Abstract
The human functional magnetic resonance imaging (fMRI) experiments performed in the Center for Magnetic Resonance Research (CMRR), University of Minnesota, were planned between two colleagues who had worked together previously in Bell Laboratories in the late nineteen seventies, namely myself and Seiji Ogawa. These experiments were motivated by the Blood Oxygenation Level Dependent (BOLD) contrast developed by Seiji. We discussed and planned human studies to explore imaging human brain activity using the BOLD mechanism on the 4 Tesla human system that I was expecting to receive for CMRR. We started these experiments as soon as this 4 Tesla instrument became marginally operational. These were the very first studies performed on the 4 Tesla scanner in CMRR; had the scanner became functional earlier, they would have been started earlier as well. We had positive results certainly by August 1991 annual meeting of the Society of Magnetic Resonance in Medicine (SMRM) and took some of the data with us to that meeting. I believe, however, that neither the MGH colleagues nor us, at the time, had enough data and/or conviction to publish these extraordinary observations; it took more or less another six months or so before the papers from these two groups were submitted for publication within five days of each other to the Proceedings of the National Academy of Sciences, USA, after rejections by Nature. Based on this record, it is fair to say that fMRI was achieved independently and at about the same time at MGH, in an effort credited largely to Ken Kwong, and in CMRR, University of Minnesota in an effort led by myself and Seiji Ogawa.
Keywords: Neuroimaging, brain imaging, MRI, High field, Ultrahigh field, 4 Tesla, 4T, 7 Tesla, 7T, functional mapping, fMRI, functional mapping
INTRODUCTION
This article is closely related to another titled “The Road to Functional Imaging and Ultrahigh Fields” that I wrote for this volume (Ugurbil, this issue). The other article focuses on the development and the use of high and ultrahigh magnetic fields in functional magnetic resonance imaging of brain activity (fMRI), and takes a longer “historical” perspective on events that shaped my career in magnetic resonance. Inevitably, however, such a topic includes the history of the development of fMRI since high fields and fMRI are intricately tied in my career. The very first human imaging experiment that I ever undertook was the experiments aimed at developing fMRI using the very first human imaging instrument my lab acquired at the University of Minnesota; this instrument was a “high field” human system operating at 4 Tesla, at a time when 1.5 Tesla was the prevalent clinical MR scanner and 3 Tesla clinical scanners of today did not exist. Until very recently, in fact, I would have been able to say I never worked on functional imaging, or any other imaging for that matter, at a field strength lower than 4 Tesla. That record was altered in the last year with work on 3 Tesla, launched due to the Human Connectome Project (Van Essen and Ugurbil, this issue).
FUNCTIONAL IMAGING
In the two decades since its discovery, blood oxygen level dependent (BOLD) fMRI has seen a revolution in its ability to image brain function, going from early experiments demonstrating relatively coarse images of activity in the visual cortex to mapping cortical columns and to “brain reading” that constructs mental experiences of an individual, all using the fact that we were endowed with a complex paramagnetic molecule sequestered in our blood vessels and that neuronal activity has spatially-specific metabolic and physiologic consequences. We at the Center for Magnetic Resonance Research (CMRR), University of Minnesota, were fortunate to be one of groups that independently initiated and conducted the experiments that introduced fMRI (Ogawa et al. 1992).
BELL LABS CONNECTION
The attempt to develop fMRI in CMRR came about because of the work Seiji Ogawa did in Bell Labs introducing the BOLD effect (Ogawa et al. 1990; Ogawa et al. 1990; Ogawa and Lee 1990)(also see Ogawa, this issue). These early experiments conducted on rats did not show functional mapping; rather, they demonstrated that metabolic perturbations such as hypoglycemia and graded levels of oxygen in the inhaled gas mixture affected the visibility of venous blood vessels. I was very much aware of this work not only because of my scientific interests at the time but also because Seiji and I knew each other well; we were colleagues that had worked together for several years in the same group in Bell Laboratories, driven with the aim of establishing in vivo applications of the magnetic resonance phenomenon.
After receiving my Ph.D. in Chemical Physics at Columbia University in 1977, and after serving four months in the Turkish army (the Marines to be specific) to fulfill my obligatory military duty, I joined the Biophysics Department in Bell Laboratories. The Department was led by Robert Shulman; I was his postdoctoral fellow, working on the development of MR spectroscopy for the study of intracellular processes in intact cells. Seiji Ogawa and Truman Brown were members of this department and were involved in the intact cell work. Later, Jan den Hollander, Sheila Cohen and Bob Gillies would join us. Gil Navon was there before my time but would visit us on occasion and participate in the effort when I was there as well. We employed 31P and 13C NMR spectroscopy to study energetics and metabolism in E. coli and yeast cells in suspension (e.g., (Ugurbil et al. 1978; Ugurbil et al. 1978; Shulman et al. 1979; Ugurbil et al. 1982)). The work from this lab together with the contemporaneous effort from the laboratory of George Radda at Oxford University pioneered in vivo magnetic resonance spectroscopy or MRS that many employ today to study metabolism in the human body using high and ultrahigh magnetic fields.
Although we specifically worked on spectroscopy studies directed at in vivo metabolism, the general scientific theme that excited us was the use of magnetic resonance to obtain information non-invasively about biological processes in intact systems. At the time, knowledge on the structure of biological molecules such as proteins, RNA and DNA were being expanded at a dizzying rate, supplementing already extensive but rapidly increasing understanding of enzyme kinetics and regulation of metabolic pathways. Such knowledge, however, was derived from preparations obtained from cell extracts; we wondered, if data garnered by these destructive techniques was applicable in the complex intracellular environment of the intact cell. Oxidative ATP synthesis, a problem I worked on in Bell Labs and continue to work on even today in Minnesota sporadically, is a good example. It requires the intact bacteria (or in eukaryotes, at least the intact mitochondria) to function. It was a hotly debated topic at the time; the Mitchell hypothesis that assigned a critical role to an electrochemical +H gradient across the bacterial (or equivalently mitochondrial) membrane was pitted against concepts of an intermediary chemical compound that mediated the coupling between the electron transport chain and the ATP synthase. The proof of the latter required isolating this compound and showing that, in an isolated preparation, it could drive the conversion of ADP and inorganic phosphate (Pi) to form ATP. The former, on the other hand, required working with intact bacteria or mitochondria. Although Mitchell and others provided evidence for the “Mitchell hypothesis” by looking at proton extrusion in mitochondrial suspensions, leading to the Chemistry Nobel prize for Peter Mitchell in 1978, arguably we were the first to detect this as a transmembrane +H gradient directly by visualizing the difference between intra- and extra-cellular pH in suspensions of E. coli (Ogawa et al. 1978; Ugurbil et al. 1978; Ugurbil et al. 1979; Ugurbil et al. 1982) and mitochondria (Ogawa et al. 1978). One can see from these references that Seiji and I worked on similar problems in the same group at about the same time. Even though most of the time we were not co-authors in the same papers, the entire group was tightly knit through the excitement, and enthusiasm we felt for the work we were engaged in.
Clearly, we ultimately thought about human experiments; chemical shift imaging (Brown et al. 1982) which came from this Bell Labs period is testimony to this ultimate aspiration. However, as good physicists and physical chemists, we had taken the reductionist approach to start with the simplest system possible, the canonical bacteria E. coli, but with the dream that one day we would ultimately achieve, with similar magnetic resonance methods, humans studies of physiological processes in vivo. Functional imaging came about as a chapter in this general saga. Seiji eventually moved from working with E. coli to working on the brain of rodents with imaging; he recently told me that he was interested in the neonatal brain and switched to imaging because he was skeptical that spectroscopy would work in such small brains. His interest in the brain fitted well to the transformation of the Biophysics Department in Bell Labs that occurred when Bob Shulman, and many others including myself, left Bell Labs. Under advise from John Hopfield, a member of our department at the time, the Biophysics Department was redirected towards neurosciences, hiring individuals like David Tank and others. Thus, Seiji found himself immersed in a neuroscience environment where his interest in the brain found a natural home.
I also abandoned cell suspensions starting at about the same time to work with perfused heart models. This model possessed inherent solutions to many difficulties encountered with cells in suspension; it had a high cell density (better for signal-to-noise ratio (SNR)) than what is achievable in cells in suspension), it had its own vasculature that enabled the delivery of ample oxygen simply through perfusion, and it could perform work and hence attain commensurate oxygen consumption over a large range by simple external manipulations, such as pacing or pharmaceutical interventions. I was not necessarily interested in the heart per se as a cardiologist would be; I was being opportunistic, scientifically speaking, in adopting it for studies of oxidative energetics. Of course, it also helped that upon my arrival in Minnesota, among the many people I talked to, cardiologists showed the greatest interest in collaborating with me, leading to close working relationships with Arthur From, Mark Petein, John Foker, and Robert Bache, and others in the University of Minnesota, all clinicians and cardiac researchers. The perfused heart experiments were followed with open-chest instrumented heart studies in large animal models, closed chest instrumented animal models and ultimately humans at 4 Tesla, demonstrating the claim I have made that we did aim to go to humans from the very beginning.
When I moved to Minneapolis in 1982, I initially started working with a vertical bore 8.4 Tesla system. Subsequently, I acquired a 4.7 Tesla horizontal bore magnet with a 40 cm bore for animal model studies, shortly after such a magnet became available for the first time. The experiments performed on these systems were spectroscopy based but also focused on technical developments. For example, plane rotation adiabatic pulses that overcome the large B1 inhomogeneity of surface coils and impart B1 independent rotations were developed during this phase of my career, with Mike Garwood as my second postdoc (e.g. (Ugurbil et al. 1987; Ugurbil et al. 1988; Ugurbil et al. 1988)), as well as the applications of these pulses to perform spatially resolved spectroscopy studies of myocardial energetics in different layers of the left-ventricular wall with Pierre-Marie Robitaille as my third post-doc (Robitaille et al. 1989; Ugurbil et al. 1989; Path et al. 1990). The quest towards extending this work to humans led us in the mid-eighties to an initiative to establish a human capable 4 Tesla MR instrument in Minnesota (see Ugurbil in this issue). However, I definitely envisioned the research on this 4 Tesla human system to go beyond the heart and beyond spectroscopy. What interested and excited me was developing new MR techniques and engineering solutions to obtain unique biological information, whatever the technique, the targeted organ, or the biological system may be. Consequently, I started thinking about, “trolling” if you like, for other potentially interesting ideas for the 4 Tesla project. It was clear from the start that the BOLD contrast based on magnetic susceptibility phenomenon and 4 Tesla together presented a unique opportunity to attempt at functional imaging in humans, since magnetic field inhomogeneities induced by susceptibility differences increase at the higher magnetic field. Thus, fMRI became my highest priority for the 4 Tesla instrument about a year before the instrument was even delivered.
IMAGING HUMAN BRAIN FUNCTION with BOLD CONTRAST
I became aware of Seiji’s work on the BOLD mechanism from various conferences that we both attended; I had seen some of his presentations (e.g. Society of Magnetic Resonance in Medicine (SMRM) meeting in August 1989 in Amsterdam) and also discussed this work informally when we met, catching up with each other. The link to oxygen metabolism, at the time my research focus, is what initially attracted my attention to the BOLD effect. Although the BOLD work was based on manipulation of oxygenation levels in the blood in the rat model through pharmaceutical and/or metabolic interventions, in his paper published in the Proceedings of the National Academy of Sciences, USA (PNAS), submitted in August 1990 and published in December 1990 (Ogawa et al. 1990), Seiji and his coworkers suggested the use of this method to possibly achieve functional imaging in the brain, in a way analogous to the PET approach but, unlike PET, using an endogenous contrast mechanism. In the Discussion section of this 1990 manuscript, Seiji stated:
“PET imaging relies on a family of tracer method for measuring different physiological quantities including blood volume, blood flow, and regional oxygen extraction (13). BOLD contrast adds to a similar, emerging set of functional MRI methodologies that are likely to be complementary to PET imaging in the study of regional brain activity.”
Clearly, experiments are performed and scientific questions are discussed and debated long before a paper describing them starts taking shape and long before such a paper is submitted, reviewed, and eventually appears in press. Thus, even before Seiji’s 1990 PNAS paper was submitted, we started talking about pursuing functional imaging together in the human brain using the 4 Tesla system I was waiting to receive in Minneapolis (see Ugurbil, in this issue). Evidence of this discussion can in fact be found in Seiji’s 1990 PNAS paper (Ogawa et al. 1990), where it is stated that
“The results shown here indicate that BOLD contrast can be used to noninvasively to monitor in real time the blood oxygenation levels of brain areas in response to central nervous system drugs that affect basal metabolism or blood flow. Although BOLD-image contrast is enhanced at high magnetic fields, the effect is observed at 4.7 T*, a field strength that is close to the highest field strength (4 T) presently available for human subjects.”
As we waited for the 4 Tesla instrument, we did not want to talk about the plans to pursue functional imaging or the excitement we felt about this prospect. We also did not consider pursuing fMRI at 1.5T because we were focused on the BOLD contrast, which, as previously stated, is a susceptibility effect. As such, we did not think BOLD contrast would be sufficiently strong at low fields like 1.5T. In principle, we were mainly right, although incomplete in our understanding of potential sources for functional imaging signals. Particularly the early fMRI experiments, performed as single slice studies using fast repetition times and large flip angles, were prone to inflow effects, mostly associated with large vessels with fast flows (Duyn et al. 1994; Segebarth et al. 1994). These and other predominantly large vessel effects (e.g. (Hoogenraad et al. 1999; Duong et al. 2003; Silvennoinen et al. 2003)) can generate strong stimulus-evoked imaging signals even at 1.5 Tesla, albeit inadequate ones if high spatial fidelity to sites of neuronal activity is desired. Nevertheless, for many questions, these early 1.5 Tesla functional maps did provide adequate accuracy and have produced numerous useful results.
The 4 Tesla instrument arrived in Minneapolis in 1990. However, the magnet was damaged in transport and had to be repaired. When the system finally became operational in CMRR, the very first experiment we started working on this system was fMRI. Had the 4 Tesla instrument been delivered earlier or had it functioned right away we would have certainly achieved fMRI earlier.
I had a relatively small group at that time. CMRR per se did not exist. CMRR came into existence around the human 4 Tesla system. A small new building was built in the Medical School campus, close to the University Hospital, to house the 4 Tesla instrument as well as the 4.7 Tesla 40 cm bore system I had acquired previously for animal model studies. Thus, my lab in the new facility became the CMRR. I asked Ravi Menon, who had joined my group as a postdoctoral fellow, to take on the functional imaging effort. Seiji visited us for the experiments and Ravi, Seiji, and Jutta Ellermann (who was also my post-doctoral fellow at the time) performed the experiments together, often taking turns as subjects. Seiji and I proved at the end to be the worst subjects with respect to seeing any stimulus induced signal changes in the brain. Jutta had the best response; images that appeared in our first paper reporting fMRI (Ogawa et al. 1992) are from her brain. David Tank from Bell Labs joined us at times, and participated in the data collection, advised us on neuroscience aspects of the studies, and wrote software for data analysis and visualization. Seong-Gi Kim also joined my group as a post-doctoral fellow later in the effort and started working with us on these early fMRI experiments. Much had to be done since the 4 Tesla system was an immature platform. We had to build human size radiofrequency (RF) coils at this high frequency for the first time (the task of Hellmut Merkle who now works at NIH); we had to implement pulse sequences virtually from scratch to collect the data (done by Ravi and later by Seong-Gi Kim and Xiaoping Hu), and develop protocols to transfer these data to other computers for analysis; we had to deal with problems of a new instrument, such as imprecise synchronization of gradients with data acquisition that led to extensive ghosting, and regulatory hurdles for performing studies at 4 Tesla for the first time. Echo Planar Imaging (EPI) that has now become the most commonly employed imaging approach for fMRI was not available generally on any system, let alone a high field 4 Tesla system. Along with functional imaging, we did pursue spectroscopy studies at 4 Tesla, initially focusing on extending our previous cardiac spectroscopy work on animal models to humans. As a result, the fMRI success was accompanied with another first: We were able to obtain transmurally resolved spectra in the human heart (Menon et al. 1992).
We started collecting data for BOLD functional imaging on humans sometime early in 1991. We had to pause several times due to instrumentation problems and/or changes. We used gradient recalled echo imaging (i.e. FLASH). Obviously, in these early experiments, we worried about whether the results were real, if they were motion artifacts, or instrumental glitches etc. We also expected the stimulation-evoked signal to be a decrease relative to the baseline state, being prejudiced because of familiarity with strongly coupled oxygen consumption increases associated with work and metabolism in other systems. We were aware of the Fox and Raichle paper reporting minimal to no oxygen consumption change with visual stimulation studies with PET based measurements of oxygen consumption (Fox et al. 1988), but, as many others in the field, we were skeptical of it. When we started to be convinced that we were seeing positive signal changes due to visual stimulation, I remember Seiji and I one day muttering something to the effect that the PET people may be right after all.
I do clearly recall a study early in the summer in 1991, with one of our colleagues who had become one of our best test subjects, spending inordinate amounts of time in the magnet with GRASS visual stimulation goggles on. We thought the data were extremely encouraging. We decided to take a break and get something to eat for dinner at a local pub in the neighborhood named Sally’s. We, that is Ravi, Seiji, Jutta, I, and our test subject colleague, sat outside on the patio. All but our test subject colleague enjoyed a drink, a beer I think. But we did not allow our test subject to have an alcoholic beverage because we worried about the effects of alcohol on the brain’s vascular response to elevated neuronal activity.
Certainly by the time we went to the SMRM annual meeting that was held in San Francisco in August 1991, we had functional images. We took a slide of the data with us and showed it to some of our close colleagues with whom we discussed the project. We knew sometime before this meeting that MGH was working on similar experiments. Lin Jelinsky, the head of Seiji’s department in Bell Labs at the time, told us that she had been visiting MGH, heard about their efforts and felt obligated to tell them of our attempts to develop BOLD based fMRI in Minneapolis. The abstract book of the 1991 SMRM annual meeting did not contain any abstracts from any group reporting results from or even attempts at fMRI with BOLD contrast; clearly, at the time of the abstract deadline, no one was able to submit or thought of submitting a BOLD fMRI abstract to this annual meeting. But Tom Brady from MGH had a plenary talk in this conference and showed functional images of visual stimulation obtained with BOLD contrast in his talk.. In this meeting, we could have shown BOLD fMRI images as well. Clearly, however, neither the MGH colleagues nor we were at a stage where we could rush to publish these unique experiments. Otherwise both groups would have submitted papers for publication by then, especially since we knew of each other’s competing efforts. Yet it took another few months before the papers from these two groups were submitted for publication within five days of each other. Likely, we were all being too cautious. But then, “extraordinary claims require extraordinary evidence1.”
We submitted our paper to Nature first. It was rejected after a few weeks without being sent to scientific review, with the usual rejection letter saying that it was not of “general interest”. I was against sending it to Nature, because I worried about the fallibility of editorial “judgment” and pre-selection in journals like Nature; I knew that this work would get enormous attention irrespective of where it was published. But my co-workers all wanted to send it to Nature. After the rejection, we recouped and sent it to Proceedings of the National Academy of Sciences, USA where it was received in March 1992 and appeared in press in July 1992 (Ogawa et al. 1992).
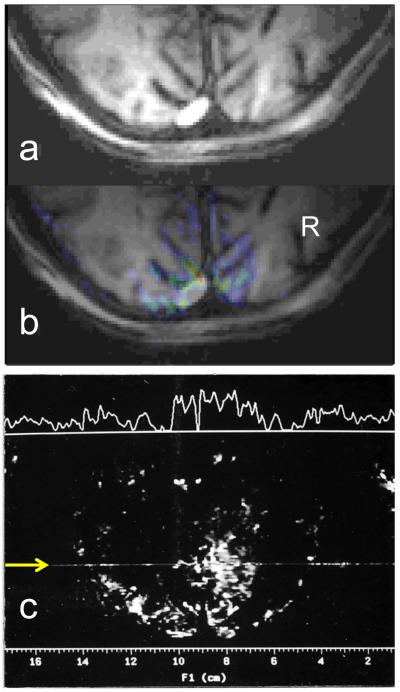
When I look at the first fMRI images we published, I must say I am impressed as to how good they were. I reproduced one of the figures we published in our first fMRI paper (Ogawa et al. 1992) in a different format in Figure 1 of this article. Figure 1b illustrates the functional image obtained with full field visual stimulation on a plane parallel to the calcarine fissure (in color), superimposed on the anatomical image (they were presented separately in the original figure in our 1992 paper (Ogawa et al. 1992)). The anatomical image is displayed separately in Figure 1a. One can appreciate that the stimulus evoked functional signals really correspond to the cortical gray matter, especially on the right hemisphere (labeled as R); on the left hemisphere, the fMRI map also extend into the sulcus where the strongest signals are seen, likely coming from blood vessels in the sulcus. This 4 Tesla fMRI image, the first ever published, demonstrates what a plethora of modeling studies and experimental data have indicated since then; namely, in gradient recalled echo BOLD images, the strongest signals come from veins on the pial surface but that at high fields, there is also a significant contribution from intracortical vessel.
Figure 1.
(a) and (b) are the images that were published as Figure 2 in the Ogawa et. al. 1992 paper; panel (b) shows the full-field visual stimulation functional image (in color) superimposed on the anatomical image in gray scale; the latter is presented also separately in panel (a). Panel (c) illustrates a functional image of hemifield visual stimulation (on minus off) from 1991, obtained with 4 mm slice and in plane resolution 1.4×1.4 mm2. The signal intensity trace along the line marked with the yellow arrow appears on top of the image
In our first fMRI paper (Ogawa et al. 1992), we showed full and hemifield visual stimulation to demonstrate the fMRI response; for the latter we did not show images, only time courses. I found out that Seiji still had a hemifield data from 1991. I decided to take the opportunity to show this early image here (Figure 1c). The hemifield image was obtained in the same plane and orientation as the images shown in Figure 1a and 1b. Unfortunately, we failed to mention the in-plane resolution of the fMRI studies in the original paper, supplying only the acquisition matrix size (64×128) without the field-of-view (FOV); these original studies were performed with a surface coil, which permitted the use of reduced FOV. From the hemifield data, I can now report that these original studies had an in plane resolution of 1.4 ×1.4 mm2. The hemifield image shown here used a 4 mm slice thickness, though in the paper we showed data from 10 mm slices.
The 4 Tesla system with which the original fMRI work was performed continued to serve as the platform for our human fMRI studies until about 1999 when we developed a 7 Tesla system (Ugurbil this issue) for advanced fMRI studies and installed a 3 Tesla commercial system (Siemens) to support neuroscience applications of fMRI. The original 4 Tesla magnet was retired at this time because its cryogenic system (which required a helium reliquification and circulation) was discontinued and was no longer supported. This magnet became an historically important “statue” in the courtyard of our center (Figure 2).
Figure 2.
The picture shows the author next to the 125 cm bore 4 Tesla magnet from Siemens that was originally installed in CMRR in 1990. This magnet is now a garden “statue” in the courtyard of CMRR, University of Minnesota.
fMRI in CMRR after ITS INTRODUCTION
In some quarters of the cognitive science community, there was a rush to apply the fMRI technique without much thought given to the underlying mechanisms of the functional mapping signals. We disagreed with this approach and followed the initial work on fMRI with numerous fundamental studies into the origin of fMRI signals. We reported for the first time the presence of large vessel confound in the functional images (Menon et al. 1993; Kim et al. 1994), and the first paper on modeling the extravascular BOLD effect (Ogawa et al. 1993) which indicated that increasing magnetic fields would provide improved accuracy of functional signals and increased functional contrast. In our modeling paper, where we also presented experimental data, we noted the presence of spontaneous fluctuations in the fMRI time series for the first time. Discussing these temporal fluctuations in this 1993 paper, we stated:
“If the oscillations represent a neural origin, it may be possible to measure spatial correlations of the responses across the cortex. This would provide an MRI method of mapping patterns of functional connections between cortical areas.”
We observed and puzzled over these oscillations. We developed the first techniques to remove components of these fluctuations due to respiration and cardiac pulsation (Hu and Kim 1994; Hu et al. 1995). We even noted cases where the oscillations in the fMRI time series were altered during task versus the pre-task baseline state. Biswal and colleagues (Biswal et al. 1995), however, were the first to demonstrate the spatial correlation of these oscillations, ascribing them to neuronal activity based on the existence of the correlation between left and right primary motor cortices. We followed through our 1993 observations also demonstrating the existence of long-range spatial correlations (Mitra et al. 1995; Mitra et al. 1997), but raised the possibility of vascular contributions.
Working at 4 Tesla gave us numerous advantages. We performed for the first time true single trial event related fMRI, without averaging over trials (Richter et al. 1997; Richter et al. 2000). We pursued high fidelity mapping at the level of cortical columns (Menon et al. 1997). In that endeavor, I was influenced by the work of Apostolos Georgopoulos, a colleague who had joined the faculty at the University of Minnesota shortly after we demonstrated fMRI, and with whom I started collaborating. In a series of ground-breaking studies (Georgopoulos et al. 1986; Georgopoulos and Grillner 1989; Georgopoulos et al. 1989; Georgopoulos et al. 1992; Georgopoulos et al. 1993), Apostolos had demonstrated, using electrophysiological recordings, the presence of directionally tuned cells in the primary motor cortex. He summarized his findings in the abstract of one of his papers (Georgopoulos et al. 1986) as
“When individual [directionally tuned] cells were represented as vectors that make weighted contributions along the axis of their preferred direction (according to changes in their activity during the movement under consideration) the resulting vector sum of all cell vectors (population vector) was in a direction congruent with the direction of movement.”
This type of a forward predictive model linking neuronal activity with behavior was very attractive to me. Instead of the myriad of fMRI studies that followed the introduction of fMRI collecting empirical data on functional cartography, bound to be useful at some point, but without contributing much to mechanistic understanding of brain function, I wanted to perform studies analogous to what Apostolos achieved with electrophysiology. To construct such forward models, however, I believed ultimately we had to get to the level of elementary computational units represented at least by cortical columns, such as the orientation columns of V1, and direction tuned columns of MT. This in turn provided the motivation for my steady drive towards improved accuracy of functional imaging signals. In this effort, I regard one of our papers (Duong et al. 2001) as critically important. This work used a technique called FAIR developed in CMRR for measurement of cerebral blood flow (CBF) and CBF based functional imaging (Kim 1995); it demonstrated for the first time that blood flow is regulated at the level of orientation columns in the cat visual cortex (Duong et al. 2001). At the time, this was a hotly debated topic. There was the concept that the “brain waters the entire garden for the sake of a thirsty flower.” But in the Duong et al 2001 paper, we showed that the brain really waters the thirsty flower while it sprinkles generously around it. This implied that physiology co-operates with us and permits the mapping of columnar organizations using CBF changes coupled to neuronal activity; by implication, BOLD based fMRI should be able to achieve the same if the spatial inaccuracies introduced by the draining vein effects can be suppressed. CBF imaging did not have enough contrast-to-noise ratio to access columnar level activity in the human brain. Thus, we had to pursue BOLD based fMRI. We suppressed the large vessel confound using spin echo (SE), rather than the most commonly employed gradient recalled echo BOLD technique, in combination with ultrahigh fields (e.g. (Lee et al. 1999; Duong et al. 2002; Yacoub et al. 2003; Yacoub et al. 2005)); we pointed out that the SE technique was virtually useless at low fields because of the expected small magnitude of the effect as well as the presence of the large vein confound due to the blood contribution to functional mapping signals (Lee et al. 1999; Duong et al. 2003) and above. We demonstrated that the spatially-non-specific blood effects in SE BOLD persist even up to 4 Tesla but become negligible at 7 Tesla and above (Lee et al. 1999; Duong et al. 2003). We finally accomplished robust imaging of ocular dominance columns at 7 Tesla (Yacoub et al. 2007) in the human brain, after earlier results at 4 Tesla by us (Menon et al. 1997) and others (Cheng et al. 2001; Goodyear and Menon 2001), followed by the first time imaging of orientation columns in human area V1 (Yacoub et al. 2008; Shmuel et al. 2010) and the axis of motion selective features of human area MT (Zimmermann et al. 2011) using 7 Tesla. The MT work utilized for the first time the 3D GRASE sequence (Feinberg and Oshio 1991), which provides largely SE contrast, and demonstrated that these organizations could be mapped in three dimensions and with laminar resolution in the human brain. As in many other cases, this work relied on extensive data on animal studies showing feasibility of fMRI with laminar differentiation (e.g. (Duong et al. 2000; Harel et al. 2006; Zhao et al. 2006)).
Apostolos also succeeded, in collaboration with us, in predicting behavior with the population vector construct in humans using a maze task that had directionality embedded in it; these studies were performed first at 4 Tesla (Gourtzelidis et al. 2005) and, subsequently, at 7 Tesla (Jerde et al. 2008), with the latter providing significantly larger number of uniquely tuned voxels for a given direction. This is the first example of the type of “encoding and decoding” studies (see review by (Naselaris et al. 2011)) that are currently generating great interest in the fMRI community. However, I regard these accomplishments as a prelude to a new era where whole brain high resolution fMRI, at or approaching columnar and layer resolution, will provide a wealth of independently informative voxels that will be used in many different and powerful algorithms, including decoding and encoding approaches, to garner detailed mechanistic information on brain function.
CONCLUSION
Clearly, the last two decades of fMRI have demonstrated its huge impact in the study of human brain function. It continues to rapidly develop due to innovative data analysis methods and improved data acquisition and instrumentation. This history is an integral part of the history of our center in the University of Minnesota, the Center for Magnetic Resonance Research or simply CMRR, from the very beginning since, I believe it is fair to say that fMRI experiments were first attempted and achieved at about the same time at CMRR in an effort led by myself and Seiji Ogawa, and at MGH in an effort credited largely to Ken Kwong.
Footnotes
This is an expression popularized by Carl Sagan after its use in “Encyclopaedia Galactica”. Carl Sagan (writer/host). Cosmos. PBS. December 14, 1980. No. 12.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NOTE: I would like to mention that I have intentionally avoided reading articles written by others to date and already available online for this Neuroimage issue; I wanted to avoid somehow responding to claims, real or imagined, in my article. I also would like to emphasize that this article is specifically focused on the Minnesota experience as expected for this issue; it is certainly not a general review of field and not even a complete review of the work carried out in CMRR to date on functional brain imaging.
REFERENCES
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci U S A. 1982;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron. 2001;32(2):359–374. doi: 10.1016/s0896-6273(01)00477-9. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution. Proc Natl Acad Sci U S A. 2001;98(19):10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements [In Process Citation] Magn Reson Med. 2000;43(3):383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: Gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med. 2003;49(6):1019–1027. doi: 10.1002/mrm.10472. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Vaughan JT, Merkle H, Kim SG. High-resolution, spin-echo BOLD, and CBF fMRI at 4 and 7 T. Magn Reson Med. 2002;48(4):589–593. doi: 10.1002/mrm.10252. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Moonen CT, van Yperen GH, de Boer RW, Luyten PR. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5 T. NMR in biomedicine. 1994;7(1-2):83–88. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Oshio K. GRASE (gradient- and spin-echo) MR imaging: a new fast clinical imaging technique. Radiology. 1991;181(2):597–602. doi: 10.1148/radiology.181.2.1924811. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science. 1992;256(5064):1692–1695. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Grillner S. Visuomotor coordination in reaching and locomotion. Science. 1989;245(4923):1209–1210. doi: 10.1126/science.2675307. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Lurito JT, Petrides M, Schwartz AB, Massey JT. Mental rotation of the neuronal population vector. Science. 1989;243(4888):234–236. doi: 10.1126/science.2911737. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233(4771):1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Taira M, Lukashin A. Cognitive neurophysiology of the motor cortex. Science. 1993;260(5104):47–52. doi: 10.1126/science.8465199. [DOI] [PubMed] [Google Scholar]
- Goodyear BG, Menon RS. Brief visual stimulation allows mapping of ocular dominance in visual cortex using fMRI. Hum Brain Mapp. 2001;14(4):210–217. doi: 10.1002/hbm.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourtzelidis P, Tzagarakis C, Lewis SM, Crowe DA, Auerbach E, Jerde TA, Ugurbil K, Georgopoulos AP. Mental maze solving: directional fMRI tuning and population coding in the superior parietal lobule. Exp Brain Res. 2005;165(3):273–282. doi: 10.1007/s00221-005-2298-6. [DOI] [PubMed] [Google Scholar]
- Harel N, Lin J, Moeller S, Ugurbil K, Yacoub E. Combined imaging-histological study of cortical laminar specificity of fMRI signals. Neuroimage. 2006;29(3):879–887. doi: 10.1016/j.neuroimage.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hoogenraad FG, Hofman MB, Pouwels PJ, Reichenbach JR, Rombouts SA, Haacke EM. Sub-millimeter fMRI at 1.5 Tesla: correlation of high resolution with low resolution measurements. J Magn Reson Imaging. 1999;9(3):475–482. doi: 10.1002/(sici)1522-2586(199903)9:3<475::aid-jmri17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hu X, Kim SG. Reduction of signal fluctuation in functional MRI using navigator echoes. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1994;31(5):495–503. doi: 10.1002/mrm.1910310505. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34(2):201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Jerde TA, Lewis SM, Goerke U, Gourtzelidis P, Tzagarakis C, Lynch J, Moeller S, Van de Moortele PF, Adriany G, Trangle J, Ugurbil K, Georgopoulos AP. Ultra-high field parallel imaging of the superior parietal lobule during mental maze solving. Exp Brain Res. 2008;187(4):551–561. doi: 10.1007/s00221-008-1318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-G. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kim SG, Hendrich K, Hu X, Merkle H, Ugurbil K. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed. 1994;7(1-2):69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- Lee SP, Silva AC, Ugurbil K, Kim SG. Diffusion-weighted spin-echo fMRI at 9.4 T: microvascular/tissue contribution to BOLD signal changes. Magn Reson Med. 1999;42(5):919–928. doi: 10.1002/(sici)1522-2594(199911)42:5<919::aid-mrm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Menon RS, Hendrich K, Hu X, Ugurbil K. 31P NMR spectroscopy of the human heart at 4 T: detection of substantially uncontaminated cardiac spectra and differentiation of subepicardium and subendocardium. Magn Reson Med. 1992;26(2):368–376. doi: 10.1002/mrm.1910260216. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Ugurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol. 1997;77(5):2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Tank DW, Ugurbil K. 4 Tesla gradient recalled echo characteristics of photic stimulation-induced signal changes in the human primary visual cortex. Magn Reson Med. 1993;30(3):380–386. doi: 10.1002/mrm.1910300317. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Ogawa S, Hu X, Ugurbil K. The nature of spatiotemporal changes in cerebral hemodynamics as manifested in functional magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1997;37(4):511–518. doi: 10.1002/mrm.1910370407. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Thompson DJ, Ogawa S, Hu X, Ugurbil K. Spatio-temporal Patterns in fMRI Data Revealed by Principal Component Analysis and Subsequent Low Pass Filtering. Proc. Int. Soc. Mag Reson Med. 1995;2:817. [Google Scholar]
- Naselaris T, Kay KN, Nishimoto S, Gallant JL. Encoding and decoding in fMRI. Neuroimage. 2011;56(2):400–410. doi: 10.1016/j.neuroimage.2010.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Kay AR, Tank DW. Brain Magnetic Resonance Imaging with Contrast Dependent on Blood Oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM. Magnetic Resonance Imaging of Blood Vessels at High Fields: in Vivo and in Vitro Measurments and Image Simulation. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, Ugurbil K. Functional Brain Mapping by Blood Oxygenation Level-Dependent Contrast Magnetic Resonance Imaging. Biophys J. 1993;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Rottenberg H, Brown TR, Shulman RG, Castillo CL, Glynn P. High-resolution 31P nuclear magnetic resonance study of rat liver mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(4):1796–1800. doi: 10.1073/pnas.75.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Shulman RG, Glynn P, Yamane T, Navon G. On the measurement of pH in Escherichia coli by 31P nuclear magnetic resonance. Biochimica et biophysica acta. 1978;502(1):45–50. doi: 10.1016/0005-2728(78)90130-5. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Path G, Robitaille PM, Merkle H, Tristani M, Zhang J, Garwood M, From AH, Bache RJ, Ugurbil K. Correlation between transmural high energy phosphate levels and myocardial blood flow in the presence of graded coronary stenosis. Circ Res. 1990;67(3):660–673. doi: 10.1161/01.res.67.3.660. [DOI] [PubMed] [Google Scholar]
- Richter W, Somorjai R, Summers R, Jarmasz M, Menon RS, Gati JS, Georgopoulos AP, Tegeler C, Ugurbil K, Kim SG. Motor area activity during mental rotation studied by time-resolved single-trial fMRI [In Process Citation] J Cogn Neurosci. 2000;12(2):310–320. doi: 10.1162/089892900562129. [DOI] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, Kim SG. Time-resolved fMRI of mental rotation. Neuroreport. 1997;8(17):3697–3702. doi: 10.1097/00001756-199712010-00008. [DOI] [PubMed] [Google Scholar]
- Robitaille PM, Lew B, Merkle H, Sublett E, Lindstrom P, From AH, Garwood M, Bache RJ, Ugurbil K. Transmural metabolite distribution in regional myocardial ischemia as studied with 31P NMR. Magn Reson Med. 1989;10(1):108–118. doi: 10.1002/mrm.1910100110. [DOI] [PubMed] [Google Scholar]
- Segebarth C, Belle V, Delon C, Massarelli R, Decety J, Le Bas JF, Decorps M, Benabid AL. Functional MRI of the human brain: predominance of signals from extracerebral veins. Neuroreport. 1994;5(7):813–816. doi: 10.1097/00001756-199403000-00019. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Chaimow D, Raddatz G, Ugurbil K, Yacoub E. Mechanisms underlying decoding at 7 T: ocular dominance columns, broad structures, and macroscopic blood vessels in V1 convey information on the stimulated eye. Neuroimage. 2010;49(3):1957–1964. doi: 10.1016/j.neuroimage.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Brown TR, Ugurbil K, Ogawa S, Cohen SM, den Hollander JA. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49(1):47–60. doi: 10.1002/mrm.10355. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Brown TR, den Hollander JA, Glynn P, Shulman RG. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, A. R. Optimization of modulation functions to improve insensitivity of adiabatic pulses to variations in B1 magnitude. J Magn Reson. 1988;80:448–469. [Google Scholar]
- Ugurbil K, Garwood M, Bendall MR. Amplitude and Frequency Modulated Pulses to Achieve 90° Plane Rotations with Inhomogeneous B1 Fields. J Magn Reson. 1987;72:177–185. [Google Scholar]
- Ugurbil K, Garwood M, Merkle M, Path G, Robitaille PM, Hendrich K, Zhang J, Tristani M, Yoshiyama M, From AHL, Bache RJ. Metabolic consequences of coronary stenosis. Transmurally heterogeneous myocardial ischemia studied by spatially localized 31P NMR spectroscopy. NMR in Biomedicine. 1989;2:317–329. doi: 10.1002/nbm.1940020523. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, Rath A, Bendall MR. Amplitude and Frequency/Phase Modulated Refocusing Pulses that Induce Plane Rotations Even in the Presence of Inhomogeneous Fields. J Magn Reson. 1988;78:472–497. [Google Scholar]
- Ugurbil K, Rottenberg H, Glynn P, Shulman RG. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Rottenberg H, Glynn P, Shulman RG. Phosphorus-31 nuclear magnetic resonance studies of bioenergetics in wild-type and adenosinetriphosphatase(1-) Escherichia coli cells. Biochemistry. 1982;21(5):1068–1075. doi: 10.1021/bi00534a038. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Shulman RG, Brown TR, Shulman RG. Magnetic Resonance in Biology. Academic Press; New York: 1979. High resolution 31P and 13C NMR studies of E. coli in vivo; pp. 537–589. [Google Scholar]
- Yacoub E, Duong TQ, Van De Moortele PF, Lindquist M, Adriany G, Kim SG, Ugurbil K, Hu X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003;49(4):655–664. doi: 10.1002/mrm.10433. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105(30):10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37(4):1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Van De Moortele PF, Shmuel A, Ugurbil K. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24(3):738–750. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim SG. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30(4):1149–1160. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Goebel R, De Martino F, van de Moortele PF, Feinberg D, Adriany G, Chaimow D, Shmuel A, Ugurbil K, Yacoub E. Mapping the Organization of Axis of Motion Selective Features in Human Area MT Using High-Field fMRI. PLoS ONE. 2011;6(12):e28716. doi: 10.1371/journal.pone.0028716. [DOI] [PMC free article] [PubMed] [Google Scholar]