Abstract
Using the in vitro rearing system in conjunction with the hair tuft bioassay, NYDA and NYDA without fragrances formulations (92% wt:wt dimeticones) were 100% ovicidal (0% of treated eggs hatched) after an 8-h exposure of the eggs of the human head louse (Pediculus humanus capitis De Geer) following the manufacturer’s instructions. Comparatively, 78 and 66% of eggs similarly exposed hatched after distilled deionized water or Nix (1% permethrin) treatments, respectively. NYDA and NYDA without fragrances formulations were also statistically and substantially more ovicidal than either distilled deionized water or Nix treatments after 10, 30 min, and 1 h exposures. Only the 10 min exposure of eggs to NYDA and NYDA without fragrances formulations resulted in hatched lice that survived to adulthood (5–8% survival). Of the lice that hatched from eggs exposed to NYDA formulations for 10 min, there were no significant differences in the time it took them to become adults, female fecundity or the viability of eggs laid by surviving females. The longevity of adults, however, was reduced after the 10 min treatments of eggs with NYDA and NYDA without fragrances formulations compared with either the distilled deionized water or Nix treatments.
Keywords: ovicide, NYDA, dimeticones, Nix, human head louse
Pediculosis caused by human head lice is one of the most common parasitic infestation of humans and its occurrence is increasing, particularly among the homeless (Raoult and Roux 1999). When heavy infestations occur, pruritus, excoriation, urticaria, papules, exudations, erythema, eczema, lymphadenopathy, and scarring can appear (Mumcuoglu et al. 2009).
Insecticide resistance to currently-used pediculicides, including permethrin, synergized pyrethrins and malathion, has occurred worldwide (Gratz 1997), is increasing (Hodgdon et al. 2010, Marcoux et al. 2010) and is certainly contributing to increased incidences of pediculosis. Given this scenario, a crisis currently exists in the effective control of human lice at a time when the prevalence of pediculosis is increasing. The search for new and effective products with novel modes of action is therefore a critical need.
Because of increasing instances of resistance, particularly to the neurotoxic insecticides currently used as pediculicides, and the increasing scrutiny of the use of such products on children, there has been a trend, primarily in Europe, for the development of physical means to control head lice. Of these types of products, dimeticone-based antilouse products (silicone oils) have attracted a good amount of attention because of their low mammalian toxicity, novel modes of action that are not neurotoxic and the possibility that they will have a low potential for the development of resistance (Heukelbach et al. 2009).
To date, particularly in Europe, different dimeticone-based products are commercially available. Dimeticones are linear polydimethylsiloxanes (CH3SiO[SiO(CH3)2]nSi(CH3)2), where n is the number of repeating monomers [SiO(CH3)2] of varying chain length. The chain length substantially influences the molecular weight and the viscosity of the substance. Although dimeticones in general are known for their low surface tension, they can vary considerably in spreading characteristics. Of the different dimeticone-based products available, two products are better characterized scientifically in terms of their effectiveness and probable modes of action.
Hedrin 4% lotion (Thornton & Ross Ltd., Huddersfield, United Kingdom) is a 4% dimeticone lotion in 96% (wt:wt) decamethylcyclopentasiloxane (cyclomethicone D5). Head lice treated with this product are rapidly immobilized but small movements in their extremities over several hours indicate that death is delayed. Scanning electron microscopy coupled with X-ray microanalysis revealed that Hedrin 4% lotion was deposited in the spiracles, in some cases blocking the opening completely, and penetrated into the outer aspects of the tracheae (Burgess 2009). Given the slow onset of mortality, asphyxia was discounted as a mode of action. The inability of the louse to excrete the excess water acquired during blood feeding by transpiration via the spiracles has been suggested as a mode of action with death occurring by either prolonged immobilization or by the rupture of organs such as the gut.
The second dimeticone-based antilouse product (NYDA, G. Pohl-Boskamp GmbH&Co. Hohenlockstedt, Germany) contains a mixture of two dimeticones, one of low viscosity and the other of higher viscosity, at a final total concentration of dimeticones of 92% (wt:wt). Medium-chain length triglycerides, jojoba wax, and two fragrances make up the remaining constituents. Because of its superior spreading characteristics, NYDA rapidly enters the tracheal system, filling even the smallest branches (Richling and Bockeler 2008). Within 1 min of treatment with NYDA, lice do not show any major vital signs. This effect appears to be because of an interruption in the oxygen supply leading to suffocation. Subsequently, another NYDA-based formulation was developed without the fragrances for those individuals who prefer un-scented products. NYDA without fragrances also contains the two-phase dimeticone as well as medium-chain triglycerides and jojoba wax, but has no fragrances added. The difference between the composition of NYDA and NYDA without fragrances is made up by adjusting the medium-chain triglycerides, which is a common constituent of both formulations.
The ‘gold standard’ for any antilouse product being currently developed is that it should rapidly kill larvae and adult lice and must be ovicidal, eliminating the need of a second treatment. Initially, the developing embryo in the egg (nit) must be supplied with oxygen, principally via the aeropyles (tubules that transverse the chorion). Later, the developing tracheal system is established. The egg also processes a micropyle open-ing that allows the egg to be fertilized by sperm. It is therefore possible that both NYDA and NYDA with-out fragrances may be ovicidal because of their highly efficient spreading characteristics, leading to suffocation of the developing embryo via these exposure routes. In the current study, NYDA and NYDA with-out fragrances are examined to determine whether the absence of fragrances alters the ovicidal action of these formulations by treating three egg groups of different developmental stages (0–2, 3–5, and 5–8 d postoviposition). Different incubation times (10, 30 min, 1, and 8 h) with these two products were also investigated. During the study period, eggs and emerging larvae were kept on human hair tufts and maintained on the in vitro rearing system. Hatchability, survivorship to adulthood, developmental time to adulthood, longevity of adults, fecundity, and viability of eggs laid by surviving lice were assessed.
Materials and Methods
Human Head Lice
The SF-HL strain of permethrin-resistant human head lice (Pediculus humanus capitis De Geer, Phthiraptera: Pediculidae) was collected from infested children in Plantation and Homestead, FL, and maintained on an in vitro rearing system at the University of Massachusetts at Amherst, MA, as described by Yoon et al. (2006). Lice have been maintained without a human host on the in vitro rearing system for 24–36 generations. Permethrin-resistant lice have been selected periodically using 1% permethrin-treated filter papers (Yoon et al. 2006). Filter papers (35 mm diameter, Whatman No. 1) were immersed into 1% permethrin dissolved in acetone (wt: vol) for 10 s and air dried in a dark fume hood for 20–30 min. Mixed developmental stages (first-third instars and adults) were placed on the treated filter paper and exposed to permethrin for 5 h. Surviving lice were transferred back onto the in vitro rearing system. The SF-HL strain has been determined previously to be susceptible to Ovide (0.5% malathion), resistant to Nix (1% permethrin), and cross-resistant to DDT treatments (Yoon et al. 2003).
Chemicals
Formulations of NYDA and NYDA without fragrances were supplied by G. Pohl-Boskamp GmbH & Co. KG. Nix formulation (1% permethrin, Pfizer, Morris Plains, NJ) was obtained commercially (CVS, Amherst, MA).
Ovicidal Bioassay and the Determination of Developmental Parameters
Louse eggs deposited on human hair tufts were treated with NYDA with and without fragrances to assess their ovicidal properties. Distilled, deionized water (ddH2O) was used as a negative control. Nix formulation was used as a positive control. Eggs were laid on hair tufts (≈300 strands, 5 cm in length) over a 48 h period and collected from feeding cups containing 30 male and 30 female lice. The day that adults were placed into feeding cups was designated day 0 and the age of the developmental stage was determined from this date. After 48 h, adults were removed and the hair tufts with attached eggs were divided into three equal groups: group 1 (0–2 d-old eggs), group 2 (3–5 d-old eggs), and group 3 (6–8 d-old eggs). Group 1 was treated on day 2, group 2 was treated on day 5, and group 3 was treated on day 8 postoviposition. Tufts with attached eggs were immersed into 0.5 ml of the test treatments, swirled in a circular motion for 30 s to ensure complete egg coverage and then placed onto a glass petri dish for various exposure periods (10, 30 min, 1, and 8 h) at 31°C and 70–80% RH. At the end of the exposure period, treated tufts with attached eggs were immersed into 10 ml of diethyl ether for 5 s with agitation, immediately placed onto filter paper and dried under a stream of air (≈5 s). This treatment was necessary to remove excess formulation from hair tufts to assess the effect of different exposure times. Dried tufts with treated eggs were placed into covered sterile glass petri dishes and moved to an incubator at 31°C and 70–80% RH. All treatments were repeated three times with 60 eggs in each replicate. The number of lice that hatched from eggs was recorded daily and used to determine the percent hatchability of eggs. Undeveloped eggs and stillborn lice were recorded as dead. Hatched lice were placed onto new hair tufts that were treated, rinsed with diethyl ether, and maintain exactly as the initial treated hair tufts with attached eggs and moved to a feeding cup maintained on the in vitro rearing system. Survivorship through larval development and adulthood was determined daily. Development time from egg hatch to adult, longevity ofadults, fecundity, and percent hatch of eggs laid by surviving females were likewise recorded.
Statistical Analysis
Nonparametric Kruskal-Wallis (KW) tests were performed between treatments and controls to determine significant differences in hatchability and survivorship when there were instances of no variability in the data reported. One-way analysis of variance (ANOVA) tests were performed between treatments and controls to determine significant differences in hatchability and survivorship. Unless otherwise noted, a Tukey’s test was performed to determine differences between means if the overall F values of the ANOVA analysis were significant. Student’s t-tests were performed when only two groups of data could be compared (GraphPad Prism v.5.03, Graph-Pad Software, LA Jolla, CA). Statistical significance was established at the P < 0.05 levels for all tests.
Results
Hatchability of Treated Eggs
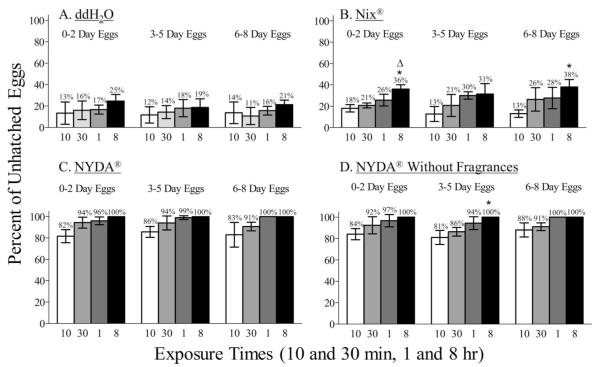
There were no significant differences in the percentages of unhatched eggs for any of the three egg groups after ddH2O treatments with exposure times from 10 min to 8 h (0–2 d: F = 1.2, df = 3, 59.75, P = 0.37; 3–5 d: F = 0.58, df = 3, 56.00, P = 0.65; 6–8 d: F = 1.2, df = 3, 50.00, P = 0.37) (Fig. 1A).
Fig. 1.
Percentages of unhatched human head louse eggs after treatments with ddH2O, Nix, NYDA, or NYDA without fragrances over time. An asterisk (*) indicates that the percentages of unhatched eggs were significantly increased compared with the 10 min exposures within a treatment group (ANOVA, P < 0.05). A triangle (Δ) indicates that the percentages of unhatched eggs were significantly increased compared with the 30 min exposures within a treatment group (ANOVA, P < 0.05).
The percentages of unhatched eggs from the 0–2, 3–5, and 6–8 d-old egg groups treated with Nix increased with longer exposure times. The percentage of unhatched 0–2 d-old eggs after the 8 h treatment was significantly greater (2.0-fold) compared with that after the 10 min treatment (F = 11.9; df = 3, 15.92; P = 0.003) (Fig. 1B). Although there was an increase (2.3- and 2.4-fold) in the percentage of unhatched eggs after the 1 and 8 h treatments of the 3–5 d-old eggs, the difference was not significant compared with that after the 10 min treatment (F = 3.5; df = 3, 66; P = 0.07). Likewise, there was a 2.2-fold increase after the 1 h treatment of the 6–8 d-old eggs that was not significant but after the 8 h treatment there was a significant increase (2.9-fold) in the percentage of unhatched eggs compared with that after the 10 min treatment (F = 4.7; df = 3, 71; P = 0.04).
The percentage of unhatched eggs in the 0–2 d-old egg group treated with NYDA was significantly increased at the 8 h exposure times (1.2-fold) compared with that after the 10 min treatment (K-W statistic = 8.3; P < 0.05) (Fig. 1C). The percentages of unhatched eggs in the 3–5 and 6–8 d-old egg groups were also increased but were not significantly different after the 1 and 8 h NYDA treatments (1.2- and 1.2-fold, respectively, for the 3–5 d-old eggs, K-W statistic = 7.7, P > 0.05, and 1.2- and 1.2-fold, respectively, for the 6–8 d-old eggs, K-W statistic = 9.7, P > 0.05). There were no significant differences in the percentages of unhatched eggs after the 30 min to 8 h exposures to NYDA for any of the egg groups (0–2 d: K-W statistic = 8.34; 3–5 d: K-W statistic = 7.67; 6–8 d: K-W statistic = 9.74. P > 0.05 for all).
The percentages of unhatched eggs in the 0–2, 3–5, and 6–8 d-old egg groups treated with NYDA without fragrances also increased with longer exposure times but were not significant (Fig. 1D). The percentage of unhatched eggs was significantly increased after the 8 h exposure to NYDA without fragrances of the 3–5d-old eggs (K-W statistic = 8.87; P = 0.05) compared with that after the 10 min treatment but there was no significant difference in the percentages of unhatched eggs after any of the exposure times to NYDA without fragrances for the 0–2 and 6–8 d-egg groups.
When the percentages of unhatched eggs were compared among the three egg groups at the same exposure time within a treatment, there were no significant differences for any of the treatments (ddH2O: 10 min, F = 0.04, df = 2, 89.67; 30 min, F = 0.38, df = 2, 58.89; 1 h, F = 0.13, df = 2, 32.56; 8 h, F = 0.68, df = 2, 40.00. Nix: 10 min, F = 1.1, df = 2, 24.44; 30 min, F = 0.42, df = 2, 77.33; 1 h, F = 0.30, df = 2, 47.56; 8 h, F = 0.76, df = 2, 54.22. NYDA: 10 min, K-W statistic = 0.64; 30 min, K-W statistic = 0.85; 1 h, K-W statistic = 3.2; 8 h, K-W statistic = 0. NYDA without fragrances: 10 min, K-W statistic = 1.56; 30 min, K-W statistic = 2.04; 1 h, K-W statistic = 2.54; 8 h, K-W statistic = 0. P > 0.2 for all) (Fig. 1). Therefore, these data were combined to compare the percent of unhatched eggs among the four treatments.
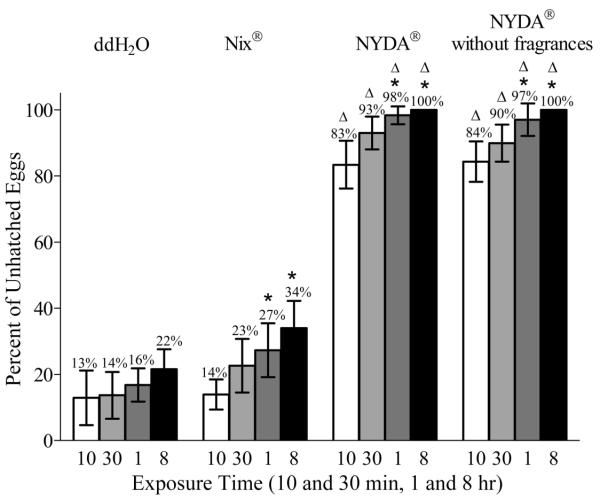
There were no significant differences in the percentages of unhatched eggs over the different exposure times for ddH2O-treated eggs (F (0–2 d) = 1.2, df = 3, 59.75; F (3–5 d) = 0.58, df = 3, 56.00; F (6–8 d) = 1.2, df = 3, 50.00; P > 0.05 for all) (Fig. 2).
Fig. 2.
Percentages of unhatched human head louse eggs determined from the data derived using combined aged egg groups. An asterisk (*) indicates that the percentages of unhatched eggs were significantly increased compared with the 10 min exposures within a treatment group (ANOVA, P < 0.05). A triangle (Δ) indicates that the percent of unhatched eggs treated with NYDA or NYDA without fragrances significantly increased when compared with the values observed after ddH2O or Nix treatments at the same exposure time (ANOVA, P < 0.05).
The percentages of unhatched eggs increased after longer exposures to Nix and were significantly increased after the 1 and 8 h treatments (2.0- and 2.4-fold, respectively) when compared with the 10 min treatment (F = 15.2; df = 3; 45.31; P < 0.0001) (Fig. 2). The percentage of unhatched eggs after 8 h treatment was significantly increased compared with the 30 min treatment (F = 15.2; df = 3, 45.31; P < 0.05). There were, however, no significant differences in the percentages of unhatched eggs after the 1 h to 8 h exposures to Nix (F = 1.2; df = 3, 45.31; P > 0.05).
Within the same exposure time, there were no significant differences in the percentages of unhatched eggs after treatment with Nix versus ddH2O (F = 12.3; df = 7, 45.17; P > 0.05) (Fig. 2).
There were, however, significant and substantial increases (4.5- to 6.6-fold) in the percentages of unhatched eggs when eggs were treated with NYDA versus ddH2O (K-W statistic = 61.86; P < 0.0001) (Fig. 2). The percentages of unhatched eggs after the 1 and 8 h treatments were also significantly greater (1.2- and 1.2-fold) versus the 10 min treatment (K-W statistic = 26.0; P < 0.05).
There were, likewise, significant and substantial increases (4.5- to 6.5-fold) in the percentages of unhatched eggs when eggs were treated with NYDA without fragrances versus ddH2O (K-W statistic = 59.46; P < 0.0001) (Fig. 2). The percentages of unhatched eggs after the 1 and 8 h treatment were also significantly greater (1.2- and 1.2-fold, respectively) than that of the 10 min treatment (K-W statistic = 61.86; P < 0.001). Within the same exposure times, there were no significant differences in the percentages of unhatched eggs after the NYDA versus NYDA without fragrances treatments (K-W statistic = 41.93; P > 0.05). There were also no significant differences among the percentages of unhatched eggs when the 30 min, 1 and 8 h exposures were compared after either the NYDA or NYDA without fragrances treatments (K-W statistic = 16.20; P < 0.05).
Within the same exposure time, the percentages of unhatched eggs after treatment with NYDA at 10 min, 30 min, 1 and 8 h were significantly greater (6.0-, 4.1-, 3.6-, and 2.9-fold) versus eggs treated likewise with Nix (K-W statistic = 65.27; P < 0.0001) (Fig. 2). Similar results were obtained when NYDA without fragrances versus Nix treatments were likewise compared (6.1-, 4.0-, 3.6-, and 2.9-fold increases in unhatched eggs) (K-W statistic = 62.85; P < 0.0001) (Fig. 2).
Survivorship of Hatched Eggs to Adulthood
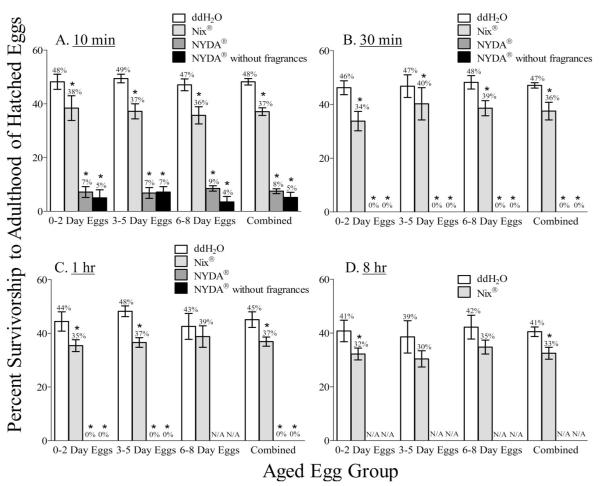
Within an exposure time and treatment, there were no statistically significant differences in the percent survivorship of lice to adulthood that hatched from any of the three treated egg groups (ddH2O: 10 min, F = 0.66, df = 2, 4.46; 30 min, F = 0.31, df = 2, 10.22; 1 h, F = 1.84, df = 2, 13.33; 8 h, F = 0.42, df = 2, 24.08. Nix: 10 min, F = 0.42, df = 2, 13.08; 30 min, F = 1.8; df = 2, 18.93; 1 h, F = 1.11, df = 2, 8.03; 8 h, F = 3.0, df = 2, 6.58. NYDA: 10 min, K-W statistic = 1.87. NYDA with-out fragrances: 10 min, K-W statistic = 2.76. P > 0.12 for all) Therefore, these data were combined for comparisons among exposure times and treatments (see “combined,” Fig. 3).
Fig. 3.
Percent survivorship to adulthood of hatched human head louse eggs treated with ddH2O, Nix, or NYDA with and without fragrances for various exposure durations. An asterisk (*) indicates that the percent survivorship was significantly decreased compared with the ddH2O treatment after Nix, NYDA, or NYDA without fragrances treatments (ANOVA, P < 0.05). N/A = data not available because of the 100% ovicidal action of NYDA and NYDA without fragrances after the 1 and 8 h exposures.
Using the combined data set, the percentage of lice surviving to adulthood after the 10 min Nix treatment was significantly decreased (1.3-fold) when compared with the 10 min ddH2O treatment (F = 667.3; df = 3, 6.24; P < 0.0001) (Fig. 3A).
The percentages of lice surviving to adulthood were significantly and substantially reduced, however, after the 10 min NYDA or NYDA without fragrances treatments when compared with the 10 min ddH2O treatment (6.0- and 9.6-fold reduction in survivorship, respectively) (NYDA: F = 667.3; df = 7, 6.24; P < 0.05. NYDA without fragrances: F = 667.3; df = 7, 6.24; P < 0.05) (Fig. 3A). The percentage of hatched lice that survived to adulthood after the 10 min NYDA treatment was not statistically different from the 10 min NYDA without fragrances treatment (F = 667.3; df = 7, 6.24; P > 0.05).
A similar significant reduction in survivorship (1.3-fold) was determined after the 30 min Nix treatment when compared with the reduction seen in the presence of the 30 min ddH2O treatment (unpaired t-test; t = 6.75; df = 16, P < 0.0001) (Fig. 3B). Nevertheless, ≈36% of the hatched lice survived to adulthood. No lice, however, survived to adulthood after the 30 min NYDA or NYDA without fragrances treatments to eggs (Fig. 3B). Similar results were obtained when eggs were exposed to these treatments for one (Fig. 3C) and 8 h (Fig. 3D): Nix treatments reduced sur-vivorship ≈1.2-fold with 30–39% of the lice surviving, and no egg hatched after the NYDA or NYDA without fragrances treatments.
Development of Lice That Hatched From Treated Eggs
Within an exposure time and treatment, there were no statistically significant differences in the development of lice that hatched from any of the three treated egg groups compared with the ddH2O treatment (data not shown). Therefore, these data were combined for comparisons among exposure time intervals and treatments (Table 1).
Table 1.
Comparative development of lice that hatched from 0- to 8-d old eggs (0-2, 3-5, and 6-8 egg groups combined) treated with ddH2O, Nix, NYDA, or NYDA without fragrances
| Measurements | Treatments |
|||
|---|---|---|---|---|
| ddH2O1 | Nixa | NYDAa,c | NYDA without fragrancea,c | |
| Development time from egg hatch to adults (days) | 11.8 ± 1.3a,b | 11.9 ± 0.9a | 12.1 ± 0.9a | 12.4 ± 1.6a |
| Longevity of adults (days) | 11.63 ± 1.0a | 12.7 ± 1.3a | 7.7 ± 1.0b | 9.6 ± 0.9a |
| Female fecundity (eggs/female/days) | 2.9 ± 0.3a | 2.9 ± 0.5a | 2.3 ± 0.3a | 3.1 ± 0.7a |
| Egg hatch from surviving females (%) | 76.2 ± 2.8a | 73.5 ± 3.9a | 74.6 ± 5.8a | 72.2 ± 2.2a |
Means ± SD were calculated from three replicate experiments (N = 3), each using 60 lice, at four exposure times. Because there were no significant differences amongst exposure times (10, 30 min, 1 and 8 h), these data were combined.
Means in the same row or column followed by the same letter are not statistically different (ANOVA, P < 0.05).
No louse survived to adulthood after egg treatment with either NYDA formulation when exposed 30 min or more.
There were no statistically significant differences in the developmental time, fecundity, or egg hatch from lice that hatched after any of the four treatments applied to eggs from 10 min to 8 h in duration (development time: F = 0.63; df = 3, 2.98; P = 0.60; fecundity: F = 0.72; df = 3, 1.62; P = 0.55; hatchability: F = 2.4; df = 3, 22.20; P = 0.09) (Table 1). The longevity of adults, however, was significantly reduced after the 10 min NYDA (1.6-fold) (F = 9.6; df = 3, 4.87; P < 0.0001) treatment when compared with either the 10 min ddH2O or Nix treatments. Although the longevity of lice treated with NYDA without fragrances was reduced (1.3-fold) compared with ddH2O or Nix, the difference was not significant (F = 9.6; df = 3, 4.87; P > 0.05).
Discussion
The ovicidal actions of NYDA and NYDA without fragrances were statistically and substantially greater than those after treatment with either ddH2O or the Nix formulation. Both NYDA formulations were 100% ovicidal (0% of the treated eggs hatched) after an 8 h exposure whereas 78 and 66% of the eggs hatched after the ddH2O and Nix treatments, respectively. These results were expected as the spreadability of the dimeticones used in the NYDA formulations and their ability to penetrate into very small diameter openings make them likely candidates as egg/embryo suffocants. Conversely, it is difficult to effectively immerse insect eggs into water as the chorion sheds water and Nix has limited ovicidal action (Yoon et al. 2006). Because there were no significant differences among groups of eggs with different developmental stages within an exposure time interval, it appears that both NYDA and NYDA without fragrances act principally as physical agents most likely suffocating the embryo, regardless of its developmental stage.
The ovicidal action of these two formulations appears to be due principally to the dimeticone constituents themselves in that both NYDA and NYDA with-out fragrances produced virtually the same effect on treated eggs. The ovicidal action of NYDA and NYDA without fragrances also appears to be dependent upon exposure times. Following the current manufacturer’s instructions, the standard 8 h exposure time resulted in 100% ovicidal action (0% of the eggs hatched) using either of the NYDA formulations. The shorter exposure times of 30 min and 1 h did not result in a significant increase of hatched eggs compared with the 8 h treatment. After the 30 min and 1 h exposures, only 7–10 and 2–3% of the treated eggs hatched, respectively, and none of these instars survived to adulthood. Only the 10 min exposure to either NYDA formulation resulted in a significant increase in hatched eggs (≈16%) compared with the 8 h exposure treatment (0%). Of the eggs that hatched after the 10 min exposure, only ≈7% survived to adulthood. Because the 10 min exposure duration is only ≈2% of the currently recommended 8 h exposure duration, the dose acquired by an egg after an 8 h treatment with either NYDA or NYDA without fragrances is expected to be substantially more than that acquired during a 10 min exposure. The dose acquired from the 8 h exposure is apparently sufficient to produce 0% egg hatch. The increases in the percentages of unhatched eggs over longer exposure time durations validate this assumption and the manufacturer’s selection of the 8 h standard exposure time duration using these two formulations. Nevertheless, a reduced incubation time of only 1 h or even 30 min did not result in a statistical significant increased hatch rate.
In addition to the substantial reduction in egg hatch, there was also a significant reduction in the survivorship to adulthood of instars that hatched from eggs after the 10 min treatment with either NYDA formulation (6- to 9-fold reduction compared with ddH2O treatment). This finding supports the contention that the NYDA formulations penetrate the egg casing and affect the developing embryo and also appear to be capable of blocking the tracheal system of first instars after eclosion.
Lastly, there were no statistical differences in the time it took lice to become adults, female fecundity or the viability of eggs laid by surviving females after egg treatment with either NYDA formulations compared with treatment with ddH2O. These findings suggest that once the louse reached adulthood there were no residual effects after the NYDA treatments of eggs and indicate that NYDA formulations were acting physically, and that any residues were probably eliminated during the first molt, and did not penetrate through the louse cuticle. Only the longevity of adults that hatched from eggs treated with NYDA formulations was significantly reduced compared with adults from ddH2O-treated eggs. This finding is not well understood, but a possible explanation could be that apoptotic processes may have been initiated early on in the louse’s development because of the stress incurred during its embryonic development after NYDA treatment of eggs. Nevertheless, the impact of reduced adult longevity is that even females that receive a sub-lethal exposure to NYDA formulations may produce substantially fewer eggs because of their shortened adult lifespan.
Acknowledgments
This work was supported in part by G. Pohl-Boskamp GmbH & Co. KG, Germany, and by a grant from the NIH/NIAID (5 R01 AI045062-06).
References Cited
- Burgess IF. The mode of action of dimeticone 4% lotion against head lice, Pediculus capitis. BMC Pharmacol. 2009 doi: 10.1186/1471-2210-9-3. doi:10.11186/1471-2210-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Document WHO/CTD/WHOPES/97.8. World Health Organization; Geneva, Switzerland: 1997. Human lice: their prevalence, control and resistance to insecticides: a review. [Google Scholar]
- Heukelbach J, Asenov A, Liesenfeld O, Mirmohammadsadegh A, Oliveira FA. A new two-phase dimeticone pediculicide shows high efficacy in comparative bioassay. BMC Dermatol. 2009 doi: 10.1186/1471-5945-9-12. doi:10.1186/1471-5945-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgdon HE, Yoon KS, Previte DJ, Kim HJ, Abo El-Ghar GE, Lee SH, Clark JM. Serial invasive signal amplification reaction for the determination of KDR frequencies in global human head louse populations for efficient resistance monitoring. Pest Manag. Sci. 2010;66:1031–1040. doi: 10.1002/ps.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux D, Palma K, Kaul N, Hodgdon H, Van Geest A, Previte D, Abo Elghar G, Yoon K, Clark JM. Pyrethroid pediculicide resistance of head lice in Canada evaluated by serial invasive signal amplification reaction. J. Cutaneous. Med. Surg. 2010;14:115–118. doi: 10.2310/7750.2010.09032. [DOI] [PubMed] [Google Scholar]
- Mumucuoglu KY, Gilead L, Ingber A. New insights in pediculosis and scabies. Expert Rev. Dermatol. 2009;4:285–302. [Google Scholar]
- Raoult D, Roux V. The body louse as a vector of reemerging human disease. Clin. Infect. Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- Richling I, Bockele W. Lethal effects of treatment with a special dimeticone formula on head lice and house crickets (Orthoptera, Ensifera: Acheta domestica and Anoplura, Phthiraptera: Pediculus humanus): insights into physical mechanisms. Arzneimittelforschung. 2008;58:248–254. doi: 10.1055/s-0031-1296501. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Gao J-R, Lee SH, Clark JM, Brown L, Taplin D. Permethrin-resistant human head lice. Pediculus capitis, and their treatment. Arch. Dermatol. 2003;139:994–1000. doi: 10.1001/archderm.139.8.994. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Strycharz JP, Gao JR, Takano-Lee M, Edman JD, Clark JM. An improved in vitro rearing system for the human head louse allows the determination of resistance to formulated pediculicides. Pestic. Biochem. Physiol. 2006;86:195–202. [Google Scholar]