Abstract
Recent advances in stem cell technology have enabled large scale production of human cells such as cardiomyocytes, hepatocytes and neurons for evaluation of pharmacological effect and toxicity of drug candidates. The assessment of compound efficacy and toxicity using human cells should lower the high clinical attrition rates of drug candidates by reducing the impact of species differences on drug efficacy and toxicity from animal studies. Methyl-β-cyclodextrin (MBCD) has shown to reduce lysosomal cholesterol accumulation in skin fibroblasts derived from patients with Niemann Pick type C disease and in the NPC1−/− mouse model. However, the compound has never been tested in human differentiated neurons. We have determined the cholesterol reduction effect of MBCD in neurons differentiated from human neural stem cells and commercially available astrocytes. The use of NSCs for producing differentiated neurons in large quantities can significantly reduce the production time and enhance the reproducibility of screening results. The EC50 values of MBCD on cholesterol reduction in human neurons and astrocytes were 66.9 and 110.7 µM, respectively. The results indicate that human neurons differentiated from the NSCs and human astrocytes are useful tools for evaluating pharmacological activity and toxicity of drug candidates to predict their clinical efficacy.
Keywords: induced pluripotent stem cells, neural stem cells, human neurons, astrocytes, skin fibroblasts, methyl-β-cyclodextrin
INTRODUCTION
Cell-based assays are commonly used in early drug discovery for high throughput screening (HTS) to identify lead compounds. They are also extensively employed in secondary and tertiary screens to confirm the activities of compounds that are initially identified from HTS using biochemical assays 1; 2. Currently, cells primarily used for compound screens are either immortalized cell lines or primary cells freshly isolated from tissues. Both primary and immortalized cells can be derived from various species, including rodents (hamster, mouse and rat) and humans, as well as from distinct tissues such as brain, heart, liver, muscle and skin. While the compound activity data obtained from animal cells is useful for guidance of drug dosing in animal model studies, compound potencies determined from human cells are expected to better predict the drug efficacy and toxicity in human clinical trials. Immortalized human cell lines are commonly cancerous and/or have accumulated significant genetic mutations. Hence, these do not provide valid model of the biological and pharmacological effects of a given compound 3. Human blood cells and skin fibroblasts are generally accessible; however, most primary cell types are not available in large quantities needed for drug screens or are prohibitively expensive. The recent availability of differentiated neurons derived from human induced pluripotent stem cells (iPSCs) provides a new paradigm for the use of human cells to determine the efficacy and potential cytotoxicity of drug candidates4; 5.
Niemann Pick Type C (NPC) disease is caused by mutations in either one of the two genes encoding the lysosomal cholesterol binding proteins NPC1 or NPC2 6; 7. NPC disease is characterized by lysosomal accumulation of unesterified cholesterol and other lipids in many cell types. Lysosomal accumulation results from the impaired transport of unesterified cholesterols from the late endosomes and/or lysosomes to the plasma membrane, trans-Golgi network and endoplasmic reticulum. The clinical manifestation of NPC disease includes progressive neurodegeneration and is often accompanied by hepatosplenomegaly. The onset of symptoms generally occurs in early childhood, leading to death within a decade. Currently, there is no cure or effective treatment for NPC disease.
Cholesterol accumulation in late endosomes and lysosomes is a direct disease manifestation that can be used as a phenotypic screen to identify cholesterol-reducing compounds8. Skin fibroblasts derived from NPC patients exhibit significant lysosomal cholesterol accumulation, and thus can serve as a cell-based disease model for compound screens. A recent high-throughput screen (HTS) using the NPC patient fibroblasts led to identification of several compounds, which significantly reduced cholesterol accumulation in late endosomes and lysosomes8. However, while neuronal degeneration is a major cause of death in NPC patients, cholesterol accumulation in skin fibroblasts does not directly contribute to the disease pathogenesis. Human neurons would thus be more desirable as a cell-based model for compound screens to evaluate activity and neuronal toxicity of drug candidates. Here, we report the application of human neurons differentiated from iPSCs derived neural stem cells (NSCs) as a model system to evaluate the pharmacological activity and cytotoxicity of MBCD. Using a biochemical cholesterol assay, the potency of MBCD has been measured in these cells and compared to skin fibroblasts, astrocytes, and a neuroblastoma cell line. We propose that the human differentiated neurons can be employed as a new screen paradigm for evaluation of compound activity and toxicity.
MATERIALS AND METHODS
Amplex-red cholesterol assay kit (catalog #: A12216) was purchased from Invitrogen. ATPlite cell viability assay kit (catalog #: 6016941) was obtained from PerkinElmer. DRAQ5 nuclear dye was obtained from Cell Signaling. Primary antibodies were purchased from GeneTex (Irvine, CA) and Cell Signaling Technology (Danvers, MA). Methyl-β-cyclodextrin (MBCD) was obtained from MP Biomedicals (Solon, OH). DyLight ® conjugated fluorescent rabbit & mouse antibodies were purchased from Epitomics (Burlingame, CA). The specific antibodies for Oct4, Sox1, Nanog and Neurofilament (NF) were obtained from Cell Signaling Technology. The antibodies for Nestin, microtubule-associated protein 2 (MAP2), and β-tubulin class III (TUJ-1) were purchased from Genetex. The 96-well black clear bottom plates (catalog #: 655090) were purchased from Greiner Bio-One (Monroe, NC).
Cells and cell culture
Human skin fibroblasts
The wild type (GM05659) and NPC1 (GM03123) human skin fibroblast cell lines were purchased from the Coriell Cell Repository (Camden, NJ). The human primary skin fibroblasts are derived directly from skin biopsies and have not been transformed although they can be passaged multiple times. The fibroblasts were cultured in DMEM medium (Invitrogen, cat# 11995-040) supplemented with 10% fetal bovine serum (FBS), 100 unit/ml penicillin in a humidified incubator with 5% CO2 at 37°C. For imaging experiments, cells were seeded at 1500 cells/well in 100 µl medium in the black/clear bottom, tissue culture treated 96-well plates and cultured overnight. Cells were seeded at 3000 cells/well in 100 µl medium in 96-well plates and cultured overnight before the LysoTracker fluorescence intensity experiment.
Neurons differentiated from human NSCs
Prior to cell plating, the dishes were coated with CELLstart from Invitrogen at 1:100 dilution in DPBS containing calcium and magnesium. Plates were rinsed with DPBS before cell seeding. The following method was used to coat plates for differentiation (1) the surface of plates were coated with poly-L-ornithine working solution at 20 µg/ml in distilled water (14 ml for T-75, 7 ml for T-25, 3.5 ml for 60-mm dish, 2 ml for 35-mm dish) and incubated overnight at room temperature, (2) the poly-L-ornithine-coated plates were washed 3 times with distilled water, and then coated with laminin working solution at 10 µg/mL in D-PBS without calcium or magnesium (14 ml for T-75, 7 ml for T-25, 3.5 ml for 60-mm dish, 2ml for 35-mm dish), (3) the laminin coated plates were incubated for 3 hours at 37°C before use or stored wrapped in parafilm for a week at 4°C.
Neural stem cells (NSC), originally derived from normal human iPSCs, were obtained from xCell Science (Novato, CA) and expanded in CellStart coated dishes. NSCs were cultured in Neurobasal media supplemented with B27, Glutamine, Non essential amino acids (NEAA) and 20ng/ml fibroblast growth factor (bFGF). To initiate differentiation of NSCs, cells were plated on poly-L-ornithine and laminin-coated 96 well plates in medium supplemented with 10ng/ml brain-derived neurotrophic factor (BDNF) and 10ng/ml glial-derived neurotrophic factor (GDNF). Cells were grown in differentiation medium for 10–12 days before proceeding to compound treatment and immunocytochemistry.
Astrocytes
Human Astrocytes (catalog # K1884, Invitrogen) are derived from normal human brain tissue and were cultured as per instructions provided by the manufacturer. Briefly, a single vial of astrocytes was immediately suspended in complete astrocyte medium (DMEM, 1X N-2, 10% FBS) and seeded into a single well of 6-well tissue culture-treated plate (catalog #353046, BD Biosciences) that had been coated with Geltrex (catalog #12760, Invitrogen). Cells were allowed to adhere for at least 24 hours at 37°C with 95% humidity and 5% CO2. The medium was exchanged every other day.
SH-SY5Y neuroblastoma cells
SH-SY5Y cell line (catalog #: CRL-2266) was obtained from ATCC. The base medium for this cell line is a 1:1 mixture of ATCC-formulated Eagle's Minimum Essential Medium, Catalog #30–2003, and F12 Medium. To make the complete growth medium, fetal bovine serum was added to a final concentration of 10% to the base medium.
Neuronal and stem cell markers
Immunofluorescent staining of differentiated neurons in 96-well plates
Cells were fixed in 100 µl 4% paraformaldehyde (PFA) for 10–20 minutes at room temperature, and then rinsed briefly with PBS before permeabilization with 0.3% Triton X-100 for 15 minutes. Non-specific protein-protein interactions were blocked with 5% donkey serum and 1% BSA. Differentiated neurons were incubated with primary antibody overnight at 4°C, unbound antibody was removed by PBS washing, and the bound antibody was detected using a goat anti-rabbit secondary antibody conjugated with DyLight 594 (catalog # 3066-1, 1:200 dilution, Epitomics). After incubation, cells were rinsed twice with PBS and nuclei were stained with Draq5 for 20 minutes and immediately imaged using an InCell2000 Analyzer (GE Healthcare) with 20× objective lens and filter sets for Cy5 and Texas Red. All secondary antibodies were tested for cross reactivity and nonspecific immunoreactivity.
Immunofluorescence staining of astrocytes in 96-well plates
Human Astrocytes (Invitrogen) were seeded into wells of a BD PureCoat black, clear bottom amine-coated plate (BD Biosciences #354717) at a density of 6400 cells/well in 100 µl of Complete Astrocyte Media (6.4× 104 cells/ml). After incubation for 6 days at 37 °C, 95% humidity and 5% CO2, immunofluorescence staining was performed as described above, with minor changes. Astrocytes were incubated in 100 µl of primary antibody solution (1:400 dilution of rabbit anti-GFAP, Sigma #G9269) and allowed to incubate overnight at 4°C and bound antibody was detected with a goat anti-rabbit secondary antibody conjugated with Dylight 594 (Epitomics). Nuclei were stained with Hoechst 33342. Cells were imaged using the InCell2000 Analyzer with 20× objective lens and the filter sets for DAPI and Texas Red.
Amplex-red cholesterol assay
Amplex-Red Cholesterol Assay Kit (Invitrogen) was used to measure the total cholesterol in patient cells. The unesterified cholesterol was determined using the same kit but without acid lipase. Esterified cholesterol was determined as the difference between the total and unesterified cholesterol values. The cells were seeded in black, tissue culture-treated 96-well plates at a density of 2500 cells/well in 100 µl medium and cultured for 24 hours. The medium in the assay plates was removed and replaced with 100 µl/well of compound dissolved in medium. After incubation for 1 or 3 days, the cells were washed twice with HBSS and 100 µl/well of reagent mixture from the cholesterol assay kit was added. The resulting fluorescence intensity after one hour incubation at 37°C was measured with an excitation wavelength of 560 (±10) and emission wavelength of 590 (±10) in a ViewLux plate reader (PerkinElmer).
ATP content assay
ATP content assay kit (ATPLite, PerkinElmer) was used to monitor compound cytotoxicity. Cells were seeded in white solid-bottom 96-well plates and treated with compounds as described above for the cholesterol assay. After incubation with compound for 1 or 3 days, 100 µl/well of reagent mixture (prepared according to the manufacturer’s instructions) was added to the assay plates followed by incubation at room temperature for 2 minutes. The luminescence signal was determined in the luminescence mode of the ViewLux plate reader.
Filipin staining
At the concentration used in the assay, Filipin dye (50 ng/ml) stains unesterified cholesterol in cells 9. Cells were washed twice with PBS and fixed with 100 µl/well of a 3.2% formaldehyde solution including Hoechst 33342 stain at room temperature (RT) for 30 minutes. After washing twice with PBS, the cells were stained with 100 µl/well of 50 ng/ml Filipin solution (freshly-dissolved in DMSO at 10 mg/ml and then diluted in PBS) at RT for 1 hour. The cells were washed twice with PBS to remove filipin solution and the plates were stored at 4°C for further imaging analysis. For imaging, cells were stained with 100 µl/well 2 µM of CellMask-Red (Invitrogen) in PBS at room temperature for 1 hour. The plates were imaged using the Incell2000 Analyzer with 20× or 40× objective lens and the filter sets for DAPI and TRITC to visualize Filipin and CellMask staining, respectively.
Data analysis
Concentration-response curves were analyzed and EC50 values calculated using Prism software (GraphPad, San Diego, CA). The bottom value in the four parameter fit was fixed at 50 % since the concentration-response curves of MBCD showed an incomplete inhibition at the bottom plateau due to the limited high concentrations used. Results in the figures are expressed as mean of triplicates ± SD unless they are specified.
RESULTS
Mature cells differentiated from iPSCs such as neurons, cardiomyocytes, and hepatocytes are desirable for use in compound screens. However, the process of generating differentiated cells from iPSCs usually takes 6 to 12 weeks before the cells are ready for compound screens. Currently, the prolonged time and complicated procedure limit broad application of stem cell derived human cells for drug discovery. We have explored the use of multi-potent neural stem cells (NSCs) as the starting point to obtain differentiated neurons for compound screens which significantly reduces the preparation time to obtain differentiated neurons. This approach begins with frozen iPSCs derived NSCs that can be plated into 96-well plates for continuous differentiation into neurons in the proper cell culture conditions. After 2–3 weeks of culture in differentiation medium, the cells are ready for characterization and compound screens. To validate this approach, we have examined the activity of MBCD on the reduction of cellular cholesterol levels in the differentiated neurons in comparison with the results obtained from primary skin fibroblasts, astrocytes and other cell lines.
Characterization of neurons differentiated from iPS cells
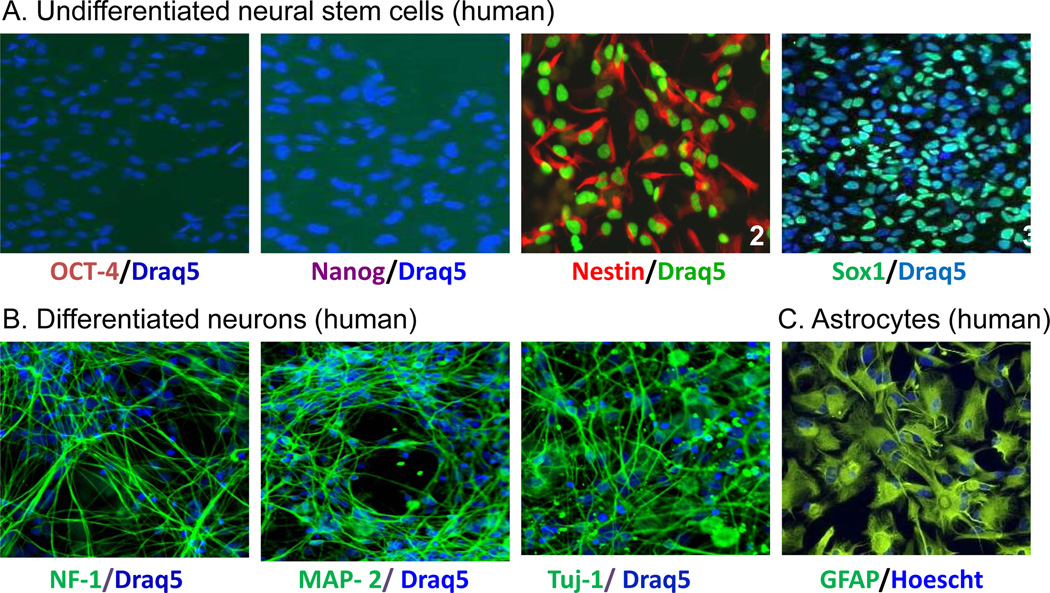
In contrast to immortalized cell lines and primary cells, it is critical to characterize the differentiated cells to confirm the cell lineage before their use in cell-based and cytotoxicity assays. Molecular markers that are commonly used for characterizing neural stem and progenitor cells are Nestin, an intermediate filament protein, and Sox2 and Sox1 (transcription factors) 10; 11. We first performed immunofluorescence staining of neural stem cells using anti-Oct4 and anti-Nanog antibodies to test for markers of pluripotency. The lack of Oct4 and Nanog staining is consistent with a more restricted lineage status (multipotency instead of pluripotency) of neural stem cells compared to iPSCs (Fig. 1A). The NSCs, maintained in media supplemented with bFGF (see Material & Methods), were positive for Nestin and Sox1, two markers of early neuroepithelial cells, prior to directed differentiation (Fig. 1A).
FIG. 1.
Fluorescence images of specific markers for stem cells and differentiated neurons. (A) Undifferentiated neural stem cells were positively stained for the NSC markers Nestin andSox1. (B) Differentiated neurons were positively stained for the neuronal marker NF-L, MAP2 & β-tubulin III. (C) Astrocytes were positively stained with GFAP. The colored words under each image represent the specific marker stained in the cells.
Switching of NSC media to media containing 10 ng/ml BDNF and 10 ng/ml GDNF (instead of bFGF) resulted in the differentiation of the majority of cells to a typical neuronal phenotype with a dense network of neurites. The neural lineages of the differentiated cells were analyzed by immunofluorescence staining using cell-type specific markers. Phenotypically, these neurons were positive for β-tubulin III, microtubule associated protein 2 (MAP2) and Neurofilament-1 (Fig. 1B). No immunoreactivity was detected in the neuronal cultures for glial fibrillary acidic protein (GFAP), an astrocyte cell marker (data not shown). Immunofluorescence staining data revealed an abundance (90%) of neurons in our culture and correlated well with the high expression of β-tubulin III by real time PCR (data not shown). Commercially available astrocytes were validated by immunofluorescence staining for GFAP (Fig. 1C).
MBCD reduces cellular cholesterol levels in both control and NPC1 fibroblasts
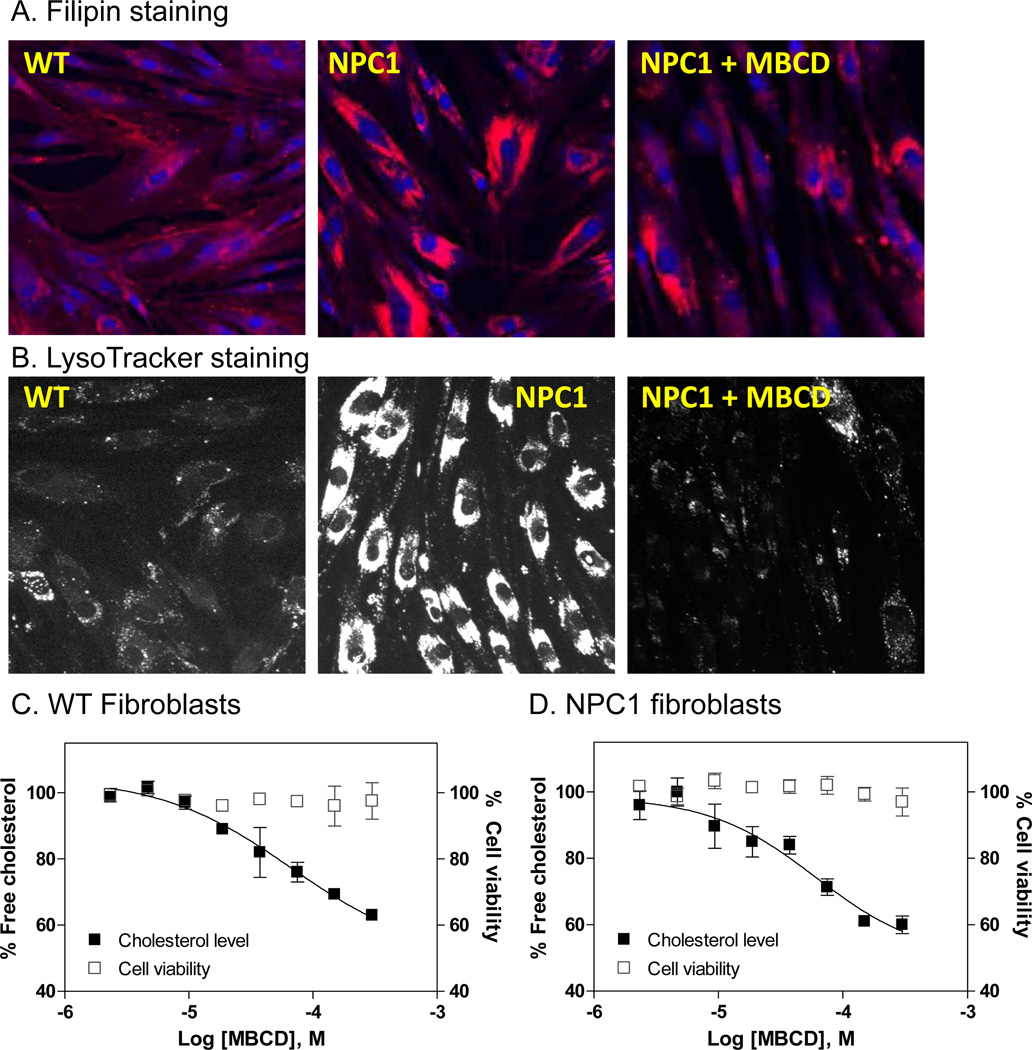
β-cyclodextrins have been reported to reduce lysosomal cholesterol accumulation in skin fibroblasts derived from NPC patients as well as in the brain of a Npc1−/− mouse model, both detected by the Filipin staining assay 12–14. The filipin dye stains unesterified free cholesterol in cells and is used for diagnosis of NPC disease 9. NPC1 fibroblasts exhibited excessive increase in Filipin fluorescence staining compared to the control cells (Fig. 2A), consistent with the published observation that the late endosomes and lysosomes are significantly enlarged in NPC1 cells due to the accumulation of free cholesterol and other lipids 15. Treatment of NPC1 fibroblasts with 300 µM MBCD significantly reduced the fluorescence staining of filipin (Fig. 2A). We then used a LysoTracker dye that specifically stains the acidic compartments in cells, which includes late endosomes and lysosomes, to confirm MBCD’s effect 15. As in the filipin staining experiment, the LysoTracker dye staining was significantly reduced after the treatment with 300 µM MBCD (Fig. 2B). These results confirmed the effect of MBCD on the reduction of cholesterol accumulation and lysosome size in NPC1 skin fibroblasts.
FIG. 2.
Effect of MBCD on skin fibroblasts derived from NPC1 patients. (A) Images of filipin staining. Treatment with 300 µM MBCD reduced the cholesterol accumulation in NPC1 skin fibroblasts. Filipin (red) stains intracellular cholesterol-laden domains and DAPI (blue) stains nuclei). (B) Images of LysoTracker staining. Treatment with 300 µM MBCD significantly reduced LysoTracker staining in NPC1 skin fibroblasts, which indicated the reduced lysosome size after the treatment. Concentration-response curve of MBCD measured in a biochemical cholesterol assay in NPC1 fibroblasts (C) and control fibroblasts (D). The solid symbols represent the percentage unesterified free cholesterol in cells that was normalized (the vehicle treated cells were used as 100% control). The open symbols represent percentage cell viability that was calculated using the vehicle treated cells as the 100% viable cells. A full inhibition of cellular cholesterol level by MBCD could not be reached because MBCD showed cytotoxicity at concentrations higher than 300 µM.
We then employed an additional assay to confirm our findings – specifically, a biochemical assay to determine the cholesterol reduction effect of MBCD in both NPC1 and control fibroblasts. The cholesterol assay uses an enzyme-coupled reporting system that consists of cholesterol oxidase, horseradish peroxidase and Amplex-red dye that involves cell wash step to remove cell culture medium before the assay. As predicted, MBCD reduced the cellular unesterified cholesterol levels in both control and NPC1 fibroblasts in a concentration-dependent manner though a complete inhibition curve at lower plateau could not be achieved due to the cytotoxicity of MBCD at concentrations higher than 300 µM. The EC50 value of MBCD for reduction of cellular cholesterol level was 66.5 µM (Fig. 2C) in the control fibroblasts that is similar to the EC50 of 60.5 µM determined in the NPC1 fibroblasts (Fig. 2D). Thus, the results indicate that normal cells could also be used to evaluate the cholesterol reduction effect of β-cyclodextrins or other compounds.
MBCD reduces cellular cholesterol levels in differentiated neurons and additional cell types
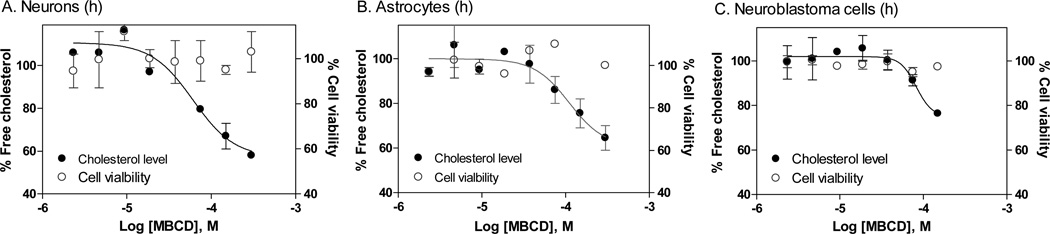
Since NPC disease primarily affects the brain with neuronal degeneration as the cause of lethality, we wanted to examine the cholesterol reduction effect of MBCD in human neurons, and more desirably in NPC disease neurons. In human neurons, the effect of β cyclodextrins on cholesterol accumulation in late endosomes and lysosomes has not been studied because these cells are not generally available. An additional layer of technical difficulty applies to differentiated neurons, which are more fragile and may not be able to tolerate experiments that require multiple steps of cell wash, fixation and dye staining included in the procedure of the Filipin staining assay. At this stage, iPSCs from NPC patients are not available though attempts are being made to produce such cells (unpublished data). As an alternative approach, we determined the effect of MBCD in normal human neurons differentiated from iPSCs. Consistent with the data in fibroblasts, MBCD reduced the cellular cholesterol levels in differentiated neurons derived from normal human iPSCs with an EC50 value of 66.9 µM (Fig. 3A and Table 1). The EC50 values reported here are similar to that determined in patient derived fibroblasts in this study (Fig. 2) or reported elsewhere 16. In human astrocytes, MBCD also reduced cellular cholesterol levels with an EC50 value of 110.7 µM (Fig. 3B). We then examined the effect of MBCD in a human neuroblastoma line, SH-SY5Y. The EC50 value of MBCD in the neuroblastoma cells was 81.9 µM (Fig. 3C), similar to those with the normal skin fibroblasts. Since MBCD is cytotoxic at concentrations higher than 300 µM, its activity in the cholesterol assay was not shown for these concentrations, thus resulting in an incomplete concentration-response curve that is missing the lower plateau. We also monitored the cytotoxicity of MBCD using an ATP content assay in parallel to the biochemical cholesterol assay to ensure the compound concentration used did not cause cytotoxicity.
FIG. 3.
Concentration-response curves of MBCD on reduction of cellular cholesterol levels in human neurons (A), astrocytes (B), and a neuroblastoma cell line, SH-SY5Y (C). The solid symbols represent the percentage unesterified free cholesterol in cells that was normalized (the vehicle treated cells were used as 100% control). The open symbols represent percentage cell viability that was calculated using the vehicle treated cells as the 100% viable cells. At the concentrations used in the experiment, MBCD did not cause cytotoxicity, however, complete inhibition of cellular cholesterol level could not be reached because MBCD at concentrations higher than 300 µM exhibited cytotoxicity.
Table 1.
Summary of MBCD activities on reduction of cellular cholesterol.
| EC50 (µM, cholesterol reduction) | |
|---|---|
| WT fibroblasts | 65.5 (48.8 to 90.5) |
| NPC1 fibroblasts | 60.5 (22.6 to 161.9 |
| Differentiated neurons | 66.9 (27.4 to 162.9) |
| Astrocytes | 110.7 (30.9 to 369.1) |
| Neuroblastoma cells | 81.9 (64.3 to 104.5) |
In parenthesis: 95 % confidence intervals
Together, our results confirmed the activity of MBCD on reduction of cellular cholesterol levels in human differentiated neurons and astrocytes. The data also demonstrated that human differentiated neurons (derived from NSCs), are a useful tool for confirmation of drug efficacy in human cells, as well as measuring compound cytotoxicity.
DISCUSSION
In last two decades, high throughput screening of molecular targets against large compound collections has become a major approach of drug discovery. Hits identified from the primary screen are confirmed in the secondary screens for target activity and selectivity. One or a few structurally appealing lead compounds subsequently undergo chemical optimization to improve potency and selectivity. The optimized compounds are then validated in the tertiary screens which are more disease relevant and often use primary cells and cell-based disease models. Finally, a few top lead compounds are tested in the animal models before entering preclinical development for drug safety and ADME properties. A recent review of failed drugs in clinical studies revealed that overall 31% of phase II and 61% of Phase III trials failed due to lack of human efficacy (data from 2007–2011) 17; 18. These once “promising” drug candidates that have good drug efficacy in animal models may have a different effect in human patients, probably due to the species difference 19. Thus, the current animal models may not always be good at predicting human efficacy, especially for certain diseases such as cancer and neuronal disorders. Therefore, the use of human cells and patient derived disease cells for drug screens and validation of lead compounds has emerged as an alternative method to animal models that may improve the success rate of drug discovery and development.
Dependent on the origins of iPSCs generated, there are two major types of differentiated human cells - normal cells and disease-specific cells. The normal human iPSCs are readily available from several commercial sources such as the NIH sponsored Coriell Cell Repository (http://www.coriell.org/stem-cells) and American Type Culture Collection (ATCC). Normal iPSCs differentiated human cells such as cardiomyocytes, hepatocytes and neurons have recently become available. Although several disease-related iPSCs have been generated and have become available, there have been many difficult cases for generation of iPSCs from patient cells. For example, the iPSCs from NPC disease cells have not been reported. Even though the disease-related iPSCs have been made, many of them do not exhibit the disease phenotype in the differentiated cells. In addition, large scale production of differentiated cells from disease related iPSCs may not be feasible in many cases. Therefore, the use of differentiated cells from normal human iPSCs, as used in this study, is a more practical approach for drug screens until the issues associated with disease-related iPSCs can be addressed. Specifically, using frozen cells that are already at an intermediate stage of differentiation, such as NSCs, for continued differentiation in microtiter plates can reduce the technical difficulties associated with a 6 week or longer differentiation process in microtiter plates, in addition to potentially reducing batch-to-batch variability in the differentiation process.
The generation of large quantities of differentiated cells with reproducible properties poses considerable challenges. In addition, it is not easy to produce disease models using cells differentiated from iPSCs. Several approaches may be employed for the assay development of cell based disease models. First, disease models can be generated by addition of toxins or chemicals that damage cells or block a specific pathway in cells. In neuronal cells, the H2O2 challenge 20, serum deprivation 21 and toxins such MPP+ 22 are commonly used to generate a model for examining neuronal protective activity of compounds. Second, a gene expression or pathway reporter assay can be set up in differentiated cells for the measurement of compound activity. Third, the cellular level of a protein, lipid or metabolite in differentiated cells can be utilized for the determination of specific therapeutic effect of compounds. In this study, we used a biochemical cholesterol assay to measure the effect of compounds on reduction of cellular cholesterol levels in human neurons differentiated from normal iPSCs.
Neuronal degeneration and massive loss of Purkinje cells in brain are closely related to the pathogenesis of NPC disease 7. Although the enlarged liver and spleen are often observed in NPC patients, peripheral symptoms are usually rare. Thus, relevant drug therapies for the treatment of NPC disease have to target the central nervous system as the pathophysiology of NPC disease mainly affects the brain. In addition, these drug candidates should be able to penetrate blood-brain barrier to exert its pharmacological action in the central nervous system. Although skin fibroblasts derived from NPC patients have been used for compound screens, neurons differentiated from human iPSCs may be more useful as the secondary assays for evaluating drug efficacy and neuronal toxicity, in addition to confirming the pharmacological action of drug candidates.
MBCD, a β-cyclodextrin, was previously reported to reduce lysosomal cholesterol accumulation in NPC1 fibroblasts and in the brain tissue of NPC1 −/− mouse model. The lifespan of these mice was also doubled after the treatment with β-cyclodextrins 12; 14. The mechanism of action of β-cyclodextrin on the reduction of lysosomal accumulation of cholesterol and other lipids is linked to a calcium dependent exocytosis of storage materials in the affected cells 23. It has also been reported that β-cyclodextrin enters the cells through endocytosis 16. Since the structure of β-cyclodextrin consists of seven sugars that are highly water soluble and impermeable to blood brain barrier, direct brain injection may be required for the therapeutic use of this compound in NPC patients. Our data demonstrates that 100 µM MBCD is nontoxic and it reduces cellular cholesterol accumulation in human neurons differentiated from iPSCs.
In conclusion, we have developed an approach to use human neurons differentiated from normal iPSCs in 96 well plate format to determine the efficacy of cellular cholesterol reduction and cytotoxicity of β-cyclodextrin compound. The application of starting neuronal differentiation from NSCs instead of iPSCs can significantly reduce the time required for cell differentiation and increase the reproducibility of cells used in a screen. As we demonstrate here for MBCD, efficacy and potential cytotoxicity of compounds can be measured in parallel in human neurons differentiated from normal iPSCs, thus providing proof-of-concept that normal iPSCs derived cells could provide valuable information for the assessment of human efficacy and cytotoxicity of drug candidates and guide future clinical development.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the Therapeutics for rare and neglected Diseases, National Center for Advancing Translational Sciences, National Institutes of Health. We would also like to thank Dr. Anand Swaroop for comments on the manuscript.
Abbreviations
- NPC1
Niemann- Pick type C disease
- iPSCs
induced pluripotent stem cells
- NSCs
neural stem cells
- MBCD
Methyl-β-cyclodextrin
- DPBS
Dulbecco's Phosphate-Buffered Saline
- MAP2
Microtubule-associated protein 2
- NF-L
Neurofilament –L
- bFGF
fibroblast growth factor
- BDNF
brain-derived neurotrophic factor
- MAP2
microtubule-associated protein 2
REFERENCES
- 1.Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 2.Michelini E, Cevenini L, Mezzanotte L, Coppa A, Roda A. Cell-based assays: fuelling drug discovery. Anal Bioanal Chem. 2010;398:227–238. doi: 10.1007/s00216-010-3933-z. [DOI] [PubMed] [Google Scholar]
- 3.Ebert AD, Svendsen CN. Stem cell model of spinal muscular atrophy. Arch Neurol. 2010;67:665–669. doi: 10.1001/archneurol.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells--opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 5.Sinnecker D, Dirschinger RJ, Goedel A, Moretti A, Lipp P, Laugwitz KL. Induced Pluripotent Stem Cells in Cardiovascular Research. Rev Physiol Biochem Pharmacol. 2012 doi: 10.1007/112_2012_6. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum AI, Maxfield FR. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116:789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum AI, Rujoi M, Huang AY, Du H, Grabowski GA, Maxfield FR. Chemical screen to reduce sterol accumulation in Niemann-Pick C disease cells identifies novel lysosomal acid lipase inhibitors. Biochim Biophys Acta. 2009;1791:1155–1165. doi: 10.1016/j.bbalip.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman AW, Demel RA, de Kruyff B, van Deenen LL. Studies on the biological properties of polyene antibiotics. Evidence for the direct interaction of filipin with cholesterol. J Biol Chem. 1972;247:1918–1929. [PubMed] [Google Scholar]
- 10.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 11.Swistowski A, Peng J, Han Y, Swistowska AM, Rao MS, Zeng X. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One. 2009;4:e6233. doi: 10.1371/journal.pone.0006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc Natl Acad Sci U S A. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci U S A. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 18.Arrowsmith J. Trial watch: phase III and submission failures: 2007–2010. Nat Rev Drug Discov. 2011;10:87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 19.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 20.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YM, Chung HT, Kim SS, Han JA, Yoo YM, Kim KM, Lee GH, Yun HY, Green A, Li J, Simmons RL, Billiar TR. Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling. J Neurosci. 1999;19:6740–6747. doi: 10.1523/JNEUROSCI.19-16-06740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng X, Chen J, Deng X, Liu Y, Rao MS, Cadet JL, Freed WJ. An in vitro model of human dopaminergic neurons derived from embryonic stem cells: MPP+ toxicity and GDNF neuroprotection. Neuropsychopharmacology. 2006;31:2708–2715. doi: 10.1038/sj.npp.1301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen FW, Li C, Ioannou YA. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS One. 2010;5:e15054. doi: 10.1371/journal.pone.0015054. [DOI] [PMC free article] [PubMed] [Google Scholar]