Mycobacterium abscessus is a challenging pathogen causing chronic respiratory infections in patients with underlying inflammatory lung diseases (such as cystic fibrosis, non–cystic fibrosis bronchiectasis, and chronic obstructive pulmonary disease) as well as in individuals with poorly defined susceptibility factors (1). This rapid growing nontuberculous mycobacterium (NTM) is in fact a complex of three subspecies—M. abscessus, M. massiliense, and M. bolletii—that are not currently distinguished by hospital laboratories but may have different clinical behaviors. For unclear reasons, infections with M. abscessus complex (MABSC) have become more common recently. Studies from Taiwan, the United States, and Australia have all reported significant increases in the prevalence of MABSC pulmonary infection over the past decade (2–4), which is of particular concern because this organism is resistant to many antimicrobial agents and responds poorly to treatment. For example, one of the larger studies of pulmonary MABSC infection examined 69 patients treated at National Jewish Health between 2001 and 2004 (5). Patients received intensive therapy that included an average of 6 months of intravenous antibiotics as well as oral antibiotics, and 24 (35%) also had surgical resection of affected lung tissue. Despite this intensive treatment, only 33 (48%) had sustained culture conversion to negative for at least 1 year after antibiotics were discontinued.
The second-generation macrolides clarithromycin and azithromycin are key components of MABSC treatment. The current American Thoracic Society/Infectious Diseases Society of America guidelines for treatment of NTM recommend use of one of these agents as part of a multidrug regimen, with no stated preference for one macrolide or the other (6). In the absence of head-to-head clinical trials, the choice of macrolide is driven by clinician preference and the potential for drug interactions. The report by Choi and colleagues (7) in this issue of the Journal (pp. 917–925) provides some interesting insights into why MABSC pulmonary infection responds suboptimally to antibiotic therapy, how the two subspecies M. abscessus and M. massiliense behave differently in vitro and in vivo, and how clarithromycin and azithromycin may differentially influence the development of macrolide resistance.
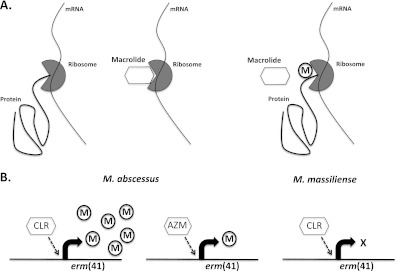
Macrolides function as antibiotics by binding to the 23S ribosomal RNA to block bacterial protein synthesis (Figure 1). Many bacteria can sense macrolides, usually through direct or indirect detection of ribosomal stalling, and express “erm” methyl transferases (erythromycin resistance methylase) that modify the ribosomal binding site for macrolides causing antibiotic resistance. In the case of M. abscessus, erythromycin resistance methylase is expressed by a novel gene, named “erm(41),” in response to low-level exposure to erythromycin or clarithromycin and mediates high-level macrolide resistance (8).
Figure 1.
Mechanism of macrolide action and inducible macrolide resistance. (A) Macrolide antibiotics bind to the 23S ribosomal RNA, preventing bacterial protein synthesis. Expression of erm (erythromycin resistance methylase) proteins in response to macrolides leads to modification of their ribosomal binding site and induction of macrolide resistance. (B) Induction of erm(41) and subsequent macrolide resistance is greater after exposure to clarithromycin (CLR) than to azithromycin (AZM) in Mycobacterium abscessus subspecies. However, neither macrolide can induce resistance in M. massiliense, because it carries a defective erm(41) gene.
Choi and colleagues examined the role of the erm(41) gene in macrolide resistance and the differential effects of clarithromycin and azithromycin in induction of erm(41)-mediated resistance using a number of complementary approaches. First, macrolide resistance was assessed, using broth microdilution, at baseline and over 14-day incubation with either clarithromycin or azithromycin for 23 M. abscessus and 24 M. massiliense clinical isolates. Inducible macrolide resistance was observed in all M. abscessus isolates and was significantly greater after exposure to clarithromycin than to azithromycin. In contrast, none of the isolates of M. massiliense (which has a nonfunctional erm(41) gene) demonstrated any inducible resistance to either antibiotic. The authors then examined erm(41) mRNA induction in response to incubation with macrolides. As expected, clarithromycin induced far higher erm(41) mRNA levels in M. abscessus than did azithromycin. Knocking out the erm(41) gene in M. abscessus eliminated the inducible macrolide resistance, whereas adding a functional erm(41) gene to M. massiliense bestowed inducible resistance to that subspecies. The authors then tested azithromycin and clarithromycin in a murine bone marrow–derived macrophage system, where azithromycin reduced M. abscessus colony-forming units significantly more than clarithromycin, but the two drugs were similarly effective for M. massiliense. Finally, the authors tested the two drugs in a murine lung infection model. Although both macrolides reduced the burden of M. abscessus organisms in the mouse lungs, azithromycin reduced the colony counts significantly more than clarithromycin. Conversely, both macrolides were equally effective when mice were infected with M. massiliense.
Although there is uncertainty about how relative increases in erm(41) mRNA induction by the two macrolides and subsequent resistance profiles detected in vitro translate to clinical outcomes and the fidelity of mouse infection model in studying human NTM disease, the results presented by Choi and coworkers arrive at the same conclusions using a number of complementary approaches: (1) inducible macrolide resistance mediated by erm(41) is important in modulating the effectiveness of macrolide treatment for M. abscessus; and (2) clarithromycin induces erm(41) to a significantly greater extent than azithromycin. The one available human study comparing treatment outcomes of M. abscessus with M. massiliense lung disease provides support to the authors’ conclusions. In that study, patients with M. abscessus infection had significantly lower rates of sputum culture conversion in response to clarithromycin-based therapy than patients with M. massiliense despite similar baseline characteristics (9). Why azithromycin should induce erm(41) to a lesser extent than clarithromycin is unclear but may relate to antibiotic-specific (and possibly multiple) ribosomal binding sites (10) or differential activation of stress pathways, similar to whiB7 in M. tuberculosis (11), which may regulate erm(41) transcription in M. abscessus.
Although sorely needed, no randomized clinical trials of treatment for M. abscessus lung infection are, to our knowledge, on the immediate horizon. Pending such studies, the work of Choi and colleagues suggests that azithromycin should be the macrolide of choice in treatment of M. abscessus pulmonary disease and that accurate subspeciation of MABSC may have important clinical implications for the management of this difficult infection.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly growing mycobacteria, an emerging cause of chromic lung disease. Am J Respir Cell Mol Biol 2010;43:387–393 [DOI] [PubMed] [Google Scholar]
- 2.Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis 2010;16:1576–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis 2010;16:294–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010;182:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011;52:565–571 [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416 [DOI] [PubMed] [Google Scholar]
- 7.Choi G-E, Shin SJ, Won C-J, Min K-N, Oh T, Hahn M-Y, Lee K, Lee SH, Daley CL, Kim S, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med 2012;186:917–925 [DOI] [PubMed] [Google Scholar]
- 8.Nash KA, Brown-Elliott BA, Wallace RJ., Jr A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 2009;53:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405–410 [DOI] [PubMed] [Google Scholar]
- 10.Kannan K, Mankin AS. Macrolide antibiotics in the ribosome exit tunnel: species-specific binding and action. Ann N Y Acad Sci 2012;1241:33–47 [DOI] [PubMed] [Google Scholar]
- 11.Morris RP, Nguyen J, Gatfield K, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2005;102:12200–12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.