Abstract
Hormone antagonist therapy for estrogen receptor positive (ER+) breast cancer patients post radical surgery and radiation therapy has a poor prognosis and also causes bone loss. 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] is a potent antitumor agent in pre-clinical studies, but caused hypercalcemia when its effective antitumor doses were used. Therefore, we investigated the effects of a less-calcemic 1α,25(OH)2D3 analog, 19-nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 (MART-10), on ER+MCF-7 cells. We demonstrate that MART-10 is 500- to 1000-fold more potent than 1α,25(OH)2D3 in inhibiting cell growth in a dose- and time-dependent manner. MART-10 is also much more potent in arresting MCF-7cell cycle progression at G0/G1 phase as compared to 1α,25(OH)2D3, possibly mediated by a greater induction of p21 and p27 expression. Moreover, MART-10 is more active than 1α,25(OH)2D3 in causing cell apoptosis, likely through a higher BAX/Bcl expression ratio and the subsequent cytochrome C release from mitochondria to cytosol. Based on our in vitro findings, MART-10 could be a promising vitamin D analog for the potential treatment of breast cancer, for example, ER+ patients, to decrease the tumor relapse rate and the side effect on bone caused by antihormone regimens. Thus, further in vivo animal study is warranted.
1. Introduction
Breast cancer ranks first globally among the most commonly diagnosed and cancer-related deaths in women [1]. Over 1.38 million new breast cancer cases and 458,400 breast cancer-related deaths have been reported worldwide in 2008. Estrogen receptor (ER), which is present in nearly 70% of all breast cancer patients, plays a crucial role in the progression of breast cancer [2]. Thus, ER antagonists, tamoxifen and raloxifene, have been widely used to treat breast cancer and have contributed to a better prognosis for ER positive (ER+) breast cancer. However, only a 50% reduction in tumor relapse has been achieved by ER antagonist therapy [3]. Furthermore, the antagonists have serious side effects on bone [4], which highlights the necessity of seeking alternative treatments for ER+ breast cancer.
Vitamin D is well known as a modulator of calcium and bone metabolism. For the past three decades, abundant evidence has been accumulated to indicate that the active form of vitamin D, 1α,25-dihydroxyvitamin D3, 1α,25(OH)2D3, or calcitriol, possesses many actions not associated with calcium and bone metabolism [5]. They include antiproliferation, antiangiogenesis, proapoptosis, prodifferentiation, and immune regulation in a cell- and tissue- specific manner [5–9].
1α,25(OH)2D3 exerts its effects through binding to vitamin D receptor (VDR). The receptor is expressed in most human cancer cell lines and its growth can be inhibited by 1α,25(OH)2D3 [10–14]. However, the clinical application of 1α,25(OH)2D3 is hindered by its lethal hypercalcemic side-effect after its systemic administration at a concentration sufficient to inhibit tumor cell growth [15]. To overcome this drawback, thousands of vitamin D analogs have been synthesized aiming to minimize its calcemic side effect while maintaining or even potentiating the antitumor activities [16, 17].
For breast cancer, 1α,25(OH)2D3 and its analogs, including EB1089, ILX 23-7533, and 22-oxa-1α,25(OH)2D3, have been shown to be effective in suppressing breast cancer cell growth in vitro and in vivo either alone or in combination with other drugs [18]. However, no significant benefit on survival has been observed in clinical trials [19, 20].
MART-10 (19-nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3) [21] has been shown to be more active in VDR transactivation [22]. Most importantly, MART-10 is far more potent in inhibiting liver and prostate cancer cell proliferation [11, 22, 23] and prostate cancer cell invasion [24], and it did not raise serum calcium in vivo in an animal model [24]. These findings suggest that MART-10 could be a good candidate for breast cancer treatment. We, therefore, study the antiproliferative and proapoptotic effects of MART-10 in ER+ MCF-7 breast cancer cells and the potential mechanisms involved.
2. Materials and Methods
2.1. Vitamin D Compounds
1α,25(OH)2D3 was purchased from Sigma (St. Louis, MO, USA). MART-10 was synthesized as previously described [21].
2.2. Cell Culture
Human breast cancer cell lines, MCF-7 and MDA-MB-231, were obtained from Bioresource Collection and Research Center (BCRC, Taiwan). Both MCF-7 and MDA-MB-231 cells were grown in DMEM (Sigma) supplemented with 5% fetal bovine serum (FBS). Culture medium was changed 3 times per week.
2.3. Cell Proliferation Assay by Cell Number Counting
Cell counting was conducted using a hemocytometer as previously described [11]. Cells were treated every two days and counted on day 7.
2.4. Western Blot for Protein Expression
The procedures for protein extraction, blocking, and detection were described previously [11]. The primary antibodies used in this study were monoclonal antibodies against VDR (D-6, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p21 (2946, Cell Signal, Beverly, MA, USA), p27 (3698, Cell Signal), cytochrome C (clone 7H8.2C12, BD Biosciences Pharmingen), Bax (554104, BD), and Bcl-2 (05-729, Millipore, Bedford, MA, USA). The secondary antibodies (1 : 5000) were anti-rabbit (111-035-003, Jackson Immunoresearch, West Grove, PA, USA) or anti-mouse secondary antibodies (Zymed 81-6520). The blots were detected using ECL reagents (WBKLS0500, Millipore, Billerica, MA, USA). Membranes were detected by VersaDoc Imaging System (Bio-Rad, Hercules, CA, USA) for analysis.
2.5. Cell Cycle Analysis by Flow Cytometry
Flow cytometry for cell cycle analysis was performed using a FACSCalibur (BD Biosciences, San Jose, CA, USA) as described previously [11, 25]. Briefly, after exposure for two days to indicated concentrations of 1α,25(OH)2D3, the cells were collected and fixed in ice-cold 75% ethanol at 20°C overnight. The fixed cells were stained in propidium iodide (PI) buffer containing 100 mM sodium citrate, 0.1% Triton X-100, 0.2 mg/mL RNase, and 50 μg/mL PI at 4°C for 1 h. Flow cytometry and cell cycle analysis were then performed using a FACSCalibur.
2.6. Apoptosis Analysis by Flow Cytometry
MCF-7 cell apoptosis was analyzed using a flow cytometer with Annexin V-FITC (fluorescein isothiocyanate) and propidium iodide (PI) staining kit (Strong Biotech Corporation, Taiwan) to distinguish early apoptotic from necrotic cells as previously described [11, 26]. Briefly, three days after the indicated concentrations of MART-10 or 1α,25(OH)2D3 treatment, MCF-7 cell apoptosis was analyzed using a flow cytometer with Annexin V-FITC (fluorescein isothiocyanate) and propidium iodide (PI) staining. Apoptosis Detection Kit (Strong Biotech Corporation, Taiwan) was applied in the present study. Briefly, cells from each sample were suspended in a mixture of 2 μL Annexin V-FITC, 2 μL propidium iodide (PI), and 100 μL AnnexinV-FITC binding buffer and then incubated at room temperature for 15 min. According to the cell density, 0.4–0.8 mL binding buffer was added. The samples were analyzed using a flow cytometer FACS Calilbur (BD Biosciences). The cell population was separated into three groups, that is, live cells with a low level of fluorescence, apoptotic cells in the earlier period with green fluorescence (Annexin V positive), and necrotic and advanced stage apoptotic cells with both red and green fluorescence (Annexin V and PI positive).
2.7. Apoptosis Analysis by TUNEL Assay
TUNEL assay was used to measure DNA fragmentation [27]. Briefly, cells were plated on autoclaved glass coverslips in six-well culture plates and treated with MART-10 or 1α,25(OH)2D3 as indicated in the figure legends. Cellular DNA was stained with apoptosis detection kits (Millipore Billerica, MA, USA), and the assay was performed according to the recommendations from the manufacturer (Millipore Billerica).
2.8. Statistical Analysis
The data from each group were compared by the student t-test. P-value < 0.05 was considered as a significant difference. Functions of Excel 2007 were used to calculate test statistics.
3. Results
3.1. VDR Expression in MCF-7 Cells
Since the genomic actions of 1α,25(OH)2D3 are mediated through VDR, we first analyzed the expression of VDR in MCF-7 cells. The expression in MDA-MB-231 cells served as a negative control [28]. As demonstrated in Figure 1(a), VDR was highly expressed in MCF-7 cells (lanes 1, 3, and 5), whereas very little or no expression (lanes 2, 4, and 6) was found in MDA-MB-231 cells as previously reported [28].
Figure 1.
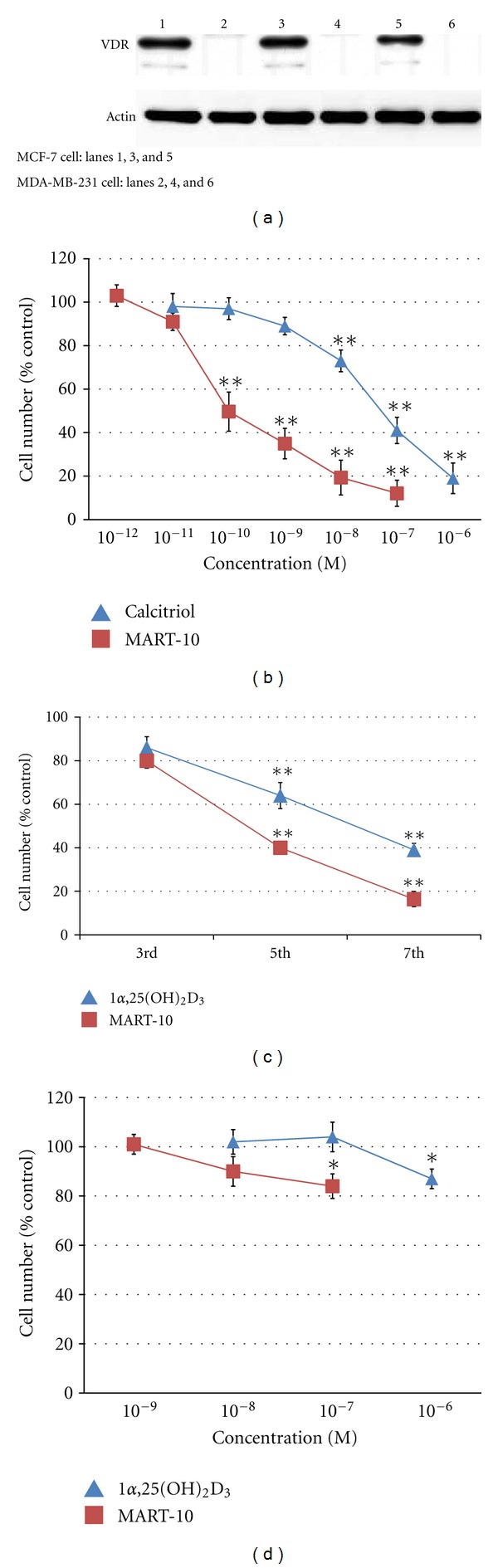
VDR expression in MCF-7 cells and MDA-MB-231 cells and the antiproliferative activity of 1α25(OH)2D3 and MART-10 in MCF-7 cells. (a) VDR expression in MCF-7 (lanes 1, 3, and 5) and MDA-MB-231 cells (lanes 2, 4, and 6) as determined by western blot method. Sixty μg proteins were added in each lane. VDR was expressed much more prominently in MCF-7 than in MDA-MB-231 cells. (b) The dose-dependent inhibitory effects of 1α25(OH)2D3 and MART-10 on the growth of MCF-7 cells. Cells were plated at 5,000 cells per cm2 in 35 mm dishes. Two days after plating, cells were treated with 1α25(OH)2D3 or MART-10 for 1 week at the indicated concentrations as described in the Materials and Methods. Cell numbers were obtained using a hemocytometer. Results are presented as the percentage of control. Each value is a mean ± SD of three to five determinations. *P < 0.05, **P < 0.001 versus control. (c) The time-dependent inhibitory effects of 1α25(OH)2D3 and MART-10 on the growth of MCF-7 cells. Cells were grown and treated with 1α25(OH)2D3 or MART-10 at the indicated concentrations two days after plating and counted on days 3, 5, and 7, respectively. Cell numbers were obtained using a hemocytometer. Results are presented as the percentage of control. Each value is a mean ± SD of three to five determinations. *P < 0.05, **P < 0.001 versus control. (d) The dose-response effects of 1α,25(OH)2D3 and MART-10 treatment on the growth of MDA-MB-231 cells. Cells plated at 5,000 cells per cm2 in 35 mm dishes were grown and treated with 1α,25(OH)2D3 or MART-10 at indicated concentrations for 1 week two days after plating as described in the Materials and Methods. Cell numbers were obtained using a hemocytometer. Results are presented as the percentage of control. Each value is a mean ± SD of three to five determinations. *P < 0.05, **P < 0.001 versus control.
3.2. Antiproliferative Effect of MART-10 and 1α,25(OH)2D3 on MCF-7 Cells
To compare the antiproliferative activity of MART-10 and 1α,25(OH)2D3 in MCF-7 cells, the cells were treated with either MART-10 or 1α,25(OH)2D3, and the cell numbers were counted on 7th day as previously described [11]. As shown in Figure 1(b), either 1α,25(OH)2D3 or MART-10 caused a dose-dependent inhibition of cell growth. However, MART-10 caused a 50 ± 9% inhibition at 10−10 M, whereas, no inhibition was observed with 10−10 M of 1α,25(OH)2D3. Only when 10−7 M 1α,25(OH)2D3 was used, a 58 ± 6% inhibition was obtained. Thus, it is concluded that MART-10 is about 500- to 1000-fold as potent as 1α,25(OH)2D3 to repress MCF-7 cell growth.
Figure 1(c) shows a time course inhibition of MCF-7 cell growth by 1α,25(OH)2D3 and MART-10 at 10−7 M. 1α,25(OH)2D3 inhibited MCF-7 cell growth by 14 ± 5, 46 ± 6 and 61 ± 3% on the 3rd, 5th, and 7th day, whereas a 20 ± 3, 60 ± 3, or 84 ± 4% growth inhibition by MART-10 was observed at the same time points. A greater inhibition by MART-10 was observed at each time point.
Figure 1(d) demonstrates that MDA-MB-231 cells were not as responsive as MCF-7 cells to 1α,25(OH)2D3 and MART-10 treatments. Only a 13 ± 6% and a 16 ± 5% inhibition were observed in the presence of 10−6 M 1α,25(OH)2D3 and 10−7 M MART-10, respectively. The results are in agreement with the VDR expression data obtained by western blot analysis showing much less expression of VDR in MDA-MB-231 cells than in MCF-7 cells (Figure 1(a)).
3.3. Induction of Cell Cycle Arrest at G0/G1 Phase and the Cyclin Dependent Kinase (CDK) Inhibitors, p21 and p27, by MART-10 and 1α,25(OH)2D3 in MCF-7 Cells
Since MART-10 and 1α,25(OH)2D3 showed a significant inhibition in the growth of MCF-7 cells, we next conducted cell cycle analysis by flow cytometry to further understand the mechanisms mediating the inhibition. When MCF-7 cells were treated with 10−8, 10−7, and 10−6 M 1α,25(OH)2D3 for two days, the fraction of cells arrested at G0/G1 phase increased by 5.81%, 13.34%, and 13.78%, respectively, whereas we observed an increase in cell arrest at G0/G1 by 10.45%, 15.36%, and 19.93% in the presence of 10−9, 10−8, and 10−7 M of MART-10, respectively, as compared to the controls (Figure 2 and Table 1). It is clear that although either 1α,25(OH)2D3 or MART-10 can significantly arrest MCF-7 cell cycle progression at G0/G1, MART-10 is much more potent than 1α,25(OH)2D3 in this respect.
Figure 2.
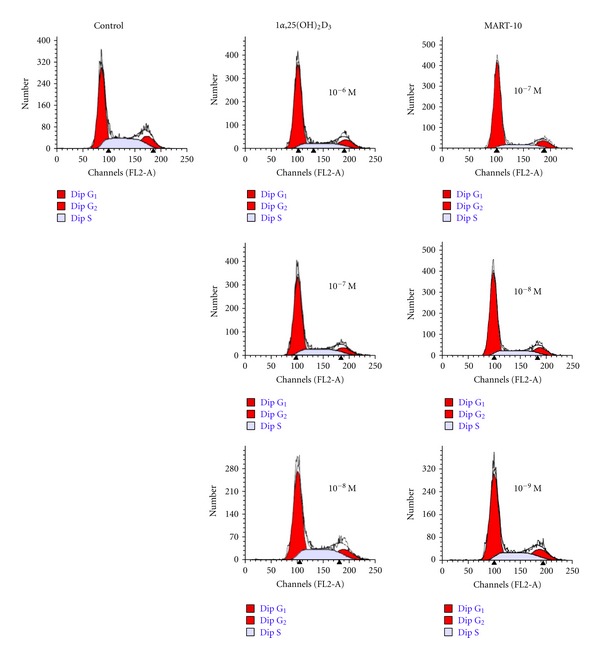
Flow cytometry analysis of cell cycle distribution for MCF-7 cells treated by 1α25(OH)2D3 and MART-10. Effects of 1α25(OH)2D3 and MART-10 on the relative distribution of MCF-7 cells at G1/G0, S and G2/M phase. MCF-7 cells were treated with 1α25(OH)2D3 from 10−8 M to 10−6 and MART-10 from 10−9 M to 10−7 M for two days before cell cycle analysis was performed with a flow cytometer. A representative DNA histogram for control, 1α25(OH)2D3-, or MART-10-treated MCF-7 cells was shown. The total DNA content of cells (x-axis) was obtained by staining with propidium iodide. Cells were analyzed by flow cytometry. The percentage of cells in each cell cycle phase was determined with the program ModFit. The first large peak represents population of cells (y-axis) in G0/G1 phase, the second small peak shows population of cells in G2/M phase, and the gray area between both peaks represents cells in S phase.
Table 1.
The distribution of different phases of MCF-7 cell cycle under the influence of 1α,25(OH)2D3 or MART-10.
| G1 | S | G2/M | |
|---|---|---|---|
| Control | 50.36% | 33.51% | 16.13% |
| 1,25D*, 10−8 M | 56.17% | 30.06% | 13.77% |
| 1,25D, 10−7 M | 63.70% | 23.85% | 12.36% |
| 1,25D, 10−6 M | 64.14% | 21.96% | 13.90% |
| M-10#, 10−9 M | 60.81% | 23.65% | 15.54% |
| M-10, 10−8 M | 65.72% | 21.32% | 12.96% |
| M-10, 10−7 M | 70.29% | 12.98% | 16.73% |
*1,25D: 1α,25(OH)2D3.
#M-10: MART-10.
Since p21 and p27 have been implicated in the G0/G1 arrest by 1α,25(OH)2D3, we next examined the expression of p21 and p27 in the presence of 1α,25(OH)2D3 or MART-10 by western blot analysis. Figure 3(a) demonstrates that p21 expression increased 1.56 ± 0.4, 1.91 ± 0.3, and 2.1 ± 0.45 time over the control group, after treating with 10−9, 10−8, and 10−7 M of 1α,25(OH)2D3 for two days, respectively, whereas 1.8 ± 0.3, 2.8 ± 0.6, and 3.1 ± 0.5 fold expressions were induced by MART-10 at 10−9, 10−8, and 10−7 M, respectively. As for p27 expression, 1α,25(OH)2D3 induced 1.29 ± 0.3, 1.66 ± 0.4, and 1.82 ± 0.45 time over the controls upon treatment with 10−8, 10−7, and 10−6 M of 1α,25(OH)2D3 for two days, respectively. MART-10 at 10−9, 10−8, and 10−7 M upregulated p27 expression 3.3 ± 0.6, 5 ± 0.9, and 5.3 ± 0.97 fold over the controls (Figure 3(b)). Taken together, we conclude that 1α,25(OH)2D3 and MART-10 are both able to upregulate p21 and p27 expression in a dose-dependent manner, and MART-10 is much more potent than 1α,25(OH)2D3.
Figure 3.
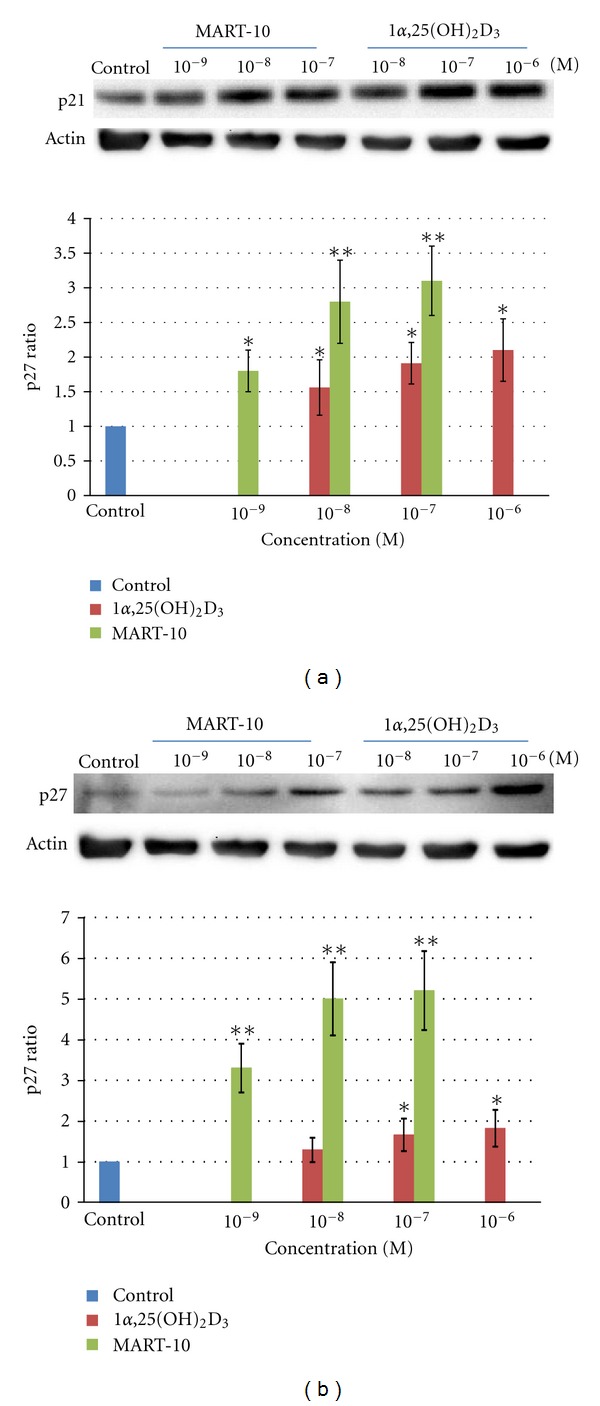
Western blot analysis for the expressions of p21 and p27 after treating MCF-7 cells with 1α25(OH)2D3 and MART-10. (a) A western blot (30 μg protein was loaded for each individual lane) depicting a typical dose-dependent upregulation of p21 protein expression in response to the treatment with 1α25(OH)2D3 or MART-10 for 2 days (upper panel). Actin was used as the loading control. The lower panel shows the average radio of the dose-dependent p21 expression relative to actin expression from three independent experiments. Each value is a mean ± SD of three independent determinations. (b) A western blot (30 μg protein was loaded for each individual lane) depicting a typical dose-dependent up-regulation of p27 protein expression in response to the treatment with 1α25(OH)2D3 or MART-10 for 2 days (upper panel). Actin was used as the loading control. The lower panel depicts the average radio of the dose-dependent p27 expression relative to actin expression from three independent experiments. Each value is a mean ± SD of three independent determinations. *P < 0.05, **P < 0.001 versus control.
3.4. Effects of 1α,25(OH)2D3 and MART-10 on MCF-7 Cell Apoptosis and Apoptotic Protein Expression
To compare the apoptotic response induced by 1α,25(OH)2D3 and MART-10 in MCF-7 cells, flow cytometry analysis coupled with staining cells with Annexin V (Annexin V-FITC) and PI was utilized [29] (Figure 4(A)). The quantitative numerical distribution of apoptotic cells from this analysis is presented in Table 2. 1α,25(OH)2D3 at 10−6 M induced MCF-7 cell apoptosis by increasing the late apoptotic cell population from 7.19% to 10.04%, while MART-10 at 10−7 M was able to increase the late apoptosis cell population from 7.19% to 13.66%. The results are in agreement with those obtained by TUNEL assay (Figure 4(B), panels a, b, c, and d). The figure shows that 8.2% and 8% apoptotic cells were generated when MCF-7 cells were treated with 10−6 M 1α,25(OH)2D3 and 10−7 M MART-10, respectively. Our results, therefore, indicate that MART-10 is about 10-fold more potent than 1α,25(OH)2D3 in the apoptotic induction of MCF-7 cells.
Figure 4.
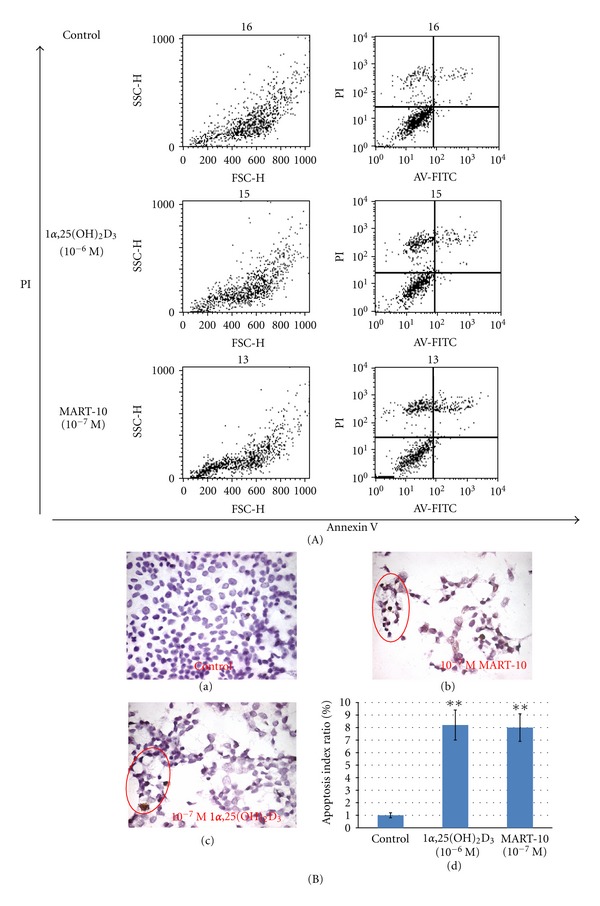
Effects of 1α25(OH)2D3 and MART-10 on MCF-7 cell apoptosis analyzed by flow cytometry with Annexin V-FITC, PI staining, and TUNEL assay. (A) Annexin V-FITC in conjunction with PI staining was used to distinguish early apoptotic (Annexin V-FITC positive, PI negative; bottom right quadrant of each panel) from late apoptotic or necrotic cells (Annexin V-FITC positive, PI positive; top right quadrant of each panel). Fluorescence intensity for Annexin V-FITC is plotted on the x-axis, and PI is plotted on the y-axis. (B) The apoptotic effects induced by MCF-7 cells were analyzed by TUNEL assay to measure the extent of DNA fragmentation visualized by fluorescence microscopy: (a) control; (b) cells treated with 10−7 M MART-10; (c) cells treated with 10−7 M 1α25(OH)2D3. The cells showing positive DNA fragmentation were circled; (d) relative apoptotic index. Each value represents the average of three determinations. *P < 0.05, **P < 0.001 versus control.
Table 2.
The distribution of different phases of MCF-7 cell cycle after 1α,25(OH)2D3 or MART-10 treatment determined by flow cytometry with Annexin V-FITC and PI staining.
| PI negative, Annexin V negative |
PI negative, Annexin V positive |
PI positive, Annexin V positive |
|
|---|---|---|---|
| Control | 76.22% | 1.59% | 7.19% |
| 1α,25(OH)2D3 (10−6 M) | 65.79% | 1.19% | 10.04% |
| MART-10 (10−7 M) |
57.87% | 1.76% | 13.66% |
Bax protein is a well-known proapoptotic protein, whereas Bcl-2 is a protein with antiapoptotic activity. Therefore, the higher Bax/Bcl-2 ratio has been used as an indicator for the expression and the subsequent release of cytochrome C into cytosol to trigger apoptosis. As shown in Figure 5(a), 10−7 M MART-10 and 1α,25(OH)2D3 increased the Bax/Bcl-2 ratio to 1.48 and 1.33 as compared to the controls, which is in agreement with a greater upregulation of cytochrome C expression over controls by MART-10 (2.35-fold) than by 1α,25(OH)2D3 (1.64-fold) (Figure 5(b)).
Figure 5.
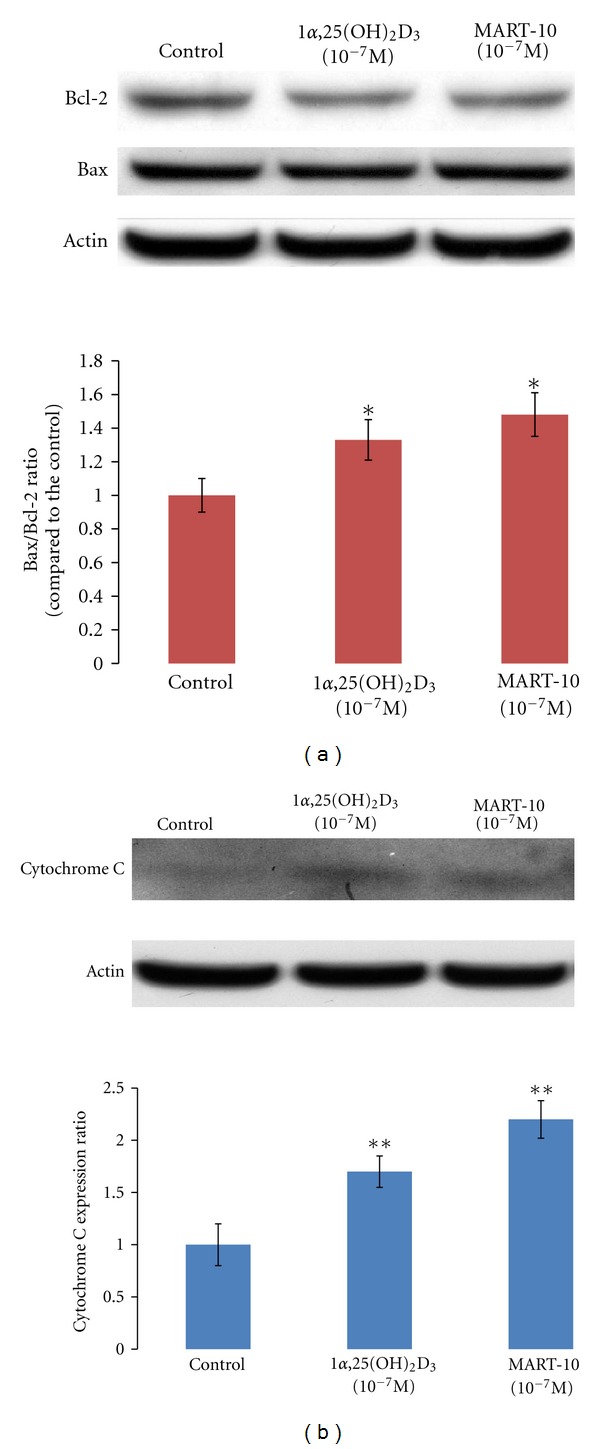
Effects of 1α25(OH)2D3 and MART-10 on the protein expression of Bcl-2, Bax, and cytochrome C in MCF-7 cells. (a) Western blot analysis of Bcl-2 and Bax expression in the untreated control, and cells treated with either 10−7 M of 1α25(OH)2D3 or MART-10 for 5 days (upper panel). Thirty μg protein was loaded in each lane. The lower panel depicts the calculated BAX/Bcl-2 ratio obtained from scanning the bands shown in the upper panel based on the control Bax/Bcl-2 ratio set as 1. (b) Western blot analysis of cytochrome C expression in cytosol after treatment with ethanol vehicle, 1α25(OH)2D3, or MART-10 (upper panel) and the expression ratio over the control (lower panel). Each value represents the average of three determinations. *P < 0.05, **P < 0.001 versus control.
4. Discussion
The focus of this study was to investigate the antiproliferative and proapoptotic activities of MART-10 in the ER+ MCF-7 breast cancer cells which express high level of VDR (Figure 1(a)). MART-10 is a new generation of 1α,25(OH)2D3 analogs with a skeleton of “2α-(3-hydroxy)propyl group” and “19-nor” integrated into one molecule. Therefore, MART-10 possesses the combined characteristics of the noncalcemic nature of the 19-nor vitamin D compounds [30] as exemplified by the FDA-approved drug Zemplar or 19-nor-1α, 25(OH)2D2 for the treatment of the secondary hyperparathyroidism, and the enhanced VDR binding property of 2α-(3-hydroxy)propyl compound [31, 32]. Similar to Zemplar, MART-10 did not raise serum calcium in an in vivo animal model [23] and was more potent than 1α,25(OH)2D3 in inducing VDR transactivation [22].
The effects of vitamin D are mainly mediated through the VDR-dependent genomic actions. Our results confirm the high level of VDR expression in MCF-7 cells and accordingly highly sensitive growth inhibitory responses to 1α,25(OH)2D3 and MART-10 in a dose- and time-dependent manner (Figures 1(b) and 1(c)). The low or no expression of VDR in MDA-MB-231 cells (Figure 1(a)) is in agreement with the low antiproliferative activity caused by 1α,25(OH)2D3 and MART-10 (Figure 1(d)) in these VDR-null cells. Thus, the results clearly suggest that VDR plays a crucial role in the response of MCF-7 breast cancer cells to 1α,25(OH)2D3. .Along this line, Lopes et al. recently reported that VDR expression was high in benign breast lesions and diminished gradually in invasive breast cancer as the tumor progressed [33]. VDR expression has also been shown to be inversely related to breast cancer incidence [34]. Collectively, the findings suggest that dysregulation of VDR expression may contribute to the incidence and progression of breast cancer.
In addition, our data, showing a greater cell growth inhibition induced by MART-10 than by 1α25(OH)2D3 on day 5 and day 7 (Figure 1(c)), suggest that the effective dose of MART-10 may be higher than that of 1α,25(OH)2D3, possibly because MART-10 is more bioavailable than 1α,25(OH)2D3 due to the nature that MART-10 is more resistant to CYP24A1 degradation [22, 23].
Our results show that although both 1α,25(OH)2D3 and MART-10 are active in inhibiting the proliferation (Figures 1(b) and 1(c)), inducing the cell cycle arrest at G0/G1 phase (Figure 2 and Table 1) and promoting the apoptosis of MCF-7 cells (Figure 4), MART-10 is far more potent than 1α,25(OH)2D3. The greater antiproliferative activity with MART-10 over 1α,25(OH)2D3 may be explained at least in part by its greater stimulatory effects on the expression of two tumor suppressor genes, p21 and p27, which act as CDK inhibitors to inhibit the progression of cells into the S phase of the cell cycle (Figure 3). This finding is consistent with several previous reports that showed that p21 and p27 were the genes targeted by 1α25(OH)2D3 and, therefore, leading to the arrest of cell growth [11, 35, 36].
As demonstrated in Figure 4 and Table 2, MART-10 is also more active than 1α,25(OH)2D3 in inducing apoptosis. Bax, a proapoptotic protein, works toward the initiation of apoptosis through promoting the release of cytochrome C from mitochondria into cytosol. Whereas, Bcl-2, an antiapoptotic protein, functions as a protector to stabilize the mitochondrial membrane from releasing cytochrome C [37]. Studying MCF-7 breast cancer cells, James et al. [38] and Simboki-Campbell et al. [39] reported that 1α,25(OH)2D3 induced apoptosis by downregulating Bcl-2 protein expression, increased TRPM-2 (clusterin) mRNA expression, and increased DNA fragmentation after 1α,25(OH)2D3 treatment. In our studies with MCF-7 cells, both 1α,25(OH)2D3 and MART-10 increased the ratio of Bax/Bcl-2 and the subsequent release of cytochrome C (Figures 5(a) and 5(b)). However, MART-10 is more potent than 1α,25(OH)2D3.
The release of cytochrome C from mitochondria to cytoplasm is a trigger of apoptosis pathway, leading to the activation of intrinsic initiator caspase 9, which in turn activates executioner caspase 3 and caspase 7 [40]. To investigate whether caspases were involved in the vitamin D-induced apoptosis in MCF-7 cells, we performed western blotting to detect the expression of the active form of caspases 3, 7, 8, and 9 in the presence of 10−7 M of 1α,25(OH)2D3 or MART-10 for 5 days. We found that none of them was detected either with or without 1α,25(OH)2D3 or MART-10 treatment (unpublished data). Our results are in agreement with the previously published observations by Narvaez and Welsh [41] and Jänicke et al. [42]. Collectively, we conclude that MART-10 and 1α,25(OH)2D3-mediated apoptosis in MCF-7 cells may be cytochrome C-related but caspases-independent, and MART-10 is more potent than 1α,25(OH)2D3 in inducing apoptosis in MCF-7 cells.
5. Conclusion
For premenopausal women with ER+ breast cancer, the choice for antihormone treatment is tamoxifen or raloxifene which binds to ER, whereas aromatase inhibitors are the major therapeutic antihormone agents for the postmenopausal women with ER+ breast cancer. The drawback of tamoxifen or raloxifene and aromatase inhibitors is that they globally attenuate estrogen receptor transactivation or estrogen synthesis. It may be undesirable for some tissues where estrogen is essential to maintain normal functions, such as bone which needs estrogen to stimulate bone formation. On the contrary, 1α,25(OH)2D3 can selectively down-regulate aromatase and ER-α expression in breast cancer cells [43, 44]. Along this line, we have performed preliminary studies indicating that MART-10 is far more potent than 1α,25(OH)2D3 in inhibiting ER-α expression in MCF-7 cells (unpublished observation). In conclusion, we show that MART-10 is much more potent than 1α,25(OH)2D3 in inhibiting cell growth through arresting cell cycle progression at G1 phase and inducing apoptosis. In addition, the more bioavailable character of MART-10 as compared to 1α,25(OH)2D3 in MCF-7 cells and its noncalcemic nature in an animal model suggest that MART-10 has potential as a superior chemotherapeutic agent to replace or to be in combination with traditional antihormone therapy for the treatment of breast cancer, such as the ER+ breast cancer patients, to decrease the tumor recurrence and eliminate the side effect on bone caused by the antihormone treatments.
Conflict of Interes
The authors declare that they have no conflict of interests.
Acknowledgments
This work was supported in part by National Science council Medical Research Grant no. 99-2314-B-182A-090-(NMRPG290031) (to K. C. Chiang), Chang Gung Memorial Hospital Research Grant (CMRPG) no. 280273 (to K. C. Chiang), National Science Council Medical Research Grant no. 101-2314-B-182-052-MY3 (NMRPG3B6331) (to S. C. Chen), Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (no. 21590022 and 24590021 to A. Kittaka), and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (no. 23790021 to M. Takano).
Abbreviations
- ER:
Estrogen receptor
- 1α,25(OH)3D:
1α,25-Dihydroxyvitamin D
- MART-10:
19-nor-2α-(3-Hydroxypropyl)-1α,25(OH)2D3
- VDR:
Vitamin D receptor
- VDRE:
Vitamin D response element
- RXR:
Retinoid X receptor
- PI:
Propidium iodide
- E2F-1:
E2F transcription factor 1
- FBS:
Fetal bovine serum
- FITC:
Fluorescein isothiocyanate.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zwart W, Theodorou V, Carroll JS. Estrogen receptor-positive breast cancer: a multidisciplinary challenge. Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 2011;3(2):216–230. doi: 10.1002/wsbm.109. [DOI] [PubMed] [Google Scholar]
- 3.Holm C, Kok M, Michalides R, et al. Phosphorylation of the oestrogen receptor α at serine 305 and prediction of tamoxifen resistance in breast cancer. Journal of Pathology. 2009;217(3):372–379. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77(11):1107–1112. doi: 10.1016/j.steroids.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochemical Journal. 2012;441(1):61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang KC, Chen TC. Vitamin D for the prevention and treatment of pancreatic cancer. World Journal of Gastroenterology. 2009;15(27):3349–3354. doi: 10.3748/wjg.15.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a review. Journal of Gastroenterology and Hepatology. 2011;26(11):1597–1603. doi: 10.1111/j.1440-1746.2011.06892.x. [DOI] [PubMed] [Google Scholar]
- 8.Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative Action of Vitamin D. Vitamins and Hormones. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 9.Chiang KC, Chen TC. The anti-cancer actions of vitamin D. Anti-Cancer Agents in Medicinal Chemistry. 2012;13:126–139. [PubMed] [Google Scholar]
- 10.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annual Review of Pharmacology and Toxicology. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 11.Chiang KC, Yeh CN, Chen HY, et al. 19-Nor-2α-(3-hydroxypropyl)-1α, 25-dihydroxyvitamin D3 (MART-10) is a potent cell growth regulator with enhanced chemotherapeutic potency in liver cancer cells. Steroids. 2011;76(13):1513–1519. doi: 10.1016/j.steroids.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Archives of Biochemistry and Biophysics. 2012;523(1):107–114. doi: 10.1016/j.abb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature Reviews Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 14.Chiang KC, Persons ES, Istfan NW, Holick MF, Chen TC. Fish oil enhances the antiproliferative effect of 1α,25- dihydroxyvitamin D3 on liver cancer cells. Anticancer Research. 2009;29(9):3591–3596. [PubMed] [Google Scholar]
- 15.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends in Endocrinology and Metabolism. 2003;14(9):423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Bouillon R, Verstuyf A, Verlinden L, et al. Non-hypercalcemic pharmacological aspects of vitamin D analogs. Biochemical Pharmacology. 1995;50(5):577–583. doi: 10.1016/0006-2952(95)00121-f. [DOI] [PubMed] [Google Scholar]
- 17.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annual Review of Pharmacology and Toxicology. 2001;41:421–442. doi: 10.1146/annurev.pharmtox.41.1.421. [DOI] [PubMed] [Google Scholar]
- 18.Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Molecular Aspects of Medicine. 2008;29(6):433–452. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Bower M, Colston KW, Stein RC, et al. Topical calcipotriol treatment in advanced breast cancer. Lancet. 1991;337(8743):701–702. doi: 10.1016/0140-6736(91)90280-3. [DOI] [PubMed] [Google Scholar]
- 20.Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. British Journal of Cancer. 1998;78(1):6–13. doi: 10.1038/bjc.1998.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono K, Yoshida A, Saito N, et al. Efficient synthesis of 2-modified 1α,25-dihydroxy-19-norvitamin D3 with Julia olefination: high potency in induction of differentiation on HL-60 cells. Journal of Organic Chemistry. 2003;68(19):7407–7415. doi: 10.1021/jo034787y. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan JN, Zheng S, Chiang KC, et al. Evaluation of 19-nor-2α(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Research. 2009;29(9):3547–3553. [PubMed] [Google Scholar]
- 23.Iglesias-Gato D, Zheng S, Flanagan JN, et al. Substitution at carbon 2 of 19-nor-1α, 25-dihydroxyvitamin D3 with 3-hydroxypropyl group generates an analogue with enhanced chemotherapeutic potency in PC-3 prostate cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2011;127(3–5):269–275. doi: 10.1016/j.jsbmb.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen TC, Persons KS, Zheng S, et al. Evaluation of C-2-substituted 19-nor-1α,25-dihydroxyvitamin D3 analogs as therapeutic agents for prostate cancer. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3–5):717–720. doi: 10.1016/j.jsbmb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Ma Q, Li J, et al. Cyclin D1 induction through IκB kinase β/nuclear factor-κB pathway is responsible for arsenite-induced increased cell cycle G 1-S phase transition in human keratinocytes. Cancer Research. 2005;65(20):9287–9293. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 26.Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. Journal of Immunological Methods. 2000;243(1-2):167–190. doi: 10.1016/s0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 27.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods. 2008;44(3):250–254. doi: 10.1016/j.ymeth.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buras RR, Schumaker LM, Davoodi F, et al. Vitamin D receptors in breast cancer cells. Breast Cancer Research and Treatment. 1994;31(2-3):191–202. doi: 10.1007/BF00666153. [DOI] [PubMed] [Google Scholar]
- 29.Jaruga E, Salvioli S, Dobrucki J, et al. Apoptosis-like, reversible changes in plasma membrane asymmetry and permeability, and transient modifications in mitochondrial membrane potential induced by curcumin in rat thymocytes. FEBS Letters. 1998;433(3):287–293. doi: 10.1016/s0014-5793(98)00919-3. [DOI] [PubMed] [Google Scholar]
- 30.Perlman KL, Sicinski RR, Schnoes HK, DeLuca HF. 1α,24-Dihydroxy-19-nor-vitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Letters. 1990;31(13):1823–1824. [Google Scholar]
- 31.Hourai S, Fujishima T, Kittaka A, et al. Probing a water channel near the A-ring of receptor-bound 1α,25-dihydroxyvitamin D3 with selected 2α-substituted analogues. Journal of Medicinal Chemistry. 2006;49(17):5199–5205. doi: 10.1021/jm0604070. [DOI] [PubMed] [Google Scholar]
- 32.Suhara Y, Nihei KI, Kurihara M, et al. Efficient and versatile synthesis of novel 2α-substituted 1α,25-dihydroxyvitamin D3 analogues and their docking to vitamin D receptors. Journal of Organic Chemistry. 2001;66(26):8760–8771. doi: 10.1021/jo010375i. [DOI] [PubMed] [Google Scholar]
- 33.Lopes N, Sousa B, Martins D, et al. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions Vitamin D pathways unbalanced in breast lesions. BMC Cancer. 2010;10:p. 483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiology Biomarkers and Prevention. 2005;14(10):2335–2339. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1α,25-dihydroxyvitamin D3 on the G1-S phase-controlling machinery. Molecular Endocrinology. 2001;15(8):1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 36.Simboli-Campbell M, Narvaez CJ, VanWeelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Research and Treatment. 1997;42(1):31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 37.Reed JC. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Seminars in Hematology. 1997;34(4) supplement 5:9–19. [PubMed] [Google Scholar]
- 38.James SY, Mackay AG, Colston KW. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 1996;58(4):395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- 39.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 1996;58(4):367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 40.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Current Opinion in Cell Biology. 2003;15(6):725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Narvaez CJ, Welsh J. Role of Mitochondria and Caspases in Vitamin D-mediated Apoptosis of MCF-7 Breast Cancer Cells. Journal of Biological Chemistry. 2001;276(12):9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 42.Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. Journal of Biological Chemistry. 1998;273(16):9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151(1):32–42. doi: 10.1210/en.2009-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swami S, Krishnan AV, Feldman D. 1α,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clinical Cancer Research. 2000;6(8):3371–3379. [PubMed] [Google Scholar]


