Abstract
Background:
This study aimed to determine the frequency of cognitive impairment and depression in our Parkinson's Disease (PD) and their relationship with disease severity and disability.
Patients and Methods:
A total of 40 PD patients and 40 age-, sex-, and educationally matched controls were studied. The Unified Parkinson Disease Rating Scale (UPDRS) Motor and Activities of Daily Living (ADL) scores and the Hoehn and Yahr (HY) stage were documented. Depression was assessed using the Zung Self-Rating Depression Scale (ZSDS), while cognition was evaluated using a composite score of the mini-mental state examination (MMSE) score and category fluency score.
Results:
A total of 55% (22/40) of PD and 10% (4 of 40) of controls had depression (P<0.001). A total of 60% of PD (24/40) and 5% of controls (2/40) had cognitive impairment (P<0.001). Both NMS coexisted in 16 of 40 PD (40%) compared with none of the controls (P<0.001). UPDRS (motor and ADL) scores and HY stage were significantly worse with impaired ZSDS scores - P 0.001. UPDRS ADL was significantly impaired by the presence of cognitive impairment. Coexisting depression and cognitive impairment were associated with significant worsening of all scores of severity and disability.
Conclusion:
Cognitive impairment and depression accompany our PD and are related to disability and worsening disease severity.
Keywords: Cognitive impairment, depression, disability, Parkinson's disease, severity
INTRODUCTION
Non-motor symptoms (NMS) of Parkinson disease (PD) are now recognized as being a common but often overlooked feature of the disease, which can impair quality of life and adversely affect long-term outcome.1 Although some of the NMS occur as late sequelae, they may antedate the onset of the hallmark motor abnormalities - bradykinesia, tremors and rigidity. The NMS include olfactory and sleep disorders, mood disorders (anxiety and depression), dysautonomia, and cognitive impairment (including dementia).
Although both depression and cognitive impairment have been found to be common in PD, the reported frequencies have varied widely due to methodological differences (including the instruments). Also, the populations under study have varied, and this may contribute to the divergent figures reported in literature. There are few reports on the impact of depression and cognitive impairment in PD in Africans and Nigerians specifically.
In a systematic review of 27 studies, the prevalence of cognitive impairment was found to be 40% by Cummings.2 Dementia and cognitive impairment have previously been reported in the Nigerian PD population.
Osuntokun and Bademosi3 reported a 5% frequency of dementia in their study of 217 Parkinsonism cases in Ibadan, while Akinyemi et al.4 in their study of 51 PD cases reported the frequency of cognitive impairment as 21.6%.
Depression is said to be the most common neuropsychiatric abnormality in PD. The prevalence worldwide has been documented as varying between 2.7% and 90%,5 though the average frequency in most surveys is 40-50%.6 Variations occur mainly as a result of differences in methodology. There are no data on frequency of depression in PD in our environment.
The biochemical basis for cognitive impairment in PD is considered to be low levels of catecholamine transferase in the mesofrontal cortex resulting from Lewy body deposition in the cortex. Also, there is reduction in the number of cholinergic neurons in the nucleus basalis of Meynert.7 On the other hand, the biochemical basis for depression in PD is thought to be predominantly due to a disruption in the dopaminergic system – dopaminergic denervation of the mesolimbic system and reduced dopamine transporter activity in the ventral striatum.8,9 Other suppositions regarding depression in PD are reduction in the noradrenergic neurons in the nucleus coeruleus and loss of serotonergic neurons in the nucleus raphe.10
Loss of serotonergic neurons has been postulated as the common biochemical basis for both depression and cognitive impairment.
This preliminary study was prompted by the paucity of data in this environment regarding cognitive impairment and depression in PD and it aimed to determine the frequency of both NMS in a clinic cohort of Nigerians with PD and to determine the relationship of either condition alone or in coexistence to PD disease severity and disability.
PATIENTS AND METHODS
Approval of the study protocol was obtained from the Health Research and Ethics committee of the Lagos University Teaching Hospital (LUTH), Lagos State, Nigeria. Study subjects were recruited from the Neurology out-patients clinic of the LUTH between January and September 2006, and were consecutively attending persons with PD. Healthy volunteer controls from the population were also recruited. In all, the study included 40 PD patients and 40 age- (±3 years), sex-, and educationally matched controls. PD was diagnosed with the UKPDS Brain Bank clinical diagnostic criteria.11 Disease and treatment characteristics were documented for all PD cases.
Controls were apparently healthy persons without any symptoms or signs of PD based on clinical examination at inclusion, and were age (±3 years) and sex-matched to cases.
All PD were evaluated in the “on” state. Disease severity was assessed using the Unified Parkinson Disease Rating Scale motor score (UPDRS M) (in accordance with the Movement Disorder Society (MDS) teaching tape12 and the Columbia scale of Hoehn and Yahr13 (HY). PD severity was classified as mild for persons in HY stages 1 and 2, moderate in stage 3, and severe in stages 4 and 5.14 Disability was measured using the UPDRS activities of daily living (UPDRS ADL) score assessed in accordance with the MDS Standardized training tools for UPDRS ADL scale.15
Cognition was assessed using a composite cognitive score (summation) of the Mini-Mental State Examination (MMSE) and the category fluency test scores.
Cognitive impairment was defined as any score below two standard deviations of the mean composite cognitive score of controls. The Zung Self-Rating Depression Scale (ZSDS) was used to assess depressive symptomatology, with a cut-off value of 40 used to define depression.16
Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 17.0 statistical software. Numerical data were presented as means±standard deviation (SD) and ranges and compared using parametric Student's t-test. Categorical variables were presented as proportions and compared using nonparametric χ2 test. Values of P<0.05 were considered statistically significant.
RESULTS
The mean age of the PD cases was 65.8±9.8 years which was comparable to the mean age of the controls (63.3 ± 10.8 years) (P=0.27) [Table 1]. The male to female ratio for cases and controls was 4:1 (32 males, 8 females). A total of 5 (12.5%) of the PD and 3 (7.5%) of the controls had no formal education, 17 PD (42.5%) and 19 controls (47.5%) were educated up to secondary school level (10 years’ formal education), while 18 (45%) PD cases and 18 (45%) controls had tertiary education (>10 years formal education) (P>0.05).
Table 1.
Comparison of baseline parameters, ZSDS, and cognitive scores in PD and controls
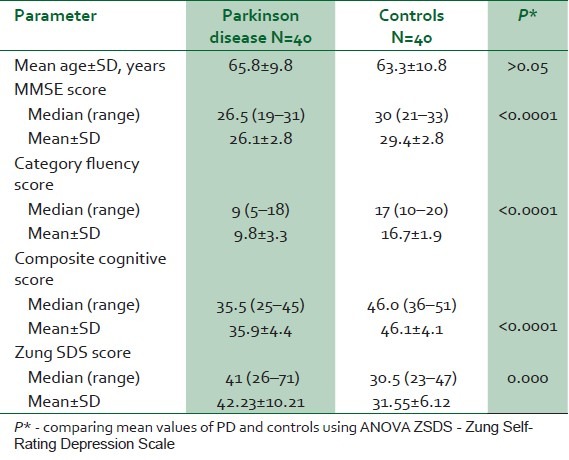
The mean ± SD disease duration in PD cases was 64.9±49.6 months (range from 6 to 192 months and median of 48 months). Disease severity was as follows - 25 (62.5%) had mild disease while 11 (27.5%) and 4 (10%) of the cases had moderate and severe disease respectively. The median HY score was 2. The mean UPDRS motor score was 41.1±17.6, while the mean UPDRS ADL score was 13.6±7.2.
Thirty five (87.5%) of the PD were on treatment while five (12.5%) were treatment naive. All treated cases were on levodopa-carbidopa (alone or in combination with dopamine receptor agonist and/or anticholinergic trihexyphenidyl). The mean duration of treatment ± SD (in months) was 48.9±63.9 with a range between 0.25 and 360 months (median 36 months). The mean levodopa dose±SD in 24 hours was 656±256.3 mg (range 250-1125 mg).
Cognitive scores (mean, ranges, median) of PD patients and controls are shown in Table 1, and Figure 1. Cognitive impairment was determined as any composite cognitive score below 38. On this basis, 60% (24/40) of PD and 5% (2/40) controls had cognitive impairment (P<0.001).
Figure 1.
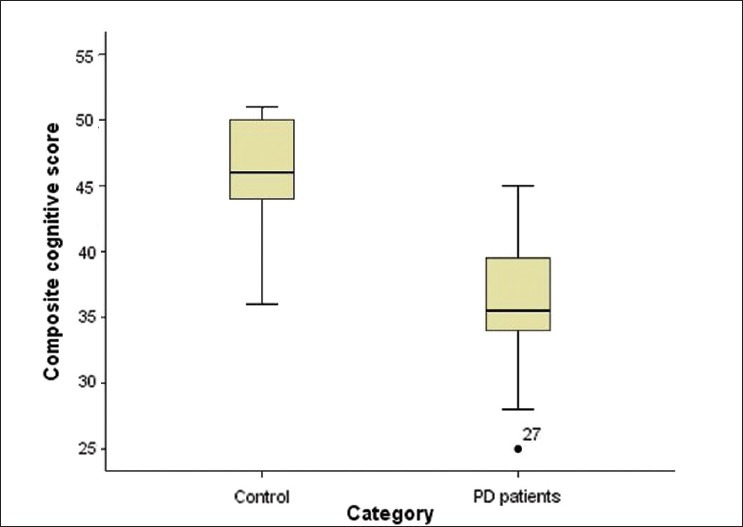
Comparison of composite cognitive scores in PD cases and controls
Mean ZSDS in PD was 42.23±10.21 significantly higher than the score in the controls - 31.55±6.12 in the controls (F=32.15, P<0.001) [Table 1]. A total of 22 PD cases (55%) and 4 controls (10%) had depressive symptomatology (χ2=16.47; P<0.001). The depressed PD cohort had a longer disease duration (74.73±51.31), though it did not differ significantly from nondepressed cases (52.78±45.88), P=0.17.
16 of 40 cases had coexisting depression and cognitive impairment compared with none of the controls (χ2=20.00, P=0.00). There was significant correlation between the ZSDS scores and the composite cognitive function score (Pearson correlation –0.570, P=0.00).
To assess the relationship between cognitive impairment and depression and PD severity and disability, the HY and UPDRS scores of PD with and without cognitive impairment and depression were compared [Table 2].
Table 2.
Relationship of cognitive function and depression to disease-related disability and severity scores in Parkinson disease
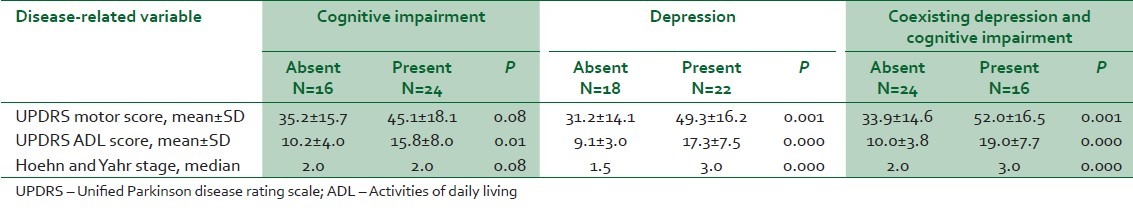
Scores of disease disability (UPDRS ADL) were significantly higher in PD with cognitive impairment. All three measures (UPDRS M, UPDRS ADL scores, and HY stage) were significantly worse in the presence of depression. Coexisting depression and cognitive impairment were also associated with significant worsening of all scores of severity and disability.
In correlation analysis, age, age at diagnosis, HY stage, UPDRS M, UPDRS ADL, MMSE, category fluency, and composite cognitive scores significantly correlated with depression, while age, age at onset, age at diagnosis, HY stage, UPDRS ADL, UPDRS M, ZSDS significantly correlated with cognitive impairment (P<0.05 in all instances).
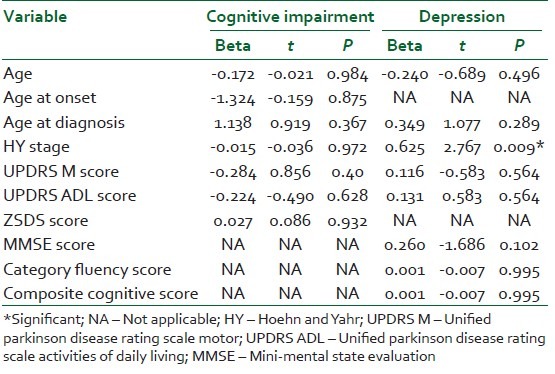
To further explore these correlations, all variables with significant correlation (P<0.05) with either depression scores or composite cognitive scores were subjected to a multivariate linear regression model. Subsequently, only the HY stage related to the ZSDS score (P=0.009). There was no relationship between any disease characteristic and cognitive score [Table 3].
Table 3.
Multivariate linear regression for determinants of cognitive function and depression in PD
DISCUSSION
Measures that can potentially ameliorate disability and improve outcome are important in the long-term management of individuals with PD. The occurrence of cognitive impairment and depression are particularly important determinants of overall morbidity and quality of life in PD.17
The frequency of cognitive impairment in this study (60%) is within the range previously reported from other studies. The prevalence of cognitive impairment in PD varies widely - ranging from 2% to 81% depending to a large extent on the on the methods (particularly with respect to operational definitions and assessment scales utilized), and the study population.18–20
In their study on Nigerians with PD, Akinyemi et al.4 obtained a frequency of 21.6% – the study excluded more severe PD, and used a combination of the Community Screening Instrument for Dementia (CSI‘D’)21,22 and the Ibadan neuropsychological test battery.23 The investigators of the CamPaIGN study24 reported a frequency of 8% using the MMSE. This relatively low frequency can be explained by the fact that only newly diagnosed PD cases were recruited for the study. The investigators however also used a pattern recognition task and the Tower of London task to assess cognitive impairment and the addition of these two instruments increased the frequency of cognitive impairment to 36%. Muslimovic et al.25 reported a frequency of 11% in a cohort of PD cases in the Netherlands using the supermarket category fluency test. Only newly diagnosed PD cases were enrolled in the study suggesting that the patients may only have early cognitive impairment and this was substantiated by the fact that the MMSE scores did not differ between the PD cases and their age- and sex-matched controls. The investigators of that study also excluded PD cases with MMSE scores less than 24. It should be noted that the overall frequency obtained by Muslimovich in his study was 23.7%. In addition to the supermarket category fluency, other neuropsychological tests were employed, and cognitive impairment was defined as poor performance on at least 3 of those tests.
Cognitive impairment and dementia in PD have been attributed to subcortical or dysexecutive dysfunction characterized by bradyphrenia, disorientation, memory impairment, visuospatial deficits, and impairment of executive functions.2 Thus, although the MMSE remains the most popular and fastest way of screening patients (PD inclusive) for cognitive impairment or dementia, it lacks a timed or executive component making this disadvantageous when screening PD patients.26 The inclusion of other tests sensitive to executive dysfunction and bradyphrenia has been proposed as the most realistic way of improving cognitive evaluation in parkinsonian patients.27 The verbal fluency test is a fast (less than 5 minutes administration time), easy, and yet clinically useful test of frontal lobe function,27 with the ability to reflect the profound difficulty of the parkinsonian patient in generating verbal actions.27
Cognitive impairment in our PD was associated with worse disability in keeping with findings of Rosenthal et al.28 They however used Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory to assess ADL. Impaired ADL is well known in the presence of dementia though the relationship between mild cognitive impairment and ADL has not been well studied.28,29
Our PD cohort also demonstrated a trend of worsening severity (measured by the UPDRS motor scores). Verbaan et al.30 found more advanced disease was associated with poorer cognitive performance in line with other studies.31–34
This is possibly a result of a combination of factors, most important of which could be natural disease progression resulting in increasing dopaminergic neuronal loss as well as catecholaminergic nerve loss in the nucleus basalis of Meynert35 with advancing PD. Furthermore, neurotoxicity from antiparkinsonian medications (including levodopa) remains a potential mechanism, although existing data are inconclusive.4,30
Depression occurred in 55% of our cohort. Assessment of depression in PD is hampered by lack of a disease-appropriate depression scale. Though different scales have been used with sufficient reliability, a scale taking into consideration the motor slowing of PD is needed.9
The Zung Self-Depression Rating Scale36 (ZSDS) is a short self-rated scale that assesses psychological and somatic depressive symptoms. It has been widely used to screen for depression, and measure the severity of depression.37–39 It is more easily comprehended than the Hamilton Depression rating scale and the Beck Depression Inventory40 (BDI), though few validation studies in PD are available.37 It has however been validated for use in Nigerians,16 thus justifying its use in this study.
Our study findings are similar to that of Dotchin et al.41 in Tanzania – they also found the frequency of depression in their study population to be 55%, though they used the Hospital Anxiety and Depression scale. An earlier study on depression in PD out-patients in Dar-es-Salam using the BDI had concluded that depression in their PD cohort was uncommon.42 Depression in our PD cohort was associated with significantly worse disease severity and disability as has been reported by many studies.43–47 As expected, our PD cases with coexisting cognitive impairment and depression had a worse disease profile (disability and severity). Veiga et al.48 in a tertiary clinic setting (like ours) in Brazil found a frequency of 42% and their depressed PD cohort also had worse motor function severity) – ADL was however not assessed. Previous studies on relationship between depression and PD severity have given conflicting results. Though the majority of studies report a positive relationship, some report no correlation.49
CONCLUSION
This study highlights the relatively high frequencies of both cognitive impairment and depression in our PD patients, and it provides a basis for promoting routine screening for these important nonmotor manifestations that can negatively impact outcome, but for which useful pharmacological and non-pharmacological interventions exist.
We acknowledge the fact that our sample size was small and thus refer to these findings as preliminary, suggesting the need for more elaborate studies, preferably in community-based PD cases, to establish more representative data.
In addition, our cohort was derived from PD patients attending tertiary care and inherently representing more symptomatic cases. However, we utilized a control group and standardized measures including grading and staging our cases in order to explore cognitive profile and depression across the spectrum of PD that would be encountered in the population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Global Parkinson's Disease Survey Steering Committee. Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord. 2002;17:60–7. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL. Intellectual impairment in Parkinson's disease: clinical, pathological and biochemical correlations. J Geriatr Psychiatry Neurol. 1988;1:24–36. doi: 10.1177/089198878800100106. [DOI] [PubMed] [Google Scholar]
- 3.Osuntokun BO, Bademosi O. Parkinsonism in the Nigerian African: a prospective study of 217 patients. East Afr Med J. 1979;56:597–607. [PubMed] [Google Scholar]
- 4.Akinyemi RO, Okubadejo NU, Akinyemi JO, Owolabi MO, Owolabi LF, Ogunniyi A. Cognitive dysfunction in Nigerians with Parkinson's disease. Mov Disord. 2008;23:1378–83. doi: 10.1002/mds.22087. [DOI] [PubMed] [Google Scholar]
- 5.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;23:183–9. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 6.Cummings JL, Masterman DL. Depression in patients with Parkinson's disease. Int J Geriatr Psychiatry. 1999;14:711–8. [PubMed] [Google Scholar]
- 7.Jellinger KA. Post mortem studies in Parkinson's disease-is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, Kleiner- Fisman G, et al. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson's disease. J Nucl Med. 2005;46:227–32. [PubMed] [Google Scholar]
- 9.Malhi GS, Berk M. Does dopamine dysfunction drive depression? Acta Psychiatr Scand Suppl. 2007;433:116–24. doi: 10.1111/j.1600-0447.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.Ziemssen T, Reichmann H. Non-motor dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 2007;13:323–32. doi: 10.1016/j.parkreldis.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. The prevalence of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz CG, Stebbins GT, Chmura TA, Fahn S, Klawans HL, Marsden CD. Teaching tape for the motor section of the unified Parkinson disease rating scale. Mov Disord. 1995;10:263–6. doi: 10.1002/mds.870100305. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–34. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–8. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, LeWitt PA, Weiderman M. Standardized training tools for the UPDRS activities of daily living scale: newly available teaching program. Mov Disord. 2003;18:1455–8. doi: 10.1002/mds.10591. [DOI] [PubMed] [Google Scholar]
- 16.Okulate GT, Jones OB. Two depression rating instruments in Nigerian patients. Niger Postgrad Med J. 2002;9:74–8. [PubMed] [Google Scholar]
- 17.Thanvi BR, Munshi SK, Vijaykumar N, Lo TC. Neuropsychiatric non-motor aspects of Parkinson's disease. Postgrad Med J. 2003;79:561–5. doi: 10.1136/pmj.79.936.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hietanen M, Teravaine H. The effect of age of disease onset in neuropsychological performance in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:244–9. doi: 10.1136/jnnp.51.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin WE, Lowenstein RB, Resch JA, Barber AB. Parkinson's disease: Clinical analysis of 100 patients. Neurology. 1973;23:783–90. doi: 10.1212/wnl.23.8.783. [DOI] [PubMed] [Google Scholar]
- 20.Emre M. Dementia associated with Parkinson's disease. Lancet Neurol. 2003;2:229–37. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 21.Hall KS, Hendrie HC, Brittain HM, Norton JA, Rodgerss DD, Prince CS, et al. The development of a Dementia Screening Interview in two distinct languages. Int J Methods Psych Res. 1993;3:1–28. [Google Scholar]
- 22.Hall KS, Gao S, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI“D”); performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15:521–31. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Gureje O, Unverzergt FW, Osuntokun BO, Hendrie HC, Baiyewu O, Ogunniyi A, et al. The CERAD Neuropsychological Test Battery: norms from a Yoruba-speaking Nigerian sample. West Afr J Med. 1995;14:29–33. [PubMed] [Google Scholar]
- 24.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127:550–60. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 25.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson's disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 26.Grabowski TJ, Anderson SW, Cooper GE. Disorders of Cognitive Function. In: Mancall EL, editor. Continuum; Lifelong Learning in Neurology. Vol. 8. Philadelphia: Lipincott Williams and Wilkins; 2002. pp. 7–40. [Google Scholar]
- 27.Bak T. Cognitive Profiles of Parkinsonian Syndromes. Adv Clin Neurosci Rehabil. 2006;6:12–4. [Google Scholar]
- 28.Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25:1170–6. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahn DA, Sullivan EV, Sheer PK, Pfefferbum A, Heit G, Silverberg G. Differential Contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson's Disease. Arch Neuropsychol. 1998;13:575–83. [PubMed] [Google Scholar]
- 30.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HA, et al. Cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:1182–7. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, et al. Assessment of cognition in Parkinson's disease. Neurology. 2003;61:1222–8. doi: 10.1212/01.wnl.0000091864.39702.1c. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KE. Dementia in Parkinson's disease. Curr Treat Options Neurol. 2004;6:201–7. doi: 10.1007/s11940-004-0012-9. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs GA, Gemende I, Herting B, Lemke MR, Oehlwein C, Reichmann H, et al. Dementia in idiopathic Parkinson's syndrome. J Neurol. 2004;251(Suppl 6: VI):28–32. doi: 10.1007/s00415-004-1607-5. [DOI] [PubMed] [Google Scholar]
- 34.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–10. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 35.Bosboom JL, Stoffers D, Walters ECh. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111:1303–15. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 36.Zung WW. A Self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 37.Schrag A, Barone P, Brown RG, Leentjens AF, MacDonald WM, Strakstein S, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord. 2007;22:1077–92. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13:508–15. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]
- 39.Brodaty H, Luscombe G, Peisch C, Anstey K, Andrews G. A 25 year longitudinal comparison study of the outcome of depression. Psychol Med. 2001;31:1347–59. doi: 10.1017/s0033291701004743. [DOI] [PubMed] [Google Scholar]
- 40.Shumway M, Sentell T, Unick G, Bamberg W. Cognitive complexity of self-administered depression measures. J Affect Disord. 2004;83:191–8. doi: 10.1016/j.jad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Dotchin CL, Jusabani A, Walker RW. Non-motor symptoms in a prevalent population with Parkinson's disease in Tanzania. Parkinsonism Relat Disord. 2009;15:457–60. doi: 10.1016/j.parkreldis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Matuja WB, Aris EA. Motor and non-motor features of Parkinson's disease. East Afr Med J. 2008;1:3–9. doi: 10.4314/eamj.v85i1.9599. [DOI] [PubMed] [Google Scholar]
- 43.Papapetropoulos S, Ellul J, Argyriou AA, Chroni E, Lekka NP. The effect of depression on motor function and disease severity of Parkinson's disease. Clin Neuro Neurosurg. 2006;108:465–9. doi: 10.1016/j.clineuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc. 2004;52:784–8. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 45.Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry. 1992;149:443–54. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 46.Menza MA, Mark MH. Parkinson's disease and depression: the relationship to disability and personality. J Neuropsychiatry Clin Neurosci. 1994;6:165–9. doi: 10.1176/jnp.6.2.165. [DOI] [PubMed] [Google Scholar]
- 47.Oertel WH, Hoglinger GU, Caraceni T, Girotti F, Eichhorn T, Spottke AE, et al. Depression in Parkinson's disease. An update. Adv Neurol. 2001;86:373–83. [PubMed] [Google Scholar]
- 48.Veiga BA, Borges V, Silva SM, Goulart Fde O, Cendoroglo MS, Ferraz HB. Depression in Parkinson's disease: clinical-epidemiologic correlates and comparison with a controlled group of non-parkinsonian geriatric patients. Rev Bras Psiquiatr. 2009;31:39–42. doi: 10.1590/s1516-44462009000100010. [DOI] [PubMed] [Google Scholar]
- 49.Poewe W, Luginger E. Depression in Parkinson's Disease: impediments to recognition and treatment options. Neurology. 1999;52(7Suppl 3):S2–6. [PubMed] [Google Scholar]


