Abstract
Acute lower respiratory tract infection is responsible for an inordinate disease burden. Pulmonary immunity determines the outcomes of these infections. The innate and adaptive immune responses to microbes in the lung are critical to maintaining a healthy respiratory system and preventing pulmonary disease. In addition to balancing antimicrobial defense against the risk of lung injury during the immediate infection, the shaping of pulmonary immunity by respiratory infection contributes to the pathophysiology of many and even perhaps most chronic pulmonary diseases. This Pulmonary Perspective aims to communicate two interconnected points. First, tremendous morbidity and mortality result from inadequate, misguided, or excessive pulmonary immunity. Second, our understanding of pulmonary immunity is at an exciting stage of rapid developments and discoveries, but many questions remain. Further advances in pulmonary immunity and elucidation of the cellular and molecular responses to microbes in the lung are needed to develop novel approaches to predicting, preventing, and curing respiratory disease.
Keywords: respiratory tract infections, pneumonia, acute lung injury, innate immunity, adaptive immunity
Each day, a typical human adult inhales all the contaminants of approximately 11,000 liters of air, the equivalent of two elevator cars full, into the lungs. In addition, microbes and other materials from the upper airways are frequently aspirated into the lungs. Pulmonary immunity has evolved to respond to these challenges, in most cases protecting the lung from infection and maintaining respiratory function. However, when responses designed to protect the lungs during infection go awry, pulmonary disease develops.
Acute Lung Infections
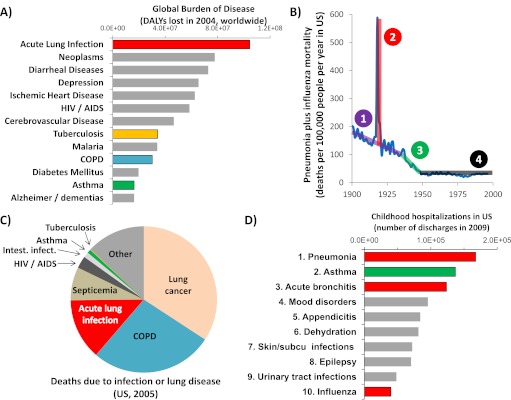
The World Health Organization uses the metric of disability-adjusted life-years to assess burden of disease, quantifying life-years lost to mortality or compromised by morbidity. Since first analyzed for 1990 (1), the greatest disease burden worldwide has consistently been reported as acute lower respiratory infection (Figure 1A, from World Health Organization data [2]). Impressive as they are, these figures underestimate the full impact of such infections, because intersections with comorbidities (such as HIV/AIDS, chronic obstructive pulmonary disease [COPD], or asthma) tend to be attributed to the underlying disease rather than lung infection.
Figure 1.
Disease caused by acute lower respiratory infection. (A) Total worldwide burden of select diseases, as assessed by disability-adjusted life years (DALYs) lost reported by the World Health Organization (WHO) (2). Diseases directly related to pulmonary immunity are highlighted with colored bars. Figures represent Standard DALYs for 2004. (B) Trends in U.S. mortality due to pneumonia and influenza through the 20th century. (Data provided by Dr. Gregory L. Armstrong, U.S. Centers for Disease Control and Prevention, Atlanta, GA, as previously reported [3]). Four distinct trends are differentiated. (C) The relative numbers of deaths in the United States attributed to any infection or lung disease, based on data from the National Vital Statistics System (7). International Classification of Diseases codes included were as follows: Lung cancer C33–C34, COPD J40–J44; Acute lung infection J10–J18; Septicemia A40–A41; HIV/AIDS B20–B24; Intestinal infections A00–A09; Asthma J45–J46; and Tuberculosis A16–A19. The category “Other” includes all deaths attributed to “Diseases of the respiratory system (J00–J98)” or “Certain infectious or parasitic diseases (A00–B99)” other than the above. (D) The top 10 most common reasons U.S. children are hospitalized, based on data from the Healthcare Cost and Utilization Project (9). Colored bars represent hospitalizations resulting from alterations in pulmonary immunity, with red reflecting acute lower respiratory infection directly. Acute Lung Infection = WHO categories Lower Respiratory Infections plus Pertussis; Alzheimer/dementias = Alzheimer and other dementias, and other diseases as identified by the WHO; COPD = chronic obstructive pulmonary disease; Depression = unipolar depressive disorders; Neoplasms = malignant neoplasms; subcu = subcutaneous.
The U.S. mortality rate due to pneumonia and influenza (3) declined through the first half of the 20th century in conjunction with sanitation, pollution, nutrition, hygiene, and education improvements that anteceded medical strategies specific to lung infection (Figure 1B, purple line). Healthier hosts better resist and overcome infection, which may be the most significant reason acute lower respiratory tract infections exert disproportionate tolls in the poorest communities (4). The trend of improvement was dramatically broken in 1918, when an influenza pandemic suddenly quadrupled the death rate (Figure 1B, red line). The general course of improvement was interrupted again in the middle of the century, in this case beneficently (Figure 1B, green line). During this time, basic research on antibiotics was effectively translated into therapies that became widely applied, and the mortality rate from pneumonia and influenza plummeted, a stirring testament to the potential for biomedical discoveries to transform the public’s health. In contrast to the rapid progress in the middle of the last century, the latter half of the century might by comparison be considered bleak. The remaining mortality rate is substantial, as detailed in the next paragraph, but there has been little or no improvement for decades (Figure 1B, black line). Some evidence suggested that pneumonia mortality was recently decreasing (5), but this apparent trend was attributed to artifactual changes in diagnostic coding rather than improvements in pneumonia outcome (6).
Acute lung infections remain a substantial concern, even in wealthy countries. Of infectious diseases, acute lower respiratory infections cause the most deaths (Figure 1C) and are the largest burden of disease (4, 7) in the United States. Among pulmonary diseases, acute lung infection is the third greatest killer (Figure 1C). Subpopulations are especially prone to pneumonia, such as the elderly and those with comorbidities (8). For those older than 65 years, pneumonia hospitalizations are increasing, and hospitalization for pneumonia carries a significantly increased risk of mortality compared with other hospitalizations (8). At the other end of the age spectrum, pneumonia is the most common reason that U.S. children become hospitalized ([9] and Figure 1D). Acute bronchitis and influenza, also acute lung infections, are third and tenth, respectively (Figure 1D). These findings are not particular to the United States, as pneumonia exerts a similarly heavy burden in Europe (10). Thus, acute lung infections are a terrible problem even in the wealthiest societies, and improved abilities to prevent or treat respiratory infection would have a profound impact. Unfortunately, in comparison with other diseases, pneumonia is dramatically understudied and receives disproportionately little attention from biomedical researchers and funding bodies (4, 11). Increased attention will improve pneumonia outcomes, as has occurred with HIV/AIDS (12), myocardial infarctions (13), and other diseases. The substantial public health consequences combined with the failure to improve pneumonia mortality over so many recent decades should be a clarion call for more and better research.
Host Response to Microbes in the Lung
The data in Figure 1 argue that novel approaches are needed to prevent or cure acute lung infection. Novel approaches will require new insights into how the deposition of microbes in the lung results in the successful eradication of the microbe and/or diminished lung function. Normally, the respiratory tract is impressively capable of fighting infection without digesting lung tissue or compromising pulmonary physiology. The inevitable exposures plus the potential for severe outcomes have put enormous selective pressures on pulmonary adaptation to infection. It is astounding that this works as well as it does most of the time, with the vast majority of all exposures causing no detectable infection and with most acute infections resulting in no long-term sequelae. However, in some cases, inadequate host defenses allow microbes to thrive or inappropriate responses compromise lung function (14).
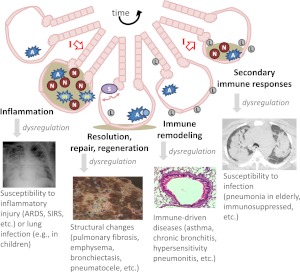
Responses to microbes in the lung are coordinated by pulmonary immunity (14). After exposure of the lungs to a microbe, immune responses begin within seconds and then continue to transition over hours, days, and weeks. Temporally distinct stages of pulmonary immunity involve different types of cells and molecules, as discussed below (Figure 2). Each stage needs to be better elucidated to understand how the lungs respond to microbes, how dysregulation of pulmonary immunity results in respiratory disease, and how cells and molecules can be rationally manipulated to prevent or cure respiratory disease.
Figure 2.
Respiratory infections and dynamic pulmonary immunity. The schematic depicts a stylized lung unit with airway and alveolar air spaces surrounded by epithelium, changing over time due to infection (red I with open arrow). The following cells are highlighted: alveolar macrophages (A), neutrophils (N), inflammatory monocyte/macrophages (m), stem cells (S), and lymphocytes (L). Different stages of the responses to infection are identified, as well as consequences of dysregulated processes in these steps.
Inflammation and Innate Immunity During Lung Infection
Small numbers of less virulent microbes can be eliminated by resident defenses in the respiratory tract, such as the mucociliary escalator and alveolar macrophages. However, virulent or numerous microbes require inflammation, which is recruited innate immunity (Figure 2). Plasma extravasation benefits host defense by enriching the area with complement, circulating antibodies, and acute phase proteins, but this accumulation of liquid is pulmonary edema and a defining feature of acute lung injury. The influx of phagocytes, including neutrophils and inflammatory monocyte/macrophages, helps destroy microbes, but many of their antimicrobial armaments can also exacerbate tissue damage and contribute to organ failure. Balance is key, and disruption of this balance leads to acute lung infections and/or acute lung injury.
The transcription factor nuclear factor (NF)-κB operates as a molecular fulcrum balancing the initial innate immune response to microbes in the lungs (14, 15). Animal experiments demonstrate that interruptions in NF-κB signaling, including mutations of NF-κB RelA (16), intracellular signaling pathways that stimulate NF-κB (17), or receptor complexes activating these pathways (18), can compromise host defenses and predispose to lung infection. Conversely, animal models in which NF-κB activity is exaggerated reveal that this transcriptional activity is sufficient to induce or exacerbate lung injury (14, 15). Similar observations have been collected from human patients. For example, children with MyD88 or IRAK-4 deficiency have defects in NF-κB activation and extreme susceptibility to severe pyogenic bacterial infections, with more than one-third of these patients dying of infection in early childhood (19). In complementary fashion, a Tlr1 polymorphism that amplifies NF-κB signaling associates with increased organ dysfunction, acute lung injury, and death among patients with sepsis (20), confirming that limiting these innate immune signaling pathways is vital. Appropriate innate immune signaling is critical to effective and safe defense against respiratory pathogens.
In which cells is NF-κB (or other innate immune signaling pathways) especially important, and through which receptor and effector molecule pathways? Bone marrow chimera studies in mice demonstrate that MyD88 signaling is necessary for optimal host defense during pneumonia both in hematopoietic cells and in nonhematopoietic cells (21). Among hematopoietic cells, the first responders are the alveolar macrophages. When pneumococcus enters the lung, alveolar macrophages mediate the initial recognition and elaboration of cytokine signals that coordinate the earliest steps of host defense (22). The subsequent recruitment of neutrophils is needed for effective immunity against diverse microbes in the lungs. Neutrophils are antimicrobial effector cells, using a combination of phagocytosis and neutrophil extracellular trap formation. In addition, they have affector roles and are capable of generating a variety of soluble mediators, including cytokines normally ascribed to CD4+ T cells during adaptive immune responses (23, 24). The significance of neutrophil-derived cytokines, or whether and when neutrophils are essential sources of which cytokines during pneumonia, has yet to be well-established. As with neutrophils, the recruitment of inflammatory monocyte/macrophages increases the phagocytic host defense capacity in the lung and also provides new sources for immunomodulating signals, with other innate leukocytes, including natural killer cells, natural killer T cells, and γδ-T cells, further modulating responses.
The leading candidates for nonhematopoietic cells with innate immune functions against respiratory infection are epithelial cells, supported by studies in murine models in which epithelial cells have interrupted NF-κB signaling (16, 25, 26). Whether and which innate immune responses are dependent on whether and which subtypes of epithelial cells remain important unanswered questions. Roles of innate immunity signaling in nonepithelial lung cells, such as endothelial cells, fibroblasts, or smooth muscle cells, are even less well understood during pneumonia. It seems highly likely that distinct lung cells will have unique and specialized roles in innate immunity, yet to be defined. Finally, cells outside of the infected lung have innate immunity functions, such as hepatocytes, which modulate plasma proteins during pneumonia to prevent the dissemination of microbes from the infected lung (27). The influence of extrapulmonary innate immunity on local responses within the lung remains to be elucidated.
Repair, Resolution, and Regeneration After Lung Injury
The changes that occur during severe pneumonia are extreme, but lungs usually withstand this inflammatory onslaught and recover to become histologically normal (Figure 2). This resolution is a remarkable success story but remains less well understood than the pathways driving inflammation and innate immunity. Defects in these pathways likely exacerbate acute lung injury and may lead to structural abnormalities in the lung like pulmonary fibrosis, emphysema, bronchiectasis, and pneumatoceles.
Resolution of acute inflammation requires clearance of the pus, both liquid and cells. Clearance of the inflammatory cells via efferocytosis removes potentially dangerous cells containing degradative and reactive mediators and also brakes the positive feedback loop characteristic of inflammation. After acute inflammation driven by bacterial products or influenza virus, alveolar macrophages perform most of the efferocytosis in the lung, and the alveolar macrophages that were present originally persist after inflammation wanes, rather than being replaced by recruited myeloid cells (28). Macrophages recognizing apoptotic cells decrease their elaboration of proinflammatory cytokines while increasing expression of antiinflammatory mediators such as transforming growth factor-β and IL-10 (29), actions that together help calm the inflammatory seas. Lipid mediators known as lipoxins, protectins, and resolvins are produced by macrophages recognizing apoptotic cells, and they in turn enhance macrophage efferocytosis in autocrine and paracrine fashion (30). Gain-of-function studies in various systems including pneumonia reveal these lipids to be capable of dampening inflammation and accelerating recovery without compromising host defense (31), but roles for the endogenous lipids are less clear because specific loss-of-function approaches are yet to be accomplished. Soothing signals also originate from recruited cells. T regulatory cells (Tregs) accumulate in the lungs during resolution stages after LPS-induced lung injury, and these cells are necessary and sufficient for the rapid elimination of inflammatory cells and pulmonary edema, likely via transforming growth factor-β elaboration (32). Other soothing signals from lymphocytes include IL-10 from CD8+ T cells (33) and amphiregulin from innate lymphoid cells (34), both of which contribute to limiting injury and enhancing recovery after influenza infection.
In addition to pathways involving specialized immune cells, structural cells of the lung also contribute importantly to repair and regeneration after injury. Inflammatory lung injury can cause the death and replacement of structural cells, and molecular and cellular pathways involved in protecting from cytotoxicity and enhancing the proliferation, migration, and differentiation of replacement cells are emerging as exciting areas related to pulmonary immunity and infection. Just as NF-κB is a signaling hub central to innate immunity, STAT3 may be a central mediator of cytoprotection (15). STAT3 induces gene expression programs that prevent cell death, promote cell migration, and enhance cell proliferation, all of which may contribute to lung protection. The mutation of STAT3 in epithelial cells of the lung increases pulmonary edema and epithelial cell apoptosis after lung infection (35, 36). Defective cytoprotection downstream of epithelial STAT3 may be responsible in part for the bronchiectasis and pneumatoceles that arise subsequent to lung infections in patients with hyper-IgE syndrome with mutations in STAT3 (37). STAT3 can be activated by a variety of signals, and during bacterial pneumonia IL-6 and especially leukemia inhibitory factor (LIF) are key upstream determinants (36, 38). The blockade of LIF during pneumonia dramatically increases lung injury in a mouse model of pneumonia, associated with transcriptome-wide changes characteristic of cell death and disruption of epithelial homeostasis (39). LIF is perhaps best known as a component of stem cell cultures, and it is also possible that its role in protecting the lung involves stem cell biology. Stem or progenitor cells in the lung will almost certainly be important to repairing the injured lung. Roles of stem cells in lung repair and regeneration are the foci of many research programs, some of which incorporate infectious challenges, and suggestive, exciting, and controversial discoveries have been recently reported (40). Pathways involved in resisting injury as well as in repair and regeneration after injury need to be further elucidated to design rational approaches for preventing or treating lung injury due to lung infection.
Immune Remodeling and Secondary Responses in the Lung
Although histologically resembling preinfection, pulmonary immunity is altered to a “new normal” or different baseline state after overcoming infection and resolving acute inflammatory processes (Figure 2). This renders the individual more resistant to the same microbe as well as microbes related to the initial infection, as elegantly demonstrated in experimental studies of mice in which a prior respiratory tract exposure to Klebsiella pneumoniae results in improved clearance of diverse K. pneumoniae serotypes during subsequent lung challenges (41). The initial exposures seed the lungs with effector memory Th17 cells that recognize widely varying K. pneumoniae serotypes but not unrelated bacteria like pneumococcus or Staphylococcus aureus. The improved host defenses require IL-17 signaling and neutrophils but not antibody, demonstrating Th17 cells to be critical players in protective heterotypic pulmonary immunity. Adult humans without ongoing infections have tremendous numbers of T cells residing in their lungs, equivalent to the total numbers in the blood (42). T cells in the lungs, nasal tissues, and blood of adults without ongoing infections respond to respiratory pathogens, such as pneumococcus and influenza, with greater responsiveness by T cells from the relevant tissues, suggesting a mucosal compartmentalization of immunity (42–45). Respiratory pathogen-reactive T cells often generate IL-17 on stimulation (42, 45), consistent with an ability to amplify neutrophil-mediated host defense in the lungs. In addition to these host defense attributes, human pneumococcus-specific T cells can have Treg activities (42, 45), capable of limiting lung injury as described above. In humans, preexisting CD4+ T cells recognizing conserved influenza antigens decrease severity of experimental infections with influenza viruses (46), supporting the notion that heterotypic immunity is an important aspect of healthy young adult responses to respiratory tract infection. Further bolstering this idea, the extraordinary susceptibility of MyD88-deficient or IRAK-4–deficient children to pneumococcus and other pyogenic bacterial infections wanes if they are fortunate enough to reach adolescence (19). The immune-remodeled lungs and heterotypic immunity that are characteristic of healthy human adults are rarely integrated into experimental investigations of respiratory disease. It will be important for future studies to determine how heterotypic immunity develops in the lung, influences pulmonary responses to microbial and other exposures, and is altered by aging and other stresses.
Intersections Among Respiratory Infection, Pulmonary Immunity, and Other Lung Diseases
Beyond the acute lung infections highlighted by red bars and trend lines in Figure 1, pulmonary immune responses designed to combat lung infection are also central components of many (or even perhaps most) other pulmonary diseases as well, as schematized in Figure 2. Furthermore, acute lower respiratory infections themselves contribute directly to the pathophysiology of chronic pulmonary diseases. Thus, research into immune responses to acute infection will have “off-target” benefits, which potentially open up new directions relevant to diverse respiratory diseases (Figure 2). For example, COPD involves extensive immunological alterations in the lung, with airways containing mixed myeloid and lymphocyte aggregates contributing to the chronic bronchitis (47). The majority of COPD exacerbations that require medical attention result from respiratory infections (48). The emphysematous loss of alveoli in COPD results from imbalances both in immuno-inflammatory pathways and in repair and regeneration pathways (49). As another example, asthma results directly from dysregulated immune responses in the lung that yield aberrant inflammatory and bronchoconstrictor responses to otherwise inconsequential challenges (50). Diverse microbial exposures influence the immunological skewing that drives asthma (50, 51). Respiratory infections make asthma worse (52), and asthma makes respiratory infection worse (53), creating a vicious cycle. Pulmonary immune responses and respiratory infections are involved in other pulmonary diseases as well, including tuberculosis, sarcoidosis, pulmonary alveolar proteinosis, cystic fibrosis, pulmonary fibrosis, pneumoconiosis, hypersensitivity pneumonitis, pulmonary arterial hypertension, and lung cancer. Pulmonary immunity, which is designed for defense against microbes in the lung, is a truly central determinant of human health. Greater insight into pulmonary immunity function and dysfunction will identify new opportunities for combating many lung diseases.
Conclusions
An astonishing burden of disease results from dysregulation of pulmonary immunity, most or all of which evolved to appropriately respond to respiratory pathogen exposures. Although our concepts of pulmonary immunity to infection are advancing rapidly, there remain far more questions than answers concerning which cells and molecules are involved, how these responses are regulated, and which steps may be meaningfully measured or manipulated to the benefit of patients or those at risk. There is a relative paucity of effort and funding focused on the biology of acute lower respiratory infection (4, 11), making this an important area for professional development and research prioritization. There has never been such a promising and exciting time to study lung infections and pulmonary immunity. Advances will guide innovative medical approaches to acute lung infection and chronic respiratory diseases.
Supplementary Material
Acknowledgments
The author thanks Drs. Lee J. Quinton and Matthew R. Jones for critical reading of the manuscript and regular discussion of these topics, Dr. Gregory L. Armstrong for sharing the data used to make Figure 1B, and Drs. R. Sharon Chinthrajah, Lester Kobzik, William W. Cruikshank, and Praveen Govender for providing disease-related images used in Figure 2.
Footnotes
Supported by National Institutes of Health grants R01-HL068153 and R01-HL079392.
Originally Published in Press as DOI: 10.1164/rccm.201206-1063PP on July 12, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murray CJ, Lopez AD. Evidence-based health policy–lessons from the global burden of disease study. Science 1996;274:740–743 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Health statistics and health information systems. 2012. [Accessed June 8, 2012]. Available from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional/en/index.html
- 3.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA 1999;281:61–66 [DOI] [PubMed] [Google Scholar]
- 4.Mizgerd JP. Lung infection—a public health priority. PLoS Med 2006;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011;124:171–178.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012;307:1405–1413 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Worktable 292f. Deaths from 358 selected causes, by 5-year age groups, race, and sex: United States, 1999–2007. 2010 [accessed 2012 Jun 1]. Available from: http://www.cdc.gov/nchs/nvss/mortality/gmwk292f.htm
- 8.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA 2005;294:2712–2719 [DOI] [PubMed] [Google Scholar]
- 9.Yu H, Wier LM, Elixhauser A. Hospital stays for children, 2009. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project Statistical Brief #118; 2011 [PubMed] [Google Scholar]
- 10.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in europe. Thorax 2012;67:71–79 [DOI] [PubMed] [Google Scholar]
- 11.Enserink M. Global health. Some neglected diseases are more neglected than others. Science 2009;323:700. [DOI] [PubMed] [Google Scholar]
- 12.Broder S. Twenty-five years of translational medicine in antiretroviral therapy: promises to keep. Sci Transl Med 2010;2:39ps33. [DOI] [PubMed] [Google Scholar]
- 13.Ewig S, Torres A. Community-acquired pneumonia as an emergency: time for an aggressive intervention to lower mortality. Eur Respir J 2011;38:253–260 [DOI] [PubMed] [Google Scholar]
- 14.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinton LJ, Mizgerd JP. NF-κB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res 2011;343:153–165 [DOI] [PubMed] [Google Scholar]
- 16.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappab RelA during pneumococcal pneumonia. J Immunol 2007;178:1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 2004;172:3377–3381 [DOI] [PubMed] [Google Scholar]
- 18.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol 2005;175:7530–7535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin Microbiol Rev 2011;24:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wurfel MM, Gordon AC, Holden TD, Radella F, Strout J, Kajikawa O, Ruzinski JT, Rona G, Black RA, Stratton S, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med 2008;178:710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 2005;33:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algul H, Schmid RM, Mizgerd JP. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am J Respir Cell Mol Biol 2011;45:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun 2011;79:3966–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, Doerschuk CM. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med 2011;183:1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol 2003;170:6257–6265 [DOI] [PubMed] [Google Scholar]
- 26.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L143–L152 [DOI] [PubMed] [Google Scholar]
- 27.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, Sabharwal V, Algul H, Akira S, et al. Hepatocyte-specific mutation of both NF-κB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest 2012;122:1758–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 2011;184:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol 2010;189:1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 2010;184:836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009;119:2898–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 2009;15:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011;12:1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, Wert SE, Whitsett JA. STAT3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol 2006;177:527–537 [DOI] [PubMed] [Google Scholar]
- 36.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2008;38:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimall J, Freeman A, Holland SM. Pathogenesis of hyper IgE syndrome. Clin Rev Allergy Immunol 2010;38:32–38 [DOI] [PubMed] [Google Scholar]
- 38.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis 2006;193:360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinton LJ, Mizgerd JP, Hilliard KL, Jones MR, Kwon CY, Allen E. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J Immunol 2012;188:6300–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotton DN. Next-generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med 2012;185:1255–1260 [DOI] [PubMed] [Google Scholar]
- 41.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 2011;35:997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 2011;6:e16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Bree GJ, Daniels H, Schilfgaarde M, Jansen HM, Out TA, van Lier RA, Jonkers RE. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis 2007;195:1718–1725 [DOI] [PubMed] [Google Scholar]
- 44.Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, Heyderman RS, Gordon SB. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax 2011;66:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. Acquisition of pneumococci specific effector and regulatory CD4+ T cells localising within human upper respiratory-tract mucosal lymphoid tissue. PLoS Pathog 2011;7:e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012;18:274–280 [DOI] [PubMed] [Google Scholar]
- 47.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 48.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355–2365 [DOI] [PubMed] [Google Scholar]
- 49.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 2005;32:367–372 [DOI] [PubMed] [Google Scholar]
- 50.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012;18:673–683 [DOI] [PubMed] [Google Scholar]
- 51.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med 2012;18:726–735 [DOI] [PubMed] [Google Scholar]
- 52.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol 2011;128:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, Schaffner W, Craig AS, Griffin MR. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005;352:2082–2090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.