biomarker–pollutant association analysis. This demonstrates the use of the novel and noninvasive biomarkers in examining airway inflammation and oxidative stress attributable to ambient pollution even in healthy and young adults.
FeNO has been widely used as a sensitive biomarker of airway inflammation (22, 34, 35). Increases in FeNO concentration have been associated with increases in air pollution levels in children and adults with asthma (13, 15, 19, 36). Our study further confirms the FeNO and air pollution association in healthy and young adults. Lowering of the airway pH has been reported to cause bronchoconstriction (37); to impair airway epithelial functions, such as ciliary motility (38); and to increase airway mucus viscosity (39). Decreases in EBC pH in patients with asthma suggests that acute asthma exacerbations may be accompanied by airway acidification that reflects inhibition of local epithelial proton pumps during airway inflammation (16). Our study is perhaps the first to examine and confirm air pollution associations with airway acidification in healthy adults.
Much of the effort to identify a biochemical basis for acute and chronic effects of air pollution has centered on the role of reactive oxygen species (ROS) induced from inhaled air pollutants, especially PM (10). These ROS may result in an increased burden of oxidative stress and proinflammatory effects, locally and distant from the site of initial injury (10, 40–42). EBC nitrite and nitrate (or the sum: nitrite plus nitrate) are metabolic oxidation products of NO that is produced primarily in the lung. Concentrations of nitrite and nitrate in EBC, hence, reflect levels of oxidative and nitrosative stress in the respiratory tract (43, 44). ROS can react with lipids to form stable compounds, such as 8-isoprotane. Increased EBC concentrations of nitrite and nitrate and 8-isoprostane have been associated with exacerbation of asthma, chronic obstructive pulmonary disease, and cystic fibrosis (45–49). However, to the best of our knowledge, there have been no published studies reporting EBC nitrite and nitrate changes in relation to air pollution exposure. Only two studies have reported increases in EBC 8-isoprostane concentration associated with air pollution exposure: one in children with asthma and one in patients with coronary heart disease (12, 17). In the present study, we observed significant increases in EBC nitrite, nitrate, and nitrite plus nitrate, each associated with increased concentrations of PM2.5, EC, OC, and sulfate with largest effects at lag 0. In contrast, EBC 8-isoprostane showed its largest effect estimates at lag 3 or lag 4 for PM2.5, sulfate, SO2, and CO. This suggests that air pollution may affect NO metabolism faster than it induces lipid peroxidation.
ROS can oxidize DNA molecules to form stable products (e.g, 8-OHdG) that are readily excreted in biofluids, such as urine. Increased 8-OHdG levels have been positively associated with premature coronary heart disease mortality (50) and with coronary artery disease but negatively associated with total serum antioxidant capacity (51, 52). In the present study, we observed a large (58%) and significant reduction in urinary 8-OHdG concentration from the pre- to the during-Olympic period and a very large (315%) reversal from the during-Olympic to the post-Olympic period. This finding supports an association between lowered whole-body oxidative stress and improved air quality. Although there has been a considerable literature reporting associations between ambient PM and oxidative stress in children and adults with asthma or other cardiorespiratory diseases (20, 36, 53–55), our study provides evidence in healthy and young adults, with both respiratory tract and whole-body biomarkers, to support the oxidative stress mechanism.
We also reported previously that biomarkers of systemic inflammation and thrombosis were significantly associated with changes in air pollution levels during the Beijing Olympics (29). This suggests a potential interactive relationship between the respiratory pathway markers demonstrated in the present data and the cardiovascular markers previously reported, all potentially contributing to clinical cardiovascular endpoints. For example, ROS characterizing oxidative stress may induce inflammation; and inflammatory mediators may engage in biochemical reactions that produce ROS. Inflammation contributes to the instability of preexisting atherosclerotic plaques or activation of platelets. Thrombosis, dependent on platelet activation, is responsible for arterial obstruction on top of such a ruptured plaque and ultimately an ischemic myocardial or cerebral event, as postulated previously (8).
Strengths and Limitations
Most previous studies exploring underlying biologic mechanisms of air pollution health effects have focused on relationships among a few biomarkers and air pollutants based on hourly, daily, or weekly variations in pollutant levels. In this study, we measured a suite of exhaled breath biomarkers of respiratory inflammation and oxidative and nitrosative stress, some of which (e.g., EBC pH, nitrite, and nitrate) are novel and have seldom been used in air pollution studies. Furthermore, we observed significant associations, in the hypothesized direction, between biomarker levels and pollutant concentrations in a homogenous group of young and healthy individuals, without potential confounding from disease, medication use, and age-related susceptibility.
However, several limitations should be noted for our study. First, although our pre-Olympic and during-Olympic measurements all occurred in summer months, the post-Olympic measurements fell in the early autumn season. We conducted a series of sensitivity analyses using different methods to adjust for temperature and RH (e.g., no adjustment, linear or natural splines adjustments with different degrees of freedom). The sensitivity analysis results indeed showed some impact on a few biomarkers (e.g., EBC nitrite) in terms of statistical significance. However, the overall lag pattern of the associations and effect estimates remained stable. Second, our measurements of air pollution were made at a fixed site near the work and residence places of the subjects and were not personal exposure measurements. Although we were not able to conduct a validation study to examine personal exposure in relation to outdoor pollution with portable samplers, we anticipated that indoor microenvironmental exposure would be highly influenced by the outdoor air because the infiltration rate was generally high in buildings during the summer and early fall in Beijing. Third, it is known that some of the biomarkers may be affected by diet. For example, a nitrate-rich diet may increase FeNO levels (and likely EBC nitrate and nitrite levels) (56, 57). However, it is unlikely that our study subjects (medical professionals) would have changed their diet during the Olympics. Hence, observed changes in biomarkers during the Olympics compared with before and after Olympics are unlikely to have resulted from dietary changes and other behavioral changes. Last, we attempted to address what specific components of the air pollution mixture (e.g., gases versus PM) and what specific constituents of PM were more “responsible” for changes in biomarker levels. However, in part because of the simultaneous shut-down of multiple pollutant sources during the Olympics and subsequent relaxation of pollution controls, it was difficult to differentiate effects of specific pollutants, particularly when many pollutants were correlated among each other. The seemingly “beneficial” effects of ozone on several biomarkers was likely caused by the fact that O3 concentrations were increased, whereas the other pollutants were substantially reduced during the Olympic air pollution control period (29). A similar seemingly protective effect of O3 has also been observed in previous studies when O3 was negatively correlated to NO2 and other pollutants (58, 59).
It is important to recognize some fundamental limitations of the EBC approach (32, 60). The most notable one is perhaps the lack of the standardization of EBC biomarker concentrations because of variable dilution during EBC collection. To minimize the variability in dilution, we collected EBC samples following the recommendation by the American Thoracic Society/European Respiratory Society Task Force on EBC (32), although we realize this cannot completely eliminate this problem. Another recognized major limitation is potential contamination of EBC biomarkers from oral microorganisms. These limitations have largely prevented the use of EBC biomarkers in clinical settings. However, in a repeated within-person comparison design like the present study, dilution and oral contamination would introduce random errors, but cannot produce the consistent relationships between the multiple EBC biomarker levels and the periods or the pollutant concentrations.
In summary, we observed significant improvements in biomarkers of pulmonary inflammation and biomarkers of respiratory and systemic oxidative stress during the Beijing Olympic air pollution control period. We also observed significant increases in levels of pulmonary inflammation, respiratory tract oxidative stress, and systemic oxidative stress, associated with increases in pollutant concentrations measured 0–24 hours to 6 days before biomarker measurements. The largest adverse biomarker changes per IQR increase in pollutant concentration (effect estimates) were seen within 24 or 48 hours for biomarkers of pulmonary inflammation (FeNO); airway acidification (EBC pH); and metabolic products of NO (EBC nitrite, nitrate, and nitrite plus nitrate). The largest effect estimates were observed slightly later (48–96 h) for biomarkers of lipid preoxidation (EBC 8-isoprostane) and DNA oxidative damage. These findings support the hypothesis that air pollution adversely affects health through acute changes in pulmonary inflammation and oxidative damage to essential biomolecules, such as lipids and DNA. The findings also demonstrate the potential utility of novel and noninvasive biomarkers used in the present study to examine biologic mechanisms of pulmonary effects in the general population consisting largely of healthy adults.
Supplementary Material
Acknowledgments
The authors are grateful to those volunteers for their commitment to the study and to the staff and students members at Peking University and Peking University First Hospital who helped with the data and sample collection.
Footnotes
Author Contributions: Conception and design, J.Z., H.K., T.Z., G.W., Y.W., P.Z., P.O.-S., S.-E.L., and M.H.; acquisition and preparation of data, G.W., Y.W., W.H., P.Z., J.G., M.H., J.Z., H.K., and D.R.; analysis and interpretation, J.Z., H.K., S.-E.L., W.H., D.R., P.O.-S., S.R.D., J.T., J.G., and W.L.; and drafting manuscript for important intellectual content, W.H., G.W., and J.Z.
Supported by NIEHS (1R01ES015864, P30ES005022, and 5P30ES007048); Health Effects Institute (4760-RPFA05-3); China National Science Foundation (20637020); Chinese Ministry of Science and Technology (grant 2008AA062503); and Chinese Ministry of Environmental Protection (grant 201009032).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201205-0850OC on August 30, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–1242 [DOI] [PubMed] [Google Scholar]
- 2.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 2007;29:879–888 [DOI] [PubMed] [Google Scholar]
- 3.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard six cities study. Am J Respir Crit Care Med 2006;173:667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunekreef B, Beelen R, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, Armstrong B, Hughes E, Jerrett M, van den Brandt P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-air study. Res Rep Health Eff Inst 2009;(139):5–71, discussion 73–89 [PubMed] [Google Scholar]
- 6.Sorensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, Loft S. Personal pm2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect 2003;111:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 2007;176:370–376 [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378 [DOI] [PubMed] [Google Scholar]
- 9.Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect 2008;116:898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nel A. Air pollution-related illness: Effects of particles. Science 2005;308:804–806 [DOI] [PubMed] [Google Scholar]
- 11.Auger F, Gendron MC, Chamot C, Marano F, Dazy AC. Responses of well-differentiated nasal epithelial cells exposed to particles: role of the epithelium in airway inflammation. Toxicol Appl Pharmacol 2006;215:285–294 [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, Wheeler A, Dales R. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect 2009;117:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. Exhaled nitric oxide in children with asthma and short-term pm2.5 exposure in Seattle. Environ Health Perspect 2005;113:1791–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ Health Perspect 2007;115:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, George SC, Shafer MM, Schauer JJ, Sioutas C. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology 2010;21:892–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 2007;357:2348–2358 [DOI] [PubMed] [Google Scholar]
- 17.Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, Freney EJ, Heal MR, Donovan RJ, Blomberg A, et al. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect 2008;116:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laumbach RJ, Kipen HM. Acute effects of motor vehicle traffic-related air pollution exposures on measures of oxidative stress in human airways. Ann N Y Acad Sci 2010;1203:107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, George SC, Kleinman MT. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect 2006;114:1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamkiewicz G, Ebelt S, Syring M, Slater J, Speizer FE, Schwartz J, Suh H, Gold DR. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax 2004;59:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin W, Huang W, Zhu T, Hu M, Brunekreef B, Zhang Y, Liu X, Cheng H, Gehring U, Li C, et al. Acute respiratory inflammation in children and black carbon in ambient air before and during the 2008 Beijing Olympics. Environ Health Perspect 2011;119:1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, Trenga CA, Larson T, Liu LJ. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect 2005;113:499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol 2002;282:C736–C743 [DOI] [PubMed] [Google Scholar]
- 24.Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, Hong YC. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect 2010;118:579–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfaro MF, Walby WF, Adams WC, Schelegle ES. Breath condensate levels of 8-isoprostane and leukotriene b4 after ozone inhalation are greater in sensitive versus nonsensitive subjects. Exp Lung Res 2007;33:115–133 [DOI] [PubMed] [Google Scholar]
- 26.Rundell KW, Slee JB, Caviston R, Hollenbach AM. Decreased lung function after inhalation of ultrafine and fine particulate matter during exercise is related to decreased total nitrate in exhaled breath condensate. Inhal Toxicol 2008;20:1–9 [DOI] [PubMed] [Google Scholar]
- 27.Ren C, Fang S, Wright RO, Suh H, Schwartz J. Urinary 8-hydroxy-2'-deoxyguanosine as a biomarker of oxidative DNA damage induced by ambient pollution in the normative aging study. Occup Environ Med 2010;68:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Han IK, Shao M, Hu M, Zhang OJ, Tang X. Pm2.5 constituents and oxidative DNA damage in humans. Environ Sci Technol 2009;43:4757–4762 [DOI] [PubMed] [Google Scholar]
- 29.Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl S, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 2012;307:2068–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kipen H, Rich D, Huang W, Zhu T, Wang G, Hu M, Lu SE, Ohman-Strickland P, Zhu P, Wang Y, et al. Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: a panel study approach. Ann N Y Acad Sci 2010;1203:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ATS/ERS ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912–930 [DOI] [PubMed] [Google Scholar]
- 32.Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, et al. Condensate AETFoEB. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 2005;26:523–548 [DOI] [PubMed] [Google Scholar]
- 33.De Martinis B, Bianchi M. Methodology for urinary 8-hydroxy-2'-deoxyguanosine analysis by HPLC with electrochemical detection. Pharmacol Res 2002;46:129–131 [DOI] [PubMed] [Google Scholar]
- 34.Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med 2000;162:981–988 [DOI] [PubMed] [Google Scholar]
- 35.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 2003a;107:1202–1208 [DOI] [PubMed] [Google Scholar]
- 36.Koenig JQ, Jansen K, Mar TF, Lumley T, Kaufman J, Trenga CA, Sullivan J, Liu LJ, Shapiro GG, Larson TV. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003;111:1625–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P. Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med 1999;159:557–562 [DOI] [PubMed] [Google Scholar]
- 38.Holma B, Lindegren M, Andersen JM. pH effects on ciliomotility and morphology of respiratory mucosa. Arch Environ Health 1977;32:216–226 [DOI] [PubMed] [Google Scholar]
- 39.Holma B. Effects of inhaled acids on airway mucus and its consequences for health. Environ Health Perspect 1989;79:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect 2005;113:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol 2005;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilliland FD, McConnell R, Peters J, Gong H., Jr A theoretical basis for investigating ambient air pollution and children's respiratory health. Environ Health Perspect 1999;107:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 2000;161:694–699 [DOI] [PubMed] [Google Scholar]
- 44.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 2002;165:1364–1370 [DOI] [PubMed] [Google Scholar]
- 45.Kostikas K, Papatheodorou G, Psathakis K, Panagou P, Loukides S. Oxidative stress in expired breath condensate of patients with COPD. Chest 2003;124:1373–1380 [DOI] [PubMed] [Google Scholar]
- 46.Robroeks CM, van de Kant KD, Jobsis Q, Hendriks HJ, van Gent R, Wouters EF, Damoiseaux JG, Bast A, Wodzig WK, Dompeling E. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin Exp Allergy 2007;37:1303–1311 [DOI] [PubMed] [Google Scholar]
- 47.Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest 2009;135:66–73 [DOI] [PubMed] [Google Scholar]
- 48.Corradi M, Pesci A, Casana R, Alinovi R, Goldoni M, Vettori MV, Cuomo A. Nitrate in exhaled breath condensate of patients with different airway diseases. Nitric Oxide 2003;8:26–30 [DOI] [PubMed] [Google Scholar]
- 49.Rihak V, Zatloukal P, Chladkova J, Zimulova A, Havlinova Z, Chladek J. Nitrite in exhaled breath condensate as a marker of nitrossative stress in the airways of patients with asthma, COPD, and idiopathic pulmonary fibrosis. J Clin Lab Anal 2010;24:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M. Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. FASEB J 1998;12:1397–1400 [PubMed] [Google Scholar]
- 51.Demirbag R, Yilmaz R, Erel O, Gultekin U, Asci D, Elbasan Z. The relationship between potency of oxidative stress and severity of dilated cardiomyopathy. Can J Cardiol 2005;21:851–855 [PubMed] [Google Scholar]
- 52.Vassalle C, Petrozzi L, Botto N, Andreassi MG, Zucchelli GC. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J Intern Med 2004;256:308–315 [DOI] [PubMed] [Google Scholar]
- 53.Corradi M, Folesani G, Andreoli R, Manini P, Bodini A, Piacentini G, Carraro S, Zanconato S, Baraldi E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med 2003;167:395–399 [DOI] [PubMed] [Google Scholar]
- 54.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Almstrand AC, Diaz-Sanchez D, Sly PD, Olin AC. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol 2008;121:903–909 [DOI] [PubMed] [Google Scholar]
- 55.Baraldi E, Carraro S, Alinovi R, Pesci A, Ghiro L, Bodini A, Piacentini G, Zacchello F, Zanconato S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax 2003;58:505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, Toren K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med 2001;95:153–158 [DOI] [PubMed] [Google Scholar]
- 57.Rava M, Varraso R, Decoster B, Huyvaert H, Moual NL, Jacquemin B, Kunzli N, Kauffmann F, Zerimech F, Matran R, et al. Plasma and exhaled breath condensate nitrite-nitrate level in relation to environmental exposures in adults in the EGEA study. Nitric Oxide 2012;27:169–175 [DOI] [PubMed] [Google Scholar]
- 58.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Emberlin J, Strachan DP. Air pollution, pollens, and daily admissions for asthma in London 1987–92. Thorax 1998;53:842–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajat S, Haines A, Goubet SA, Atkinson RW, Anderson HR. Association of air pollution with daily gp consultations for asthma and other lower respiratory conditions in London. Thorax 1999;54:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Effros RM, Casaburi R, Porszasz J, Morales EM, Rehan V. Exhaled breath condensates: analyzing the expiratory plume. Am J Respir Crit Care Med 2012;185:803–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.