Abstract
Rationale: Activation of the adenosine A2B receptor (A2BR) promotes antiinflammatory effects in diverse biological settings, but the role of this receptor in antimicrobial host defense in the lung has not been established. Gram-negative bacillary pneumonia is a common and serious illness associated with high morbidity and mortality, the treatment of which is complicated by increasing rates of antibiotic resistance.
Objectives: To test the hypothesis that absence of adenosine A2B receptor signaling promotes host defense against bacterial pneumonia.
Methods: We used a model of Klebsiella pneumoniae pneumonia in wild-type mice and mice with targeted deletion of the A2BR. Host responses were compared in vivo and leukocyte responses to the bacteria were examined in vitro.
Measurements and Main Results: A2BR–/– mice demonstrated enhanced bacterial clearance from the lung and improved survival after infection with K. pneumoniae compared with wild-type controls, an effect that was mediated by bone marrow–derived cells. Leukocyte recruitment to the lungs and expression of inflammatory cytokines did not differ between A2BR–/– and wild-type mice, but A2BR–/– neutrophils exhibited sixfold greater bactericidal activity and enhanced production of neutrophil extracellular traps compared with wild-type neutrophils when incubated with K. pneumoniae. Consistent with this finding, bronchoalveolar lavage fluid from A2BR–/– mice with Klebsiella pneumonia contained more extracellular DNA compared with wild-type mice with pneumonia.
Conclusions: These data suggest that the absence of A2BR signaling enhances antimicrobial activity in gram-negative bacterial pneumonia.
Keywords: neutrophil, extracellular traps, pneumonia, adenosine
At a Glance Commentary
Scientific Knowledge on the Subject
New therapies are needed to combat lethal lung infections by multidrug-resistant strains of gram-negative bacteria. Activation of the adenosine A2B receptor has been shown to promote antiinflammatory effects during lung injury. The role of adenosine A2B receptor signaling in the setting of pneumonia is not known.
What This Study Adds to the Field
We report that adenosine A2B receptor deficiency improves survival and enhances bacterial clearance from the lung in a mouse model of Klebsiella pneumonia. This effect was in part attributable to enhanced neutrophil extracellular trap (NET) production and extracellular killing of bacteria by A2B receptor–deficient neutrophils. Modulation of the A2B receptor may provide a novel therapeutic target for drug-resistant bacterial pneumonia.
Pneumonia is a leading cause of hospitalization in the United States and is the most common infectious cause of death (1, 2). Aerobic gram-negative bacilli are the most common cause of health care–associated pneumonia; mortality rates from gram-negative pneumonia range from 30 to 60% with antimicrobial therapy (3, 4). The emergence of multiresistant strains of gram-negative bacteria, combined with limited development of new antimicrobial therapies, has exacerbated the need for new approaches to combating these pathogens (5–7). Therapy aimed at augmenting the host response has the potential to enhance bacterial clearance and improve outcomes, and could provide a new avenue for therapeutic advances in combating gram-negative pneumonia, including infections that are caused by antibiotic-resistant pathogens.
Adenosine, a breakdown product of ATP, is a potent signaling molecule released from a variety of cells to dampen inflammation, limit tissue destruction, and promote repair (8, 9). In response to cellular stress or tissue injury, the extracellular concentration of adenosine can increase 100-fold. Adenosine acts on four widely expressed G protein–coupled receptors: A1, A2A, A2B, and A3, with variable expression among different cells. The adenosine A2B receptor (A2BR) has been shown to enhance the production of IL-6 by endothelial cells, epithelial cells, and macrophages (10, 11). On the other hand, A2BR activation attenuates inflammation by effects on endothelial adhesion molecule expression and reduced macrophage tumor necrosis factor-α production. Activation of the A2BR on endothelial cells also enhances barrier function, which limits leukocyte recruitment, rolling, and transmigration and decreases vascular leak and edema formation (12–14). Activation of A2BRs on certain macrophages inhibits their proliferation, MHC class II expression, and inducible nitric oxide synthase production (15, 16). In addition, dendritic cell immunogenicity and neutrophil oxidative burst are inhibited by A2BR agonist binding (17, 18). Although the ability of adenosine to limit tissue inflammatory damage is beneficial, it also inhibits the host response to infection and may indirectly impair bacterial clearance and recovery from infection.
Because the preponderance of evidence suggests that A2BR activation acutely inhibits inflammation, we posited that A2BR antagonism could enhance the innate immune response to invading pathogens and may represent a novel mode of antimicrobial therapy. Thus, we tested the hypothesis that the deletion of A2BR signaling promotes host defenses against bacterial pneumonia.
Methods
Animals, In Vivo Procedures, and Tissue Harvest
C57BL/6 mice and homozygous mice with β-galactosidase (β-Gal) knockin at the A2BR locus on a C57BL/6 background (12) were maintained under specific pathogen-free conditions and in compliance with institutional animal care regulations. Experimental Klebsiella pneumoniae pneumonia and generation of bone marrow–chimeric mice were performed as described (19). In vivo neutrophil depletion was induced by intraperitoneal administration of 400 μg of monoclonal antibody (mAb) against Ly6G (clone 1A8; Bio X Cell, West Lebanon, NH) or isotype control mAb (clone 2A3; Bio X Cell) as described (20). Methods for tissue harvest (21), bronchoalveolar lavage, and isolation of bone marrow neutrophils (22) and histology (19, 21) have been described previously.
Bacterial Content, Cytokine Assays, Quantitative RT-PCR, and Flow Cytometry
Sample bacterial content was determined by serial dilution and culture as previously described (19). Samples were processed for cytokine analysis as described (19, 21, 23) and cytokine levels were measured with commercial ELISA kits (DuoSet ELISA development; R&D Systems, Minneapolis, MN) or multiplex bead array kits (MILLIPLEX map; Millipore, Billerica, MA) according to the manufacturer’s instructions. For quantitative RT-PCR of the A2BR gene, RNA was isolated from sorted cells, cDNA was generated, and quantitative real-time reverse transcriptase PCR was performed as previously described (24). Cell suspensions for flow cytometric analysis and sorting were prepared as described (19, 21) according to a published gating strategy (25), as detailed in the online supplement.
Ex Vivo Assays
To opsonize bacteria, 2 × 109 bacteria were incubated in 5 ml of freshly collected mouse serum for 30 minutes at 37°C, washed with sterile water, and resuspended in Hanks’ balanced salt solution (HBSS). For all coculture experiments, 4 × 105 leukocytes and an equal number of bacteria in 200 μl of HBSS were placed in round-bottom 96-well plates (Falcon), centrifuged to achieve cell contact, and incubated at 37°C. Neutrophil and macrophage bactericidal assays were performed according to a modification of a previously described protocol (19); cells were incubated for 3 hours. In some experiments, gentamicin was added to the wells after 1 hour (1 μg/ml for 10 min at 37°C) to kill extracellular bacteria, and cells were then washed twice with HBSS and incubated for an additional 2 hours to allow intracellular killing of bacteria. In other experiments, bactericidal assays were performed in the presence of DNase (final concentration, 10 U/ml; Sigma-Aldrich, St. Louis, MO). After the incubation steps, cells were centrifuged and resuspended in 200 μl of sterile H2O to lyse neutrophils, and surviving bacteria were quantified by serial dilution and culture. Phagocytic capacity, measured as the proportion of leukocytes that contain fluorescently labeled bacteria after quenching of extracellular fluorescence, was determined as described previously (23). Oxidative burst of bone marrow neutrophils was determined by preincubation with 1 μM dihydrorhodamine 123 (Invitrogen, Grand Island, NY) in HBSS as described (26). Indicated cell stimulants were applied and resultant reactive oxygen species (ROS) production was detected with a fluorescence plate reader. Neutrophil extracellular traps were quantified as described (27) after 3 hours of coculture of neutrophils and bacteria or in bronchoalveolar lavage fluid after cells were pelleted.
Results
The A2BR Is Highly Expressed in the Setting of Gram-Negative Pneumonia
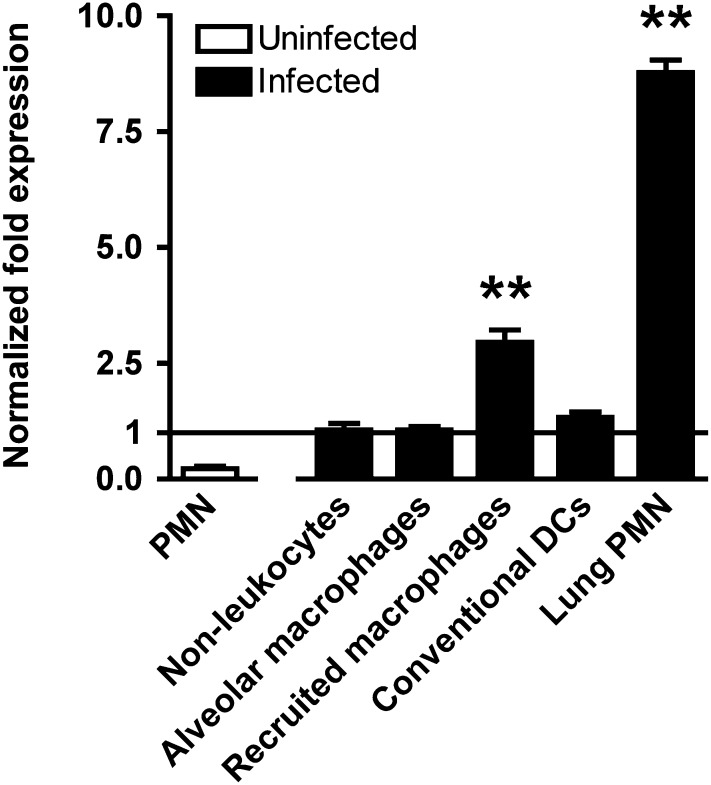
Previous studies have demonstrated that, in the uninfected lung, the A2BR is highly expressed by type II alveolar epithelial cells with relatively less expression by alveolar macrophages (24). Because the A2B receptor is also expressed on several leukocyte subsets (28), we sought to determine impact of bacterial pneumonia on the cellular distribution A2BR mRNA in the lungs. In cells sorted from lungs of mice with Klebsiella pneumonia, we found that neutrophils and recruited macrophages demonstrated nine- and threefold greater expression of A2BR mRNA as compared with nonleukocyte lung cells, indicating that neutrophils are the predominant population of cells expressing A2BR transcript in the infected lung (Figure 1). Interestingly, expression of A2BR mRNA was more than 30-fold greater in neutrophils isolated from infected lungs as compared with resting bone marrow neutrophils, suggesting that neutrophil expression of the A2BR is enhanced during infection.
Figure 1.
Adenosine A2B receptor is highly expressed in the lungs in bacterial pneumonia. Shown is relative adenosine A2B receptor mRNA expression in cells sorted from lungs on Day 3 of pneumonia. Expression is normalized to that of nonleukocyte cells in the lung. Data shown represent means ± SEM of triplicates representative of two experiments; **P < 0.001 for recruited macrophages and lung polymorphonuclear neutrophils (PMN) compared with other groups (one-way analysis of variance). DCs = dendritic cells.
Deletion of the A2BR on Bone Marrow–derived Cells Results in Improved Outcome of Gram-Negative Bacterial Pneumonia
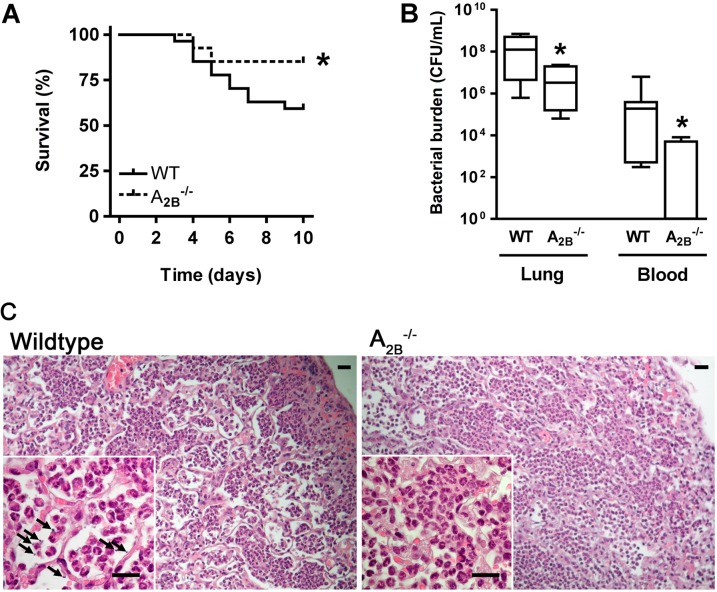
Given the high expression of the A2BR in infected lungs and its previously described antiinflammatory effects, we next compared the outcome of Klebsiella pneumonia in mice lacking the A2BR with wild-type controls. We found that A2BR–/– mice with bacterial pneumonia have a survival advantage over wild-type controls (Figure 2A). Because most deaths from the infection occurred beyond Day 4, we limited subsequent studies to the first 3 days of infection to avoid survival bias. Quantification of bacterial burden on Day 3 of infection revealed lungs of wild-type animals to contain 29-fold more viable bacteria as compared with A2BR–/– animals; the degree of bacteremia in wild-type animals similarly exceeded that of A2BR-deficient animals (Figure 2B). Lung histology revealed similar degrees of consolidation with intraalveolar neutrophils in wild-type and A2BR-deficient animals; bacteria were visible in wild-type but not A2BR-deficient lungs (Figure 2C). Because the A2BR signaling has been linked to reduced extent of lung injury in several animal models, including in response to LPS (14, 29), we also assessed the extent of lung injury in wild-type and A2BR–/– mice with bacterial pneumonia; we found A2BR-deficient mice with pneumonia to have reduced lung injury as compared with wild-type animals, a finding that we attribute to improved bacterial clearance (see Figure E2 in the online supplement).
Figure 2.
Absence of the adenosine A2B receptor is protective in Klebsiella pneumonia. (A) Survival of wild-type and A2B–/– mice after pulmonary infection with K. pneumoniae (n = 29 or 30 mice per group in two independent experiments), *P < 0.05 compared with wild-type mice (log-rank test). (B) Number of bacteria recovered from lungs and blood of Klebsiella-infected wild-type and A2B–/– mice on Day 3 postinfection (n = 6 or 7 mice per group); *P < 0.05 compared with wild-type mice (Mann-Whitney). (C) Representative lung hematoxylin and eosin stains 3 days after onset of infection show consolidation of lung parenchyma; high-power micrographs (insets) show intraalveolar cells to be polymorphonuclear cells (n = 3 mice per group). Arrows point to visible bacteria. All scale bars represent a length of 20 μm. Original magnification: (A–C) ×200; insets, ×1,000. WT = wild type.
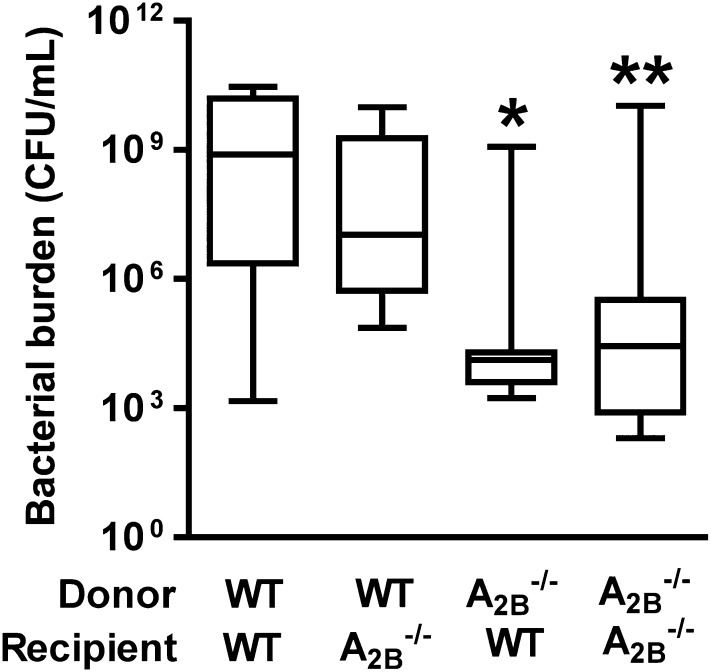
We reasoned that because the A2BR is expressed by both leukocyte and nonleukocyte populations in the infected lungs, the lack of expression on one or both of these populations may explain the protection noted in A2BR-deficient animals. To address this issue, we examined the outcome of infection in reciprocal bone marrow–chimeric animals. Consistent with prior findings, A2BR-deficient recipients of A2BR-deficient bone marrow exhibited significantly lower lung bacterial burden as compared with wild-type recipients of wild-type bone marrow. Furthermore, wild-type recipients of A2BR-deficient bone marrow demonstrated a comparable degree of protection as globally A2BR-deficient animals; conversely, both groups of recipients of wild-type bone marrow exhibited less effective bacterial clearance (Figure 3). These data suggest that deletion of the A2BR on bone marrow–derived cells mediates improved antimicrobial defense against Klebsiella pneumonia.
Figure 3.
Absence of adenosine A2B receptor on bone marrow–derived cells mediates protection against Klebsiella pneumonia. Shown is the number of bacteria recovered from the lungs of bone marrow–transplanted mice on Day 3 postinfection (n = 9–12 mice per group). Lethally irradiated recipient mice (wild-type or A2B–/–) received either wild-type or A2B–/– donor marrow for reconstitution, resulting in the four indicated groups; *P < 0.05 for A2B–/– donor/wild-type recipient versus wild-type donor/wild-type recipient; **P < 0.01 for A2B–/– donor/A2B–/– recipient versus wild-type donor/wild-type recipient (one-way analysis of variance).
Inflammatory Cell Recruitment and Cytokine Levels Do Not Differ between Wild-Type and A2BR–/– Mice
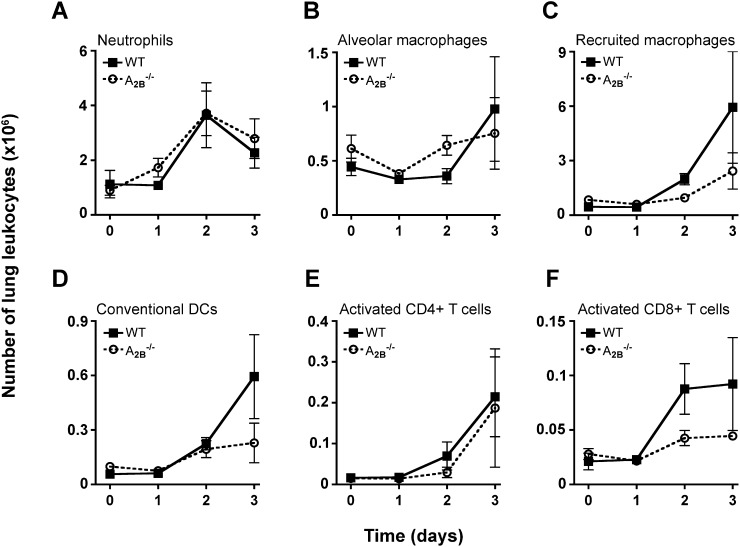
Given that bone marrow–derived cells are important for decreasing bacterial burden in infected A2BR–/– mouse lungs, we reasoned that A2BR–/– mice might exhibit enhanced cellular recruitment to the lung after infection. However, we found no significant difference between wild-type and A2BR–/– mice in the absolute number of neutrophils, alveolar macrophages, recruited macrophages, conventional CD11b+ dendritic cells, activated CD4+ T cells, or activated CD8+ T cells (Figures 4A–4F) recruited to the lung after infection. In addition, there was no significant difference in recruitment of invariant NKT cells or NK cells (see Figures E3A and E3B) to the lung between the two groups over time. Given data that A2BR signaling can inhibit leukocyte activation (17, 30), we examined the activation phenotype of lung neutrophils, macrophages, and dendritic cells and found no significant difference between wild-type and A2BR–/– mice with Klebsiella pneumonia (Figures E3C–E3H). Similarly, although A2BR signaling has been linked to enhanced production of certain inflammatory cytokines (31, 32), we found no difference between levels of these mediators in lungs of wild-type and A2BR-deficient mice with Klebsiella pneumonia (Figure E4). These data suggest that leukocyte recruitment to the lung during early infection is not significantly different between wild-type and A2BR–/– mice.
Figure 4.
Leukocyte recruitment to infected lungs does not differ between wild-type and A2B–/– mice. The absolute number of lung (A) neutrophils, (B) alveolar macrophages, (C) recruited macrophages, (D) conventional dendritic cells, (E) activated CD4+ T cells, and (F) activated CD8+ T cells recruited to wild-type and A2B–/– lungs on the indicated days postinfection, was quantified by flow cytometry. Data shown represent means ± SEM of triplicates, representative of two experiments (each with n = 4–6 mice per group per time point); no significant difference between groups was found (two-way analysis of variance). DCs = dendritic cells; WT = wild type.
A2BR-Deficient Neutrophils Demonstrate Enhanced Extracellular Bactericidal Activity
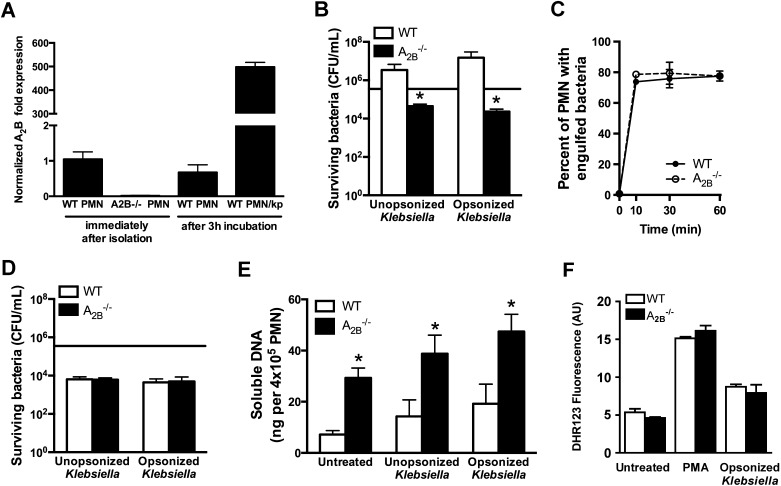
We next assessed whether functional differences between wild-type and A2BR–/– leukocytes recruited to the lung might explain the difference in outcome in response to infection. We focused on neutrophils because of their known role in the clearance of bacteria during pneumonia and because of the high expression level of A2BR mRNA on neutrophils in the setting of pneumonia (Figure 1). Because A2BR gene expression appeared to be much higher in lung neutrophils during pneumonia as compared with resting bone marrow neutrophils (Figure 1), we began by evaluating the expression of A2BR in resting neutrophils after exposure to bacteria in vitro. As compared with freshly isolated cells and neutrophils incubated ex vivo in the absence of microorganisms, neutrophils incubated with bacteria had approximately 500-fold induction of A2BR mRNA (Figure 5A). We next assessed the contribution of A2BR to neutrophil bactericidal activity. Mature neutrophils obtained from the bone marrow of uninfected A2BR-deficient donors were found to have greatly enhanced ability to kill bacteria when compared with wild-type neutrophils, with more than 80-fold greater bactericidal activity against nonopsonized K. pneumoniae and more than 600-fold against serum-opsonized bacteria, a condition that likely mimics in vivo conditions more closely (Figure 5B).
Figure 5.
Ex vivo antibacterial function of wild-type and A2B–/– neutrophils. Ex vivo assays were done to compare wild-type and A2B–/– bone marrow neutrophils. (A) Relative expression of A2BR mRNA in resting bone marrow neutrophils after exposure to bacteria; (B) combined (extracellular and intracellular) bactericidal activity, *P < 0.01 (Mann-Whitney); (C) phagocytic capacity; (D) intracellular bactericidal activity; (E) neutrophil extracellular trap (NET) production, *P < 0.05 (one-way analysis of variance); (F) oxidative burst. Data for all panels represent means ± SEM of triplicates or quadruplicates representative of two to four experiments for each panel. Horizontal line in (B) and (D) represents initial bacterial load per well. PMA = phorbol myristate acetate; PMN = polymorphonuclear neutrophils; Kp = Klebsiella pneumoniae; WT = wild type.
To determine the mechanism of enhanced bacterial killing by A2BR-deficient neutrophils, we next examined the phagocytic capacity and intracellular bactericidal activity of bone marrow–derived neutrophils from wild-type and A2BR–/– mice. We found no difference in phagocytosis of bacteria between wild-type and A2BR-deficient neutrophils (Figure 5C). In addition, the intracellular killing of bacteria did not differ significantly between the two groups (Figure 5D). Taken together with evidence of greatly enhanced killing in Figure 5A, these data suggest that A2BR–/– neutrophils have greater bactericidal activity against extracellular, but not intracellular, bacteria as compared with wild-type neutrophils.
A major mechanism of neutrophil extracellular antimicrobial activity is the production of chromatin-based extracellular traps (NETs) (33). We quantified NET production by wild-type and A2BR–/– neutrophils ex vivo, and found that A2BR–/– neutrophils produce fourfold more NETs at baseline, 2.5-fold more NETs in response to unopsonized Klebsiella, and 2.3-fold more NETs in response to opsonized Klebsiella than wild-type control neutrophils (Figure 5E). Because oxidative burst production is an essential antimicrobial function of neutrophils and can occur in the extracellular space, we also compared neutrophil oxidative burst between wild-type and A2BR–/– neutrophils, and found no difference in ROS production in response to phorbol myristate acetate or bacteria (Figure 5F). These data suggest the enhanced extracellular bacterial killing observed in A2BR-deficient neutrophils may be the result of enhanced NET production.
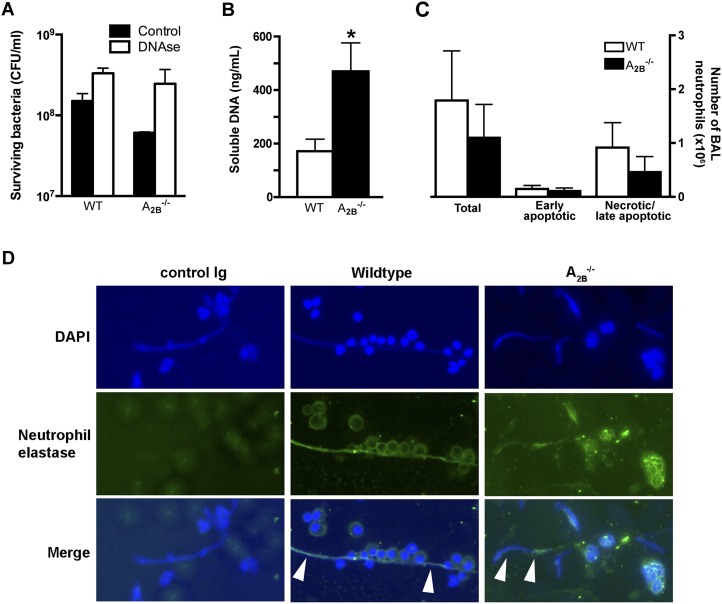
Next, we sought to assess the relevance of enhanced NET production to improved host defenses in A2BR-deficient hosts to bacterial pneumonia. We found that, when resting bone marrow neutrophils from wild-type and A2BR–/– mice were incubated with bacteria ex vivo, degradation of extracellular DNA resulted in impaired bacterial killing; furthermore, the difference in bactericidal activity between wild-type and A2BR-deficient neutrophils was abrogated (Figure 6A). We then assessed whether the ex vivo finding of enhanced NET production in A2BR–/– neutrophils is operational in vivo. To achieve this, we quantified extracellular DNA in the alveolar spaces of mice with bacterial pneumonia, reasoning that neutrophils are the predominant cells in this compartment during infection. We found threefold more extracellular DNA in the bronchoalveolar lavage fluid of A2BR–/– mice with pneumonia as compared with wild-type controls (Figure 6B). This difference was not attributable to differences in the total number of neutrophils in the bronchoalveolar lavage fluid of animals with pneumonia, or to DNA release from dead cells, because the number of apoptotic and necrotic neutrophils did not differ between A2BR–/– and wild-type mice (Figure 6C). Finally, we assessed the bronchoalveolar lavage fluid of wild-type and A2BR–/– mice with pneumonia by immunofluorescence microscopy, and found the extracellular DNA to consist of strands of DNA associated with neutrophil elastase (Figure 6D), the typical appearance of NETs (34). These data suggest that the improved bactericidal clearance in A2BR-deficient mice with bacterial pneumonia is, at least in part, attributable to enhanced NET production and extracellular killing of bacteria by A2BR-deficient neutrophils.
Figure 6.
Neutrophil extracellular trap (NET) production in wild-type and A2B–/– mice. (A) Role of NETs in bacterial killing ex vivo. Resting bone marrow neutrophils from uninfected animals were incubated with bacteria and surviving bacteria were quantified. (B) Extracellular DNA content in bronchoalveolar lavage of wild-type and A2B–/– mice on Day 3 postinfection, *P < 0.05 (Mann-Whitney). (C) Total live and apoptotic count of harvested bronchoalveolar lavage neutrophils. Data represent means ± SEM (n = 9 mice per group). Data shown in (A–C) represent means ± SEM for triplicates or quadruplicates from n = 4–10 mice per group. (D) Immunofluorescence microscopy of bronchoalveolar lavage on Day 3 of infection. Arrowheads show NETs, identified as strands of extracellular DNA that contain neutrophil elastase. Original magnifications: left and right panels, ×200; middle panels, ×100. BAL = bronchoalveolar lavage; DAPI = 4′,6-diamidino-2-phenylindole; WT = wild type.
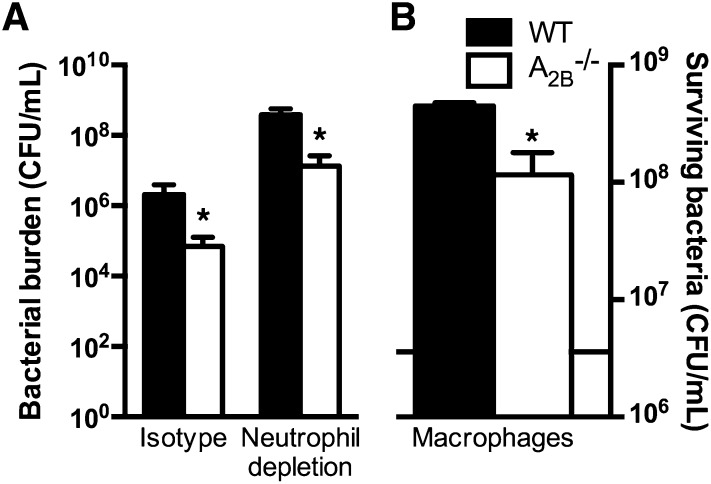
Last, we sought to determine whether enhanced neutrophil bactericidal activity is sufficient to explain the improved outcome of A2BR-deficient hosts as compared with wild-type hosts. To address this point, we rendered wild-type and A2BR–/– animals neutropenic before intratracheal challenge with K. pneumoniae. As expected, both groups of animals had a 2- to 3-log greater lung bacterial content when rendered neutropenic; in addition, we found that, even in the absence of neutrophils, A2BR deficiency resulted in improved bacterial clearance from the lungs (Figure 7A). Similar to findings in previous studies (35), we found that lung macrophages from A2BR-deficient hosts displayed better bacterial killing as compared with wild-type macrophages (Figure 7B). Taken together, our data suggest that the improved host defense of A2BR-deficient hosts is mediated by bone marrow–derived leukocytes that include, but are not limited to, enhanced neutrophil bactericidal mechanisms.
Figure 7.
Contribution of nonneutrophil leukocytes to host defense in wild-type and A2B–/– mice. (A) Number of bacteria recovered from lungs of Klebsiella-infected wild-type and A2B–/– mice after administration of isotype control or neutrophil-depleting monoclonal antibody on Day 3 after infection (n = 8–10 mice per group); *P < 0.05 compared with wild-type mice (Mann-Whitney). (B) Ex vivo bactericidal activity of alveolar macrophages harvested from wild-type and A2B–/– mice against K. pneumoniae; horizontal line in (B) represents initial bacterial load per well. *P < 0.05 compared with wild-type mice (Mann-Whitney).
Discussion
The present work identifies the A2BR as a regulator of host defense in gram-negative bacterial pneumonia. We report that A2BR deficiency improves survival and enhances bacterial clearance in a mouse model of Klebsiella pneumonia and that this effect is mediated by bone marrow–derived cells. The absence of the A2BR on leukocytes did not influence leukocyte recruitment or expression of inflammatory cytokines, but was associated with altered antibacterial effects of myeloid cells, including enhanced NET production and bactericidal activity.
The extracellular concentration of adenosine, normally in the nanomolar range, can increase to the micromolar range during inflammation as a result of accelerated turnover of ATP. Because the affinity of the A2BR for adenosine is 50- to 100-fold lower than the high-affinity states of A1, A2A, and A3 receptors, signaling via the A2B receptor may be selectively activated in the setting of inflammation (8, 9). The role of A2BR in disease pathogenesis is highly complex, being determined by rates of local production and clearance of adenosine, the extent of expression of A2BR by resident and recruited cells, and the repertoire of these cells for the other adenosine receptors, which compete with A2BR for adenosine. A2BR signaling has been reported to be broadly antiinflammatory in several models of acute lung injury; in particular, A2BR deficiency is associated with increased lung inflammation after challenge with bleomycin, LPS, ischemia–reperfusion, and ventilator-associated lung injury (29, 36–38). The reported mechanisms of these effects have varied markedly between these models and have included alteration of leukocyte traffic, reduction in vascular permeability mediated by bone marrow–derived cells, and attenuation of the neutrophil oxidative burst (14, 37, 38); on the other hand, work has shown A2BR expression on bone marrow–derived cells to paradoxically enhance neutrophil migration to the lung interstitium in response to LPS (39). We speculate that the reported differences in the mechanisms of A2BR effects in these models reflect differences in dynamics of production and degradation of adenosine, and differences in the number of adenosine-responsive cells as well as their adenosine receptor repertoire in the context of different injuries. Because bacterial pneumonia is an important cause of acute lung injury in the clinical setting, the present study expands current knowledge by providing evidence of a regulatory role for A2BR signaling that impairs the host responses to pneumonia. In particular, our observations and the prior reports in the literature are consistent with the paradigm that some of the purinogenic mechanisms that are beneficial in noninfectious lung injury inhibit host inflammation in response to tissue injury and are harmful in the context of active infection.
Whereas the role of adenosine in dampening inflammation has been an area of active research, the relevance of adenosine signaling to host defense against infection has received less attention. Studies of adenosine receptor signaling in animal models of intraperitoneal sepsis have reported contradictory findings: A2BR deletion was found to increase expression of inflammatory cytokines and worsened survival (40); conversely, A2BR blockade, global deletion, or selective deletion from myeloid cells was associated with accelerated bacterial clearance and improved outcome (35). Because unchecked inflammation and failure to control infection can both lead to death, minor differences in experimental protocols might influence the outcome of such experiments. These differences may relate to the size of the infectious inoculum that determines the relative contribution of bacterial growth on the one hand, and the systemic acute phase response on the other, to mortality in sepsis models. To our knowledge, ours is the first study of the role of adenosine signaling in pneumonia.
Several lines of evidence support a biologically important effect of adenosine signaling on neutrophil functions, as reviewed (18). One study reported inhibition of neutrophil oxidative burst with an A2BR agonist (41). We did not observe an increase in oxidative burst in A2BR-deficient neutrophils; our results may represent cross-talk between neutrophil adenosine receptors, with maintenance of A2AR signaling in A2BR–/– neutrophils, because A2AR activation strongly inhibits oxidative burst (9, 18). Our study also relates to work demonstrating that A2BR-deficient macrophages exhibit enhanced phagocytosis and bacterial clearance in a model of sepsis (35). This study concluded that A2BR–/– neutrophils did not demonstrate enhanced antibacterial activity, but only phagocytic capacity as a measure of bactericidal activity. Our data are in accordance with these findings; we found that A2BR-deficient neutrophils exhibit no significant difference in phagocytic capacity, but demonstrate enhanced extracellular killing of bacteria that is not affected by phagocytosis.
NETs are composed of chromatin and antimicrobial cytoplasmic and granular proteins such as elastase and catalase. Exuded NETs form a fibrillar matrix that entrap invading pathogens and bring them into the proximity of antimicrobial proteins, thus facilitating killing (34). Our data suggest a mechanism by which the production of NETs can be modulated by adenosine. The mechanism of NET production is not yet fully defined; it has been suggested that neutrophil populations are heterogeneous in terms of their ability to produce NETs (42). It is possible that A2BR deficiency induces the development of a larger fraction of neutrophils that are able to undergo NETosis; alternatively, adenosine may modulate the amount of NET exuded per neutrophil.
Our work has several implications for future research. First, the signaling mechanisms that lead to NET production are only beginning to be defined; the signaling that links A2BR activation to modulation of NET formation is therefore of interest. Second, a number of gram-negative bacilli including Klebsiella possess virulence factors that inhibit neutrophil phagocytosis (43, 44); augmenting extracellular killing by enhancing NETosis via modulation of the A2BR has the potential to overcome these phagocytosis-resistant variants. Finally, a number of A2BR agonists and antagonists are under development as pharmaceutical agents; the present work lays the groundwork for use of these agents as a novel therapeutic option in pneumonia caused by multidrug-resistant gram-negative bacteria.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Virginia Flow Cytometry Core for assistance with cell sorting, and the NIH Tetramer Facility.
Footnotes
Supported by NIH grants HL073848 and HL098329.
Author Contributions: K.E.B. designed and performed experiments, analyzed data, and wrote the first draft of the manuscript; R.E.C. and M.D.B. assisted with experimental design, data acquisition, and analysis; J.L. assisted with experimental conception and hypothesis delineation; B.M. conceived the project and oversaw the experimental design, data acquisition and analysis, and writing of the manuscript.
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201204-0622OC on September 20, 2012
Author disclosures are available with the text of this article at www.atsjournals.org
References
- 1.Miniño A, Murphy S, Xu J, Kochanek K. Deaths: final data for 2008 [National Vital Statistics report]. Hyattsville, MD: National Center for Health Statistics; 2011 [PubMed] [Google Scholar]
- 2.Murphy S, Xu J, Kochanek K. Deaths: preliminary data for 2010. Hyattsville, MD: National Center for Health Statistics; 2012 [Google Scholar]
- 3.Bartlett JG, O’Keefe P, Tally FP, Louie TJ, Gorbach SL. Bacteriology of hospital-acquired pneumonia. Arch Intern Med 1986;146:868–871 [PubMed] [Google Scholar]
- 4.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416 [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1–12 [DOI] [PubMed] [Google Scholar]
- 6.Woodford N, Turton JF, Livermore DM. Multiresistant gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 2011;35:736–755 [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 2009;9:228–236 [DOI] [PubMed] [Google Scholar]
- 8.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest 2006;116:1835–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 2011;11:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 2011;60:669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil–epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 2001;107:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 2006;116:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 2004;104:3986–3992 [DOI] [PubMed] [Google Scholar]
- 14.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008;111:2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, Celada A. IFN-γ up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol 1999;162:3607–3614 [PubMed] [Google Scholar]
- 16.Xaus J, Valledor AF, Cardo M, Marques L, Beleta J, Palacios JM, Celada A. Adenosine inhibits macrophage colony-stimulating factor–dependent proliferation of macrophages through the induction of p27kip-1 expression. J Immunol 1999;163:4140–4149 [PubMed] [Google Scholar]
- 17.Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, Linden J, Ernst PB. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol 2009;182:4616–4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol 2012;32:856–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrad B, Park SJ, Akangire G, Standiford TJ, Wu T, Zhu J, Mohan C. The lupus-susceptibility locus, Sle3, mediates enhanced resistance to bacterial infections. J Immunol 2006;176:3233–3239 [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, Mehrad B. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 2010;185:6190–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med 2007;175:1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SJ, Wiekowski MT, Lira SA, Mehrad B. Neutrophils regulate airway responses in a model of fungal allergic airways disease. J Immunol 2006;176:2538–2545 [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell–derived IFN-γ is essential to host defense in neutropenic invasive aspergillosis. J Immunol 2009;182:4306–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cagnina RE, Ramos SI, Marshall MA, Wang G, Frazier CR, Linden J. Adenosine A2B receptors are highly expressed on murine type II alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L467–L474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods 2012;375:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol 2012;844:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A2b adenosine receptors in immunity and inflammation. Trends Immunol 2009;30:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 2010;184:5271–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 2012;26:376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor–mediated posttranscriptional mechanism. J Immunol 2005;175:8260–8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JM, Kurtz CC, Black SG, Ross WG, Alam MS, Linden J, Ernst PB. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J Immunol 2011;186:6746–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 34.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009;30:513–521 [DOI] [PubMed] [Google Scholar]
- 35.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol 2011;186:2444–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol 2011;186:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, Laubach VE. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg 2010;140:871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 2008;118:3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konrad FM, Witte E, Vollmer I, Stark S, Reutershan J. Adenosine receptor A2B on hematopoietic cells mediates LPS-induced migration of PMNs into the lung interstitium. Am J Physiol Lung Cell Mol Physiol 2012;303:L425–L438 [DOI] [PubMed] [Google Scholar]
- 40.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol 2010;185:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Hoeven D, Wan TC, Gizewski ET, Kreckler LM, Maas JE, Van Orman J, Ravid K, Auchampach JA. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J Pharmacol Exp Ther 2011;338:1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 2011;18:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998;11:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahly H, Aucken H, Benedi VJ, Forestier C, Fussing V, Hansen DS, Ofek I, Podschun R, Sirot D, Sandvang D, et al. Impairment of respiratory burst in polymorphonuclear leukocytes by extended-spectrum β-lactamase–producing strains of Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis 2004;23:20–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.